Current status of developments in the field of respiratory syncytial virus vaccines
- Authors: Kotomina T.S.1
-
Affiliations:
- Institute of Experimental Medicine
- Issue: Vol 102, No 2 (2025)
- Pages: 239-264
- Section: REVIEWS
- URL: https://microbiol.crie.ru/jour/article/view/18831
- DOI: https://doi.org/10.36233/0372-9311-675
- EDN: https://elibrary.ru/LFGPNA
- ID: 18831
Cite item
Abstract
Respiratory syncytial virus (RSV) is the leading cause of upper respiratory tract infections in children and the elderly. The only specific treatment approved for RSV is the monoclonal antibody Palivizumab for passive immunoprophylaxis in high-risk infants. Sixty years after the virus was discovered, several safe RSV vaccine candidates have been licensed. This was facilitated by research to identify the structure of RSV, to study the basic functions of RSV components as well as the mechanisms of innate and acquired immune responses to infection. The negative result of a clinical trial of formalin-inactivated RSV vaccine in children, which resulted in the death of several vaccinated individuals, was taken into account.
The aim of the study was to summarize data from studies of RSV vaccine candidates in laboratory animals and in clinical trials on different age groups.
Articles for the analysis of preclinical and clinical trials of RSV vaccines were found, using the PubMed search engine with “respiratory syncytial virus and vaccine” as the keywords. The selection criteria were that original articles should contain information on preclinical and clinical studies, the latter including phase I–IV randomized controlled trials. From 1967 to the present year, 296 articles summarizing data from studies of RSV vaccine candidates and 1788 articles summarizing data from animal trials of vaccine candidates were found. The review summarizes data from preclinical studies of vaccine candidates and their developers, vaccine formulations, animal models on which the studies were conducted, as well as a brief description of the main findings. Data on clinical trials of vaccine candidates are presented, including target populations, clinical trial number and sources where the results of these trials were published.
Full Text
Introduction
Among severe acute human respiratory diseases, respiratory syncytial virus (RSV) accounts for approximately 22% of cases. The number of deaths annually ranges from about 55,000 to 199,000, including 50,000–75,000 among children under 5 years of age [1, 2]. After RSV disease, no lasting immunity is formed, due to which the body is not resistant to subsequent infections [3, 4]. The reasons why this happens have not yet been established. In 2023, GlaxoSmithKline (UK) and Pfizer (USA) licensed subunit vaccines for the prevention of RSV infection in people over 60 years of age. Developer Moderna (USA) has submitted a messenger RNA (mRNA) vaccine for the prevention of RSV infection in the elderly. Many alternative vaccine candidates are in various stages of clinical trials.
The aim of the review is to summarize data on the development of RSV vaccines using different platforms and methodological approaches, the results of their preclinical studies in different animal models and clinical trials in different age groups.
General characterization of respiratory syncytial virus
RSV belongs to the order Mononegavirales, family Pneumoviridae (pneumoviruses), genus Orthopneumovirus. Until 2016, the virus was referred to the family Paramyxoviridae [5]. Another representative of the pneumovirus family is human metapneumovirus. Both pneumoviruses occupy a leading position in the structure of morbidity in children from birth to one year of age, the elderly and immunocompromised persons [6]. RSV infection is accompanied by severe pneumonias and bronchiolitis. According to expert estimates, in 2019, there were about 3.6 million human hospitalizations due to RSV infection and 101,400 related deaths among children aged 0–60 months worldwide [7].
RSV structure.
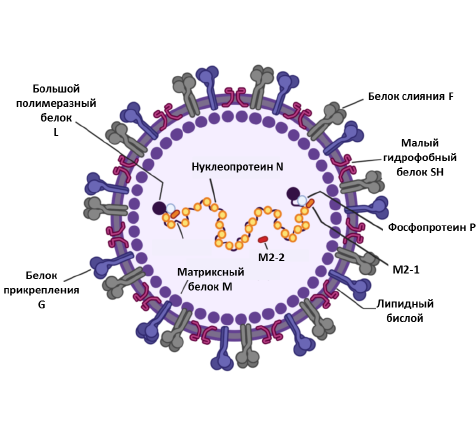
The RSV genome is unsegmented with a length of 15.2 bp, has a negative polarity, and encodes 11 viral proteins. The RSV viral envelope contains 3 transmembrane glycoproteins: attachment protein G, fusion protein F, and small hydrophobic protein SH. On the inner side of the viral envelope is the matrix protein M. The viral RNA is encapsulated by a nucleoprotein (N) and a large protein (L). Phosphoproteins P and M2-1 mediate transcription of viral RNA. M2-2 regulates viral RNA synthesis. The figure was adapted from [16].
RSV virions are pleomorphic: spherical forms with a diameter of 100–350 nm and filamentous forms with a length of up to 10 μm and a diameter of 60–200 nm are distinguished [8, 9]. The genetic material of RSV is represented by single-stranded RNA with negative polarity with a size of 15.2 kb. The RSV genome is unsegmented and contains 10 open reading frames encoding 9 structural and 2 non-structural proteins. The M2 gene contains 2 open reading frames and encodes 2 proteins: M2-1 and M2-2. The viral RNA is packaged inside a nucleocapsid of helical symmetry type, which is a ribonucleoprotein complex (RNP) (Figure). Replication occurs with the participation of an RNA-dependent RNA polymerase consisting of a large L protein and phosphoprotein P (cofactor L). P-, L- and M2-1-proteins participate in the transcription process [10, 11]. RSV refers to enveloped viruses whose genetic material is surrounded by a protein envelope and a lipid bilayer acquired from the host cell during virus assembly and budding. Matrix protein M is located between the capsid and the outer lipid bilayer. The integral membrane fusion protein F, attachment protein G, and small hydrophobic SH protein are embedded in the outer lipid bilayer. The F protein belongs to class I glycoproteins. Its main function is to allow virus entry into the target cell. Prior to interaction with the target cell, protein F is located on the surface of RSV in the “pre-F” conformation and acquires the “post-F” conformation due to the fusion of the viral and cell membrane [12].
The M2-2 protein is involved in replication and transcription [13]. NS1 and NS2 proteins are not part of the virion; their main role is to act as antagonists of α-/β-interferons, which are produced by the human body in response to viral infection [14, 15].
Prototypes of RSV vaccines
RSV was first discovered in 1956 [17], and for more than 60 years, work has been underway to develop a vaccine against RSV. The successful experience with formalin-inactivated vaccines against poliomyelitis, measles and parainfluenza was also applied to the first RSV vaccines, but the attempts were unsuccessful and new approaches to this problem were required.
Most RSV vaccines are developed on the basis of one of the most conserved RSV proteins, F, because this surface protein is the antigenic target for virus-neutralizing antibodies [18]. Approaches in which F-protein is stabilized in pre-F or post-F form have been developed [19, 20]. However, more often developers choose pre-F because virus-neutralizing antibodies are induced in greater amounts to this form of F-protein under conditions of natural infection or immunization [21].
History of RSV vaccines
In the 1950s, formalin inactivation of the virus was recognized as a successful technique [22]. In the United States in the 1960s, pilot trials of formalin-inactivated RSV vaccine (FI-RSV) were first conducted in infants and then large-scale clinical trials of the vaccine in children of different ages. The youngest cohort included children aged 2–7 months. Children in the experimental group (aged 4 months to 10 years) received 2 doses of FI-RSV intramuscularly, while those in the control groups received trivalent parainfluenza vaccine [23]. A 4-fold increase in RSV antibody levels was observed in 68% of those vaccinated with FI-RSV compared with control groups [23]. During the RSV circulation season, children receiving FI-RSV were more severely ill (7.9%) than controls (4.7%) [23]. An increased course of the disease was observed in children younger than 2 years of age [23]. Another study involved children aged 2–7 months; after FI-RSV vaccination, a 6-fold increase in neutralizing antibodies was observed compared with the control group [24]. In the winter period following the vaccination (1966/1967), 21 out of 30 children in the group of FI-RSV-vaccinated children became ill from natural exposure to RSV, whereas in the control group, it only happened in 5% of cases. Sixteen children in the FI-RSV group required hospitalization, while 2 infants died [24]. When comparing the results of trials among different age groups, it was found that children in the youngest group were at the most risk of severe disease progression when immunized with FI-RSV in the case of natural RSV infection. The development of vaccine-induced disease progression was found to be due to the fact that children were initially immunized with FI-RSV. One argument was that children who had previously been ill with RSV did not develop a state of immunopathology following immunization with FI-RSV after RSV infection. According to another argument, intramuscular injection of live RSV to RSV-naïve children did not show a protective effect of vaccination, but also did not develop a state of immunopathology. Thus, when developing RSV vaccine candidates, it is necessary to characterize in detail the phenotype of emerging memory T cells, as well as memory B cells with certain antigenic specificity.
After the unsuccessful FI-RSV clinical trials, attempts to develop a live attenuated vaccine began. The logic of this strategy is that during immunization, RSV will replicate exclusively in the upper respiratory tract, as a result of which the synthesized viral antigen will undergo intracellular processing and presentation on the surface of antigen-presenting cells, which will contribute to the formation of a balanced T- and B-cell immune response. When infants are vaccinated, the immune response will be formed locally in the respiratory mucosa where low levels of maternal IgG antibodies are observed [25]. The main advantage of using such technology is the method of administration by nasal spraying, which is recommended for use in pediatrics.
At the initial stages, certain difficulties were encountered in the creation of live attenuated vaccines due to the peculiarities of RSV cultivation, thermolability and its low viability. Live attenuated RSV can be obtained in several ways: by repeated passaging of the virus at reduced temperature, or by mutagenesis, which involves treatment of viruses with special mutagens, or by introducing mutations into the wild-type virus. To attenuate RSV, manipulations in SH or G proteins are commonly performed [26]. However, clinical trials of vaccine candidates obtained by mutagenesis have shown that a balance between sufficient viability of the vaccine strain and its immunogenicity cannot be achieved. In the 1980s, under the leadership of N.P. Leshchinskaya, a low-temperature strain obtained from Prof. P.M. Chanok (USA) was modified by conducting an additional 16 consecutive passages at a reduced temperature. During intranasal immunization with this vaccine candidate in children aged 1–2 years, clinical reactions of moderate severity were observed, which correlated with a 4-fold increase in specific anti-RSV antibodies. High antibody titers persisted only for 6-8 weeks after vaccination and then decreased to baseline levels [27]. Thus, due to attenuation mechanisms, it was not possible to obtain a ready-made vaccine preparation providing maintenance of long-term protective immunity. At the same time, the development of vaccine-induced pathology has not been shown with the use of live attenuated vaccines. Due to the failure of classical methods, alternative methods of producing RSV vaccines began to be developed.
Current approaches to the development of RSV vaccines
After the unsuccessful FI-RSV clinical trial, vaccine development in this area slowed down as new candidates underwent rigorous safety testing. On the other hand, the involvement of new approaches and technologies to combat RSV has led to notable advances in vaccine prophylaxis.
Vaccine candidates against RSV that have undergone clinical trials have been presented in detail in many reviews [28–31]. Note that such reviews should be updated every year due to the relevance and importance of the issue of vaccination coverage in populations that require RSV vaccine. It is important to select relevant animal species for preclinical trials to test the efficacy and safety of vaccines. In the case of RSV vaccines, a model of vaccine-induced enhancement of disease progression needs to be specifically studied. This problem is discussed in detail in the review by G. Zhang et al. [32]. The totality of a number of indicators characterizing innate and acquired immunity, which are associated with the severity of RSV infection in different animals, is presented in the review by S.B. Drysdale et al. [33]. This review collected data on trials of RSV vaccine candidates in laboratory animals, which were subsequently tested in humans.
mRNA vaccines
Nucleic acid-based vaccines, particularly mRNA vaccines, are an alternative to traditional vaccines. They consist of synthetic mRNA molecules with a structure that allows synthesizing the target protein of the antigen when it enters the target cell. When developing mRNA vaccines, special importance is given to mRNA delivery systems, since mRNA molecules are unstable and are subject to degradation by extracellular ribonucleases. Lipid nanoparticles are more commonly used, but other delivery strategies are being developed. For example, cell-penetrating peptide technology that allows mRNA to be delivered targeted to antigen-presenting cells for induction of an effective immune response [34].
The vaccine candidate mRNA-1345 (Moderna Inc., USA) encodes surface antigen F in a pre-F conformation, which is delivered using lipid nanoparticles (LNPs) [35]. The Spikevax vaccine (Moderna Inc.), used to immunize patients 12 years of age and older against COVID-19, was developed based on the results obtained from mRNA-1345 studies [36]. In 2024, a mRNA-1345 vaccine candidate called mResvia was approved by the U.S. Food and Drug Administration (FDA) for immunization of patients over 60 years of age. The developer initially created several vaccine candidates containing the mRNAs required to elicit expression of protein F in the pre-F conformation (mRNA-1777 and mRNA-1172) [37, 38]. The vaccine candidate mRNA-1345 was derived from genetic modification of the mRNA within mRNA-1172 performed to increase F protein expression and alter its distribution in infected cells [39, 40]. In 2023, the results of a phase III clinical trial (ConquerRSV, NCT05127434) of mRNA-1345 (subsequently mResvia) were announced, according to which the efficacy of this vaccine was 83.7%. The study evaluated the clinical effect of immunization against two symptoms associated with lower respiratory tract disease in humans caused by RSV [41]. Clinical trials of the vaccine candidate mRNA-1345 in children aged 5 months to 2 years are currently underway (NCT05743881). Clinical trials are underway for the safety and efficacy of mRNA-1345 in immunized pregnant and postpartum infants (NCT06143046).
In 2024, phase I clinical trial studies of the world’s first bivalent mRNA vaccine IN006 (InnoRNA, China), approved not only by China’s National Drug Administration but also by the FDA, began. This mRNA vaccine targets the expression of F-protein in the pre-F conformation of both RSV serotypes, A and B. Lipid nanoparticles are used as delivery system1.
Phase I and II clinical trial studies of mRNA vaccine candidates LNP CL-0059 and LNP CL-0137 mRNA vaccines against RSV (Sanofi, France) are currently underway. The study description reports that two different lipid nanoparticles are being tested for mRNA delivery (NCT05639894).
Vector vaccines
Vector vaccines are based on carrier vectors for delivery of RSV antigens to target cells and induction of an immune response against the virus antigens in the insert. RSV vaccines using modified Ankara smallpox vaccine viruses (MVA), adenoviruses, bovine parainfluenza viruses, Sendai virus and influenza viruses as vectors are currently undergoing clinical trials.
In studies at the Institute of Experimental Medicine (Russia), live influenza vaccine (LIV) serves as the vector. Three polyepitope cassettes of RSV were selected for integration into the genome of attenuated influenza virus [42]. The first cassette contains the fragment F243-294, with the antigenic site II of the RSV F-protein to which the monoclonal antibody Palivizumab binds. The other two cassettes are targeted primarily at the activation of cytotoxic T lymphocytes during vaccination and are represented by epitopes within the M2-1 protein of RSV.
Experiments on laboratory mice revealed that recombinant vaccine strains of RSV encoding a polyepitope T-cell cassette induce the development of a stable, fully functional RSV-specific systemic and local CD8+ T-cell immune response that protects immunized animals from RSV reproduction in the lungs. Immunization with a T-cell vector vaccine has been shown to induce the formation of tissue-resident memory T cells to the built-in immunodominant CD8+ T-cell epitope [43]. At the same time, the incorporation of the RSV F243-294 fragment into the HA molecule of influenza virus was found to be insufficient for induction of protective levels of RSV-specific antibodies in mice. However, immunization with such a chimeric virus prevents the development of RS-induced pulmonary pathology [44].
At the Smorodintsev Research Institute of Influenza (Russia), the developers use a modified influenza virus A/PR/8/34, weakened by shortening the NS1 protein to 124 amino acid residues, as a vector for delivery of the RSV F-protein transgene containing antigenic sites II and IV [45]. The resulting vaccine candidate RSV/Flu-01E has undergone phase I clinical trials (NCT05970744) in volunteers of two age groups — 18–59 years of age and elderly over 60 years of age and phase II clinical trials in volunteers over 60 years of age.
The vaccine candidate MVA-BN-RSV (Bavarian Nordic A/S, Denmark) is engineered based on modified Ankara vaccinia virus (MVA). The recombinant vaccinia strain MVA expresses F- and G-proteins of both subtypes (A and B) of RSV, as well as N- and M2-proteins of RSV. The results of phase I clinical trials showed that immunization with MVA-BN-RSV induced the production of humoral and cellular immune response against RSV in elderly (50–65 years old) and in adults (18–49 years old) [46]. In a phase II clinical trial (NCT02873286), MVA-BN-RSV was found to be well tolerated by immunized individuals over 55 years of age and resulted in a durable immune response that persisted for at least 6 months after a single vaccine administration. The results of a second immunization one year after the first immunization showed that the level of T-cell immune response was higher or similar to that observed after the first immunization [47]. However, in a phase III clinical efficacy trial in individuals over 60 years of age, it was recognized that MVA-BN-RSV immunization did not meet the study endpoint and was ineffective2.
In the RSV001 study (NCT01805921), two recombinant vaccines were researched: PanAd3-RSV and MVA-RSV (ReiThera, Italy). PanAd3-RSV was developed based on monkey adenovirus, while MVA-RSV was based on modified Ankara vaccinia virus [48, 49]. The following proteins were used as RSV antigens: F (F0 ΔTM — full-length, F — without transmembrane domain), N and M2-1, which are delivered to the target cell by replication-defective monkey adenovirus vector (PanAd3) or MVA vector [50]. The vaccine candidates PanAd3-RSV and MVA-RSV administered in different combinations by different routes (intramuscular and intranasal administration of PanAd3, intramuscular administration of MVA) were well tolerated and immunogenic in adult humans (18–50 years of age) [51]. In the RSV001 study (NCT01805921), induction of humoral and cellular immune response after immunization was recorded in elderly individuals (60–75 years) [52].
The vaccine candidate VXA-RSV-f (Vaxart, USA) was developed based on adenovirus subtype 5 expressing RSV F-protein and a molecular adjuvant in the form of double-stranded RNA [53]. A phase I clinical trial (NCT02830932) was conducted in humans aged 18–49 years and ended in 2018. Results to date have not been published3.
The vaccine candidate Ad26.RSV.Pre-F (Janssen, Belgium) was designed on the basis of adenovirus type 26 expressing F-protein in the pre-F conformation [54]. In phase I and II clinical trials (NCT03502707), different vaccine administration regimens, including combination with recombinant F-protein, were tested on the elderly (over 60 years of age). Those immunized with the combined regimen showed a more intense production of humoral and cellular immune response compared to the group injected with Ad26.RSV.preF alone [55]. A phase II (NCT03339713) clinical trial evaluated the combined administration of Ad26.RSV.preF and seasonal influenza vaccine (Fluarix, GSK). The vaccine was shown to demonstrate an acceptable safety profile and no evidence of immune interference in the elderly (over 60 years of age) [56]. A phase I/a (NCT03303625) clinical trial (NCT03303625) of Ad26.RSV.Pre-F showed that immunization in RSV-seropositive children aged 12–24 months and adults (18–50 years) produced RSV-specific neutralizing antibodies that persisted for 7 months. In addition, those immunized with Ad26.RSV.Pre-F were less susceptible to RSV infection in vivo [57].
ChAd155-RSV (GlaxoSmithKline, UK) is based on chimpanzee adenovirus-155 and encodes RSV proteins F, N and M2-1 [58]. The vaccine candidate was pre-tested on calves, in which the mechanisms of immune response to RSV are closest to children. After calf vaccination, neutralizing antibodies to RSV were induced, there was no evidence of vaccine-mediated disease enhancement, and protection against bovine RSV was demonstrated [59]. In a phase I/II clinical trial (NCT02927873), RSV-seropositive children aged 12–23 months were immunized with ChAd155-RSV and followed for 2 years after vaccination. As a result, a dose-dependent increase in RSV neutralizing antibody titers was observed [60].
The published results of phase I/II clinical trials (NCT03636906) of ChAd155-RSV in children aged 6–7 months showed that immunization caused induction of the humoral immune response in immunized individuals, and no signs of vaccine-mediated disease enhancement developed [61].
The vaccine candidate MEDI-534 (MedImmune, currently AstraZeneca, USA) is based on a chimeric bovine and human parainfluenza virus type 3 (PIV3) expressing the RSV F-protein [62]. In a phase I clinical trial (NCT00345670) involving children with previous RSV, this live attenuated intranasal vaccine was safe but immunogenicity was very low [63]. However, when tested in RSV seronegative infants, the target population for this vaccine, MEDI-534 was well tolerated by the immunized and induced an immune response against RSV in approximately 50% of cases and against the PIV3 vector in all cases [64].
The vaccine candidate SeVRSV (NIAID, USA) is a replication-capable Sendai virus, a strain of murine parainfluenza virus type 1 (PIV-1) that expresses the F-protein of RSV. Based on a phase I clinical trial (NCT03473002), it was found that immunization with SeVRSV in humans aged 18–45 years results in a low level of immune response to RSV. This fact is explained by the presence of pre-existing immunity to the vector in this age group [65].
Live attenuated vaccine
There are several approaches to the development of live attenuated RSV vaccines. The traditional approach is based on the sensitivity of the virus to certain reproductive temperatures or chemical agents. An alternative approach is based on the use of reverse genetics techniques to produce attenuated replication-competent virus. Vaccines developed using reverse genetics techniques have been tested on infants as young as 4 weeks of age [66, 67]. Live attenuated vaccines are considered safe for children who have not previously had RSV, as their use does not cause a vaccine-induced enhancement of the disease course after RSV infection [24, 68]. Furthermore, live attenuated vaccines are usually available as nasal drops. Once in the respiratory tract, they replicate there, regardless of the presence of maternal antibodies, ensuring the formation of both humoral and cellular immune response [69, 70].
One way to obtain an attenuated strain of RSV is deletion of the SH gene. Several vaccine variants have been developed in this way.
The vaccine candidate rA2cp248/404/1030∆SH was designed with several temperature-sensitive mutations, with mutations obtained by passaging the virus at low temperature, and with a deletion of the SH gene. In an immunogenicity study of rA2cp248/404/1030∆SH on RSV-seronegative children 1–2 months of age, high immunogenicity of the vaccine was observed. The vaccine candidate was weakly immunogenic in RSV-seropositive children and adults [67].
The vaccine candidate MEDI-559 (MedImmune, currently AstraZeneca, USA) differs from the previous candidate by the presence of 39 synonymous nucleotide substitutions [71]. Both vaccine candidates, rA2cp248/404/1030∆SH and MEDI-559, were tested on healthy children aged 5–24 months who had no previous history of RSV. However, the results of the studies revealed insufficient genetic stability of both candidates, a tendency to reversion of individual point mutations leading to decreased temperature sensitivity, which was observed in more than one third of the vaccine virus isolates [71, 72].
Vaccine candidate RSVcps2 (NIAID, NIH/Wyeth, USA) is a stabilized version of the MEDI-559 vaccine. According to the results of phase I clinical trials (NCT01852266 and NCT01968083) in children aged 6–24 months, RSVcps2 was well tolerated and had moderate immunogenicity [73].
An attenuated live vaccine phenotype can be achieved by deletion of the M2-2 RSV gene. Deletion of M2-2 results in a shift in the viral RNA synthesis program, which increases the level of viral RNA transcription and viral antigen expression, but decreases viral genome replication [74].
Two vaccine candidates MEDI/ΔM2-2 (NIAID, USA) and LID/ΔM2-2 (NIAID, USA) were evaluated [75, 76]. Both variants contain the M2-2 deletion and both are derived from different RSV cDNA derivatives of the same A2 subtype, differing by 21 nucleotide sequences located at different sites in the genome. In the vaccine candidate LID/ΔM2-2, non-translated regions of the SH gene were deleted and synonymous substitutions (silent mutations) were added to the SH protein of RSV. The inserted mutations do not affect the phenotype of the assembled virus, as confirmed by experiments in mice [77]. Vaccination with both candidates stimulated the production of RSV-neutralizing antibodies, with LID/ΔM2-2 considered more effective because it provided a slight increase in replication. On average, the peak titer of virus secreted by vaccinated individuals was 100-fold higher in those who received LID/ΔM2-2 [75, 76]. Therefore, it was proposed to modify the vaccine candidate LID/ΔM2-2 by introducing either an additional mutation in the L protein causing temperature sensitivity or 5 point mutations in the N, F, and L proteins, which were previously found in the attenuated RSV strain and associated with adaptation to reduced temperature. Because of active replication, the LID/ΔM2-2 candidate was modified. However, the vaccine candidate LID/cp/ΔM2-2 was subsequently found to be hyperattenuated, had a low infectious titer, and stimulated antibody production in only a fraction of clinical trial participants [76].
The vaccine candidate LID/ΔM2-2/1030s was created by adding the genetically stabilized mutation 1030s. The results of phase I clinical trials (NCT02237209, NCT02040831) on children aged 6–24 months established a high titer of neutralizing antibodies in immunized individuals, which was clinically equivalent to the antibody titer produced by natural RSV infection, according to expert estimates [78].
A modified LID-based vaccine candidate, D46/NS2/N/ΔM2-2-HindIII, was engineered to achieve a similar phenotype to the MEDI/ΔM2-2 candidate. Overall, it was more attenuated than LID/ΔM2-2 but exhibited high viral titers [76].
In a study by S.S. Stobart et al., a recombinant strain of RSV OE4 (RSV-A2-dNS1-dNS2-ΔSH-dGm-Gsnull-line19F) with increased levels of antigen F in the pre-F conformation was shown to exhibit thermostability and immunogenicity despite strong attenuation in the airways of cotton rats [79].
Another promising strategy involves NS1 and NS2 deletions that disrupt the host cell TGFβ signaling pathway, transforming the immune response to enhance viral replication [80, 81].
Phase I phase I clinical phase trials (NCT03227029 and NCT03422237) of the RSV/ΔNS2/Δ1313/I1314L vaccine candidate (Sanofi, France) have established the immunogenicity and protective efficacy of the vaccine in RSV-seronegative children aged 6-24 months [82]. A phase III clinical trial (NCT06252285) of the RSV/ΔNS2/Δ1313/I1314L vaccine candidate is currently underway with 6300 children aged 6–22 months.
Subunit vaccines
Most subunit RSV vaccines under development contain a surface F-protein in the pre-F conformation [83]. The immunogenicity of such vaccines is enhanced by the use of adjuvants or multiple immunizations [84]. Given the composition of subunit vaccines and the failure of FI-RSV clinical trials, special attention is paid to safety. Immunization with FI-RSV has been found to result in insufficient production of antibodies with neutralizing activity. Subsequent signs of vaccine-mediated disease enhancement are attributed, among other things, to poor stimulation of Toll-like receptors in B-cells, which entails a lack of affinity maturation of antibodies [85]. Thus, affinity maturation due to Toll-like receptor stimulation is a key factor in helping to prevent a vaccine-induced disease amplification state. Subunit vaccines are currently being developed for pregnant women, the elderly, and adolescents who have previously had RSV to reduce the risk of vaccine-mediated disease enhancement [30, 86].
The vaccine candidate DS-Cav1 (NIAID, USA) is based on the F-protein in the pre-F conformation [87]. In a phase I clinical trial of DS-Cav1 in adults aged 18-50 years (NCT03049488), it was found that immunized individuals showed an increase in RSV-F-specific antibodies with neutralizing activity. Moreover, the level of neutralizing antibodies was above the baseline level for 10 months after immunization [88]. In this study, aluminum hydroxide was used as an adjuvant.
The vaccine candidate DepoVax-RSV or DPX-RSV (ImmunoVaccine Technologies Inc., Canada) was developed based on the ectodomain of the SH (SHe) protein of RSV. This peptide is administered with DepoVax adjuvant, which ensures prolonged exposure of the antigen to the immune system. According to the results of Phase I clinical trials (NCT02472548), an increase in anti-SHe-specific antibodies was recorded after DPX-RSV administration in immunized people aged 50–64 years old [89].
In May 2023, the FDA approved the first Arexvy vaccine (GlaxoSmithKline, UK) for people over 60 years of age. This vaccine contains RSV F-protein in a pre-F conformation and is administered together with adjuvant AS01E to provide a protective effect against RSV subtypes A and B. According to the results of phase I clinical trials (NCT03814590 and NCT04090658) of Arexvy on the elderly aged 60–80 years, it was found that immunization promoted the formation of RSV-specific IgG antibodies, neutralizing antibodies, and CD4+ T cells which were detectable in the immunized after one year [90, 91]. The results of a phase II clinical trial (NCT04657198) documented an increase in immunization efficacy after revaccination of study participants 1.5 years after the first immunization [92]. A 2b randomized open-label efficacy and safety study of Arexvy was announced between 2023 and June 2025. Participants in the study included people aged 18 years and older who had undergone lung or kidney transplantation and were at increased risk of lower respiratory tract disease compared to healthy people aged 50 years and older (NCT05921903). One study in a phase III clinical trial (NCT04732871) reported an increase in neutralizing antibody titers to RSV and cases of immunization-induced adverse events [93]. The most recently published data from a phase III clinical trial (NCT04886596) evaluated the efficacy of Arexvy immunization in 24,967 participants during 2 epidemiologic seasons of RSV circulation. The efficacy of Arexvy immunization was about 67.2% for lower respiratory tract disease and about 78.8% for severe lower respiratory tract disease caused by RSV [94].
Based on the interim results of a phase III clinical trial (NCT05035212), another subunit vaccine for the prevention of RSV infection in the elderly, Abyrsvo (Pfizer, USA), which contains RSV F-protein in the pre-F conformation, was approved. According to the results of phase I clinical trials of Abyrsvo, immunized people produced RSV-specific IgG antibodies to A- and B- strains of RSV [95]. In phase II clinical trials Abyrsvo was tested in non-pregnant and pregnant women (NCT04071158 and NCT04032093). A phase III clinical trial (NCT04424316) in pregnant women has recently been completed. Infants born to mothers immunized during pregnancy were found to have a lower incidence of RSV-associated diseases than those born to unimmunized mothers [96]. The phase III clinical trial RENOIR (NCT05035212) determined that the efficacy of prevention of RSV-induced lower respiratory tract disease was about 66.7% [97]. The MONET (NCT05842967) phase III clinical trial immunized people aged 18-59 years with chronic diseases at risk for severe RSV. The vaccine was well tolerated, safe and immunogenic [101]. Overall, the rates of RSV-specific neutralizing antibodies in immunized individuals from the MONET study were not lower than those in immunized older adults over 60 years of age from the RENOIR study (NCT05035212).
Vaccines based on virus-like particles
Vaccines based on virus-like particles are considered to be a subclass of subunit vaccines. These vaccines are synthesized by self-assembly of nanoparticles that exhibit multiple copies of a selected viral antigen on their surface. A high level of humoral and cellular immune response is achieved not only due to the multiple repetition of antigen sites, but also due to the immunostimulatory properties of the matrix for immobilization of nanoparticles [99]. The absence of viral genome in the composition of these vaccine candidates makes them safe due to their inability to reproduce viable virions.
The vaccine candidate ResVax (Novavax, USA) is based on the F-protein of RSV and is a vaccine based on virus-like particles. The vaccine is being developed to protect infants by vaccinating expectant mothers, children aged 6 months to 5 years and elderly individuals over 60 years of age. A phase II clinical trial (NCT02247726) found that immunization of healthy pregnant women induced the production of neutralizing antibodies to RSV that were effectively transmitted to the newborn [100]. The success of this phase of research led to the inclusion of the vaccine candidate ResVax in the PREPARE study program (a multicenter, randomized, placebo-controlled phase III study, NCT02624947). The clinical trial involved follow-up of infants born at the very beginning of the epidemic season of RSV circulation from mothers immunized with ResVax in the third trimester [101]. It turned out that ResVax immunization was not effective. Despite this, it was stated that ResVax reduced hospitalizations in children associated with RSV-related lower respiratory tract infections by 44%. Furthermore, a 39.4% reduction in RSV-specific lower respiratory tract infections and a 58.8% reduction in RSV-associated hypoxemic respiratory failure in infants younger than 3 months of age have been reported [101].
The vaccine candidate SynGEM (Virtuvax, The Netherlands) is a mucosal vaccine containing an F-protein in a pre-F conformation bound to bacteria-like particles derived from Lactococcus lactis. Due to the bacteria-like particles, the RSV vaccine antigen appears in a more natural conformation and an enhanced immune response against RSV is observed [102]. Clinical trials of the vaccine without adjuvant were ineffective in the elderly group. Vaccination contributed to a 61% reduction in hospitalizations in the elderly with chronic obstructive pulmonary disease. According to the results of phase I clinical trials, no increase in neutralizing antibodies in sera, which recognize epitopes within the unique Ø site of the F-protein, was observed in immunized individuals [103, 104]. Nevertheless, the vaccine induced the production of palivizumab-like antibodies. Currently, clinical trials of this vaccine have been suspended.
Vaccine candidate V-306 (Virometrix, Switzerland) contains a peptide consisting of mimetics of the F-protein RSV mimicking antigenic site II. The peptide is conjugated to synthetic nanoparticles made of self-organizing lipopeptides. In a Phase I clinical trial (NCT04519073), the V-306 vaccine was found to be safe and immunogenic in healthy women aged 18–45 years old [105].
Conclusion
RSV is the cause of severe respiratory diseases in children of the 1st year of life, immunocompromised people and the elderly [106, 107]. The clinical presentation of RSV varies from mild upper and lower respiratory tract infections to pneumonias and bronchiolitis in children.
To date, the FDA has approved the use of 3 vaccines for the prevention of RSV infection in humans. The bivalent subunit vaccine Abrysvo (Pfizer, USA) is approved for use in pregnant women and the elderly. Arexvy subunit vaccine (GlaxoSmithKline, UK) and mRESVIA mRNA vaccine (Moderna, USA) are approved for use in elderly people over 60 years of age.
The development and efficacy studies of RSV vaccines for children, who represent the main target group, are ongoing. In this case, the main problems include the need to vaccinate infants at an early stage (2-4 months), the revealed effect of enhancing the course of the disease associated with the introduction of FI-RSV with alum adjuvant, and difficulties in achieving the required indicators of prophylactic efficacy. For a long time, the only specific treatment for RSV infection was the monoclonal antibody drug Palivizumab, usually prescribed to people at high risk of severe forms of the disease. In this case, a significant factor is the high cost of treatment with this drug, which makes it unaffordable in countries with the highest incidence of RSV infection [108].
In 2022–2023, the monoclonal antibody drug Nirsevimab (AstraZeneca, UK; Sanofi, France) was approved and recommended for children in cases of severe RSV infection and its complications [109].
Vaccines are currently being developed for women of reproductive age and pregnant women to protect a cohort of naïve infants, as well as to protect the elderly and children. Vaccines based on virus-like particles, vector, subunit and live attenuated vaccines are in various phases of clinical trials.
Up-to-date information on vaccine candidates in preclinical studies is presented in Table 1. Information on candidates studied in different phases of clinical trials is summarized in Table 2.
Table 1. Results of preclinical trials of RSV vaccine candidates
Vaccine (developer) | Vaccine composition (adjuvant) | Animal models (method of administration) | Research and results | Source |
Live attenuated vaccines | ||||
RSV-MinL4•0 (Codagenix, USA) | RSV-Min L – vaccine candidate obtained by introducing silent mutations (codon deoptimization) (without adjuvant) | African green monkeys (i.n. + i.t.). | RSV-MinL4•0 is more attenuated than RSV A2 when injected with 2 × 106 PFU (106 i.n. and i.t. each): the value of peak viral titers in tracheal smears in the group immunized with RSV-MinL4 is 100 times lower than in the wt RSV group. When RSV A2 was infected with RSV at a dose of 2 × 106 PFU on day 104 of the experiment, the protective potential of RSV-MinL4•0 was revealed: the peak values for virus isolation on day 6 in swabs from the trachea and from the oropharynx were 1000-fold and 100-fold lower than in the naive monkey group, respectively | [110] |
IT-RSVΔG (Intravacc, Netherlands) | RSV isolate of strain 98-25147-X (pRSV-X) in which the gene encoding the G-protein (ΔG) has been deleted (without adjuvant) | Cotton rats (i.n.). | pRSV-X ΔG was more attenuated compared to the pRSV-X vector when injected with 105 TCID50: peak titers in the lungs and noses were 4-5 times lower than in the pRSV-X group. A single vaccination with rRSV-X ΔG protected rats against RSV-X virus infection at a dose of 106 TCID50: on day 5 after the challenge trial, virus was undetectable in the lungs of immunized animals, whereas in the placebo group the virus multiplied up to 5 log10 TCID50 in the lungs. Vaccination with pRSV-X ΔG did not result in the development of bronchopulmonary pathology, and histopathologic signs of lesions were significantly reduced compared to the naive control group | [111] |
MV-012–968 (Meissa, USA) | RSV-A2-dNS1-dNS2-ΔSH-dGmGsnull-line19F codon deoptimization of NS1, NS2 genes; deleted SH gene (ΔSH). F protein in a “pre-fusion” | BALB/c mice (i.n.) | When MV-012-968 was administered at a dose of 106 focal forming units (FFU), the viral load in the lungs was significantly lower than in mice infected with RSV A2 and RSV A2-19F. MV-012-968 vaccination protected mice from RSV A2-line19F infection at a dose of 105 FFU: no virus was isolated in the MV-012-968 group 5 days after the challenge trial. Lung mucus production in candidate MV-012-968 was significantly lower than in RSV A2-line 19F and RSV A2-del-M2 at 5 days after the challenge trial. | [79] |
Cotton rats (i.n.). | Replication of MV-012-968 (OE4) administered at a dose of 105 FFU was absent in the noses and lungs of cotton rats. MV-012-968 (OE4) completely protected rats from RSV-A2-line19F infection at a dose of 106 FFU on day 42 after vaccination. In contrast to FI-RSV, vaccination with MV-012-968 (OE4) did not induce histopathologic changes in the lungs after RSV infection | [19] | ||
RSV ΔNS2/Δ1313/I1314L (NIAID/NIH, Sanofi, USA) | Candidate with ts mutations in the L-protein (deletion at position 1313, compensatory mutation I1314L that results from passaging the virus at progressively higher temperatures); NS2 deletion (ΔNS2) (without adjuvant) | BALB/c mice (i.n.) | Candidate RSV ΔNS2/Δ1313/I1314L is hyperattenuated in mice. The RSV/Δ1313 variant multiplied significantly weaker in the respiratory tracts of mice when administered at a dose of 106 PFU compared with wt RSV. This animal model is not suitable for evaluating the immunogenicity and protective efficacy of hyperattenuated vaccine candidates | |
Juvenile chimpanzees (i.n. + i.t.) | When chimpanzees were immunized with RSV ΔNS2/Δ1313/I1314L at a dose of 106 PFU each (i.n. + i.t.), peak viral load values were 3-25-fold lower compared with the highly attenuated candidates MEDI-559 (rA2 cp248/404/1030ΔSH) and cps2 (a genetically stabilized version of MEDI-559) in nasal flushes. In contrast, RSV ΔNS2/Δ1313/I1314L virus titer in bronchoalveolar lavage (BAL) and tracheal washings was 20-fold higher than in the comparison groups. The vaccine induced high levels of RSV virus-neutralizing antibodies; however, experiments on protection against RSV infection were not performed in this study | [113] | ||
Recombinant vector vaccines | ||||
MVA-BN RSV (Bavarian Nordic BN, Denmark) | MVA (modified Ankara vaccine), which includes genes encoding F, G, N, and M2-1 (RSV A) and G (RSV B) (without adjuvant) | BALB/c mice (i.n.) | Mice were immunized with MVA-BN RSV at a dose of 1 × 108 TCID50 at 3 week intervals and 2 weeks later were infected with RSV A2 at a dose of 1 × 106 PFU. No virus was detected in the lungs 4 days after the challenge trial. In the groups in which selective depletion of CD4 and CD8 T cells was used, virus replication in the lungs was 600- to 2,000-fold weaker than in the control group | [114] |
PanAd3-RSV и MVA-RSV (ReiThera, Italy) | PanAd3 (monkey adenovirus) and MVA, which include genes encoding F, N, M2-1 (RSV) (without adjuvant) | Cotton Rats (i.n., i.m.). | PanAd3-RSV (5 × 108 viral particles) and MVA-RSV (1 × 107 PFU) were administered in different combinations at an interval of 4 weeks. In all groups, rats were protected against RSV administered at a dose of 1 × 105 PFU: the virus did not replicate in the lungs on the 5th day. Challenge virus did not reproduce in the nares in the PanAd3-RSV/MVA (i.m.) and PanAd3-RSV/PanAd3-RSV groups. Pathologic changes in the lungs after the challenge trial were less pronounced in the PanAd3-RSV/MVA (i.m.) group compared to the FI-RSV group, whereas the other groups showed histopathology similar to the naive control group after the challenge trial. | [115] |
Calves (2-4 weeks) (i.n., i.m.) | PanAd3-RSV (5 × 1010 viral particles) and MVA (2 × 107 PFU) were administered to calves in different combinations at 4 week intervals. On day 6 after the challenge trial, RSV administered at a dose of 104 PFU did not replicate in the respiratory tract of calves from the PanAd3-RSV/MVA (i.m.), PanAd3-RSV (i.m.)/PanAd3-RSV (i.m.), PanAd3-RSV/PanAd3-RSV (i.m.), and PanAd3-RSV (i.m.)/MVA (i.m.) groups. In the PanAd3-RSV/PanAd3-RSV group, the virus reproduced in the lungs. In the PanAd3-RSV/MVA (i.m.) group, virus was not detected in the nares and no macroscopic lung lesions were found. Calves immunized with the heterologous prime-boost scheme developed less massive infiltration of polymorphonuclear neutrophils in the lungs and BAL after the challenge trial compared to calves from the homologous prime-boost groups | [115] | ||
Macaque (i.n., i.m.). | PanAd3-RSV (5 × 1010 viral particles) and MVA (2 × 107 PFU) were administered to macaques in different combinations at 8-week intervals. Production of interferon-γ and interleukin-4 in peripheral blood mononuclear cells was increased 6-fold after boost. Interleukin-4 production in mononuclear cells was higher in the PanAd3-RSV/MVA (i.m.) group compared with the PanAd3-RSV (i.m.)/MVA (i.m.) group. 1 week after the 2nd immunization, the mononuclear cells were dominated by a population of CD4+ T cells. Only macaques immunized with PanAd3-RSV produced RSV-specific IgA. Macaques immunized with PanAd3-RSV (i.m.) had a 40-fold higher titer of neutralizing antibodies compared with PanAd3-RSV. The protective effect of immunization was not evaluated | [50] | ||
Ad26.RSV.F and Ad35.RSV.F (Janssen Vaccines, Netherlands) | Adenoviruses of types 26 and 35 that include the gene encoding F in the pre-F conformation (without adjuvant) | BALB/c mice (i.m.) | Double immunization of mice at 4-week intervals with candidate Ad26.RSV.F and Ad.35.RSV.F in different combinations at a dose of 1010 viral particles stimulated the production of RSV F-specific T-cell immune response at week 12: high levels of interferon-γ, tumor necrosis factor-α and interleukin-2 CD8+ T-cell production was in the Ad.35.RSV.F/Ad26.RSV.F and Ad.26.RSV.F/Ad35.RSV.F groups. The immune response in mice in all groups developed Th1 type: high IgG2a/IgG1 ratios were recorded compared to the FI-RSV group. High level of neutralizing antibodies in Ad.35.RSV.F/Ad26.RSV.F and Ad.26.RSV.F/Ad35.RSV.F groups | [54] |
| Cotton rats (i.m.) | Double immunization with Ad26.RSV.F and Ad.35.RSV.F variants according to the scheme of homologous and heterologous prime-boost at a dose of 1010 viral particles induced high levels of virus-neutralizing antibodies and fully protected animals from RSV replication in the respiratory tract without causing pathological changes in lung tissues after the challenge trial. The possibility of reducing the vaccine dose was evaluated by immunizing animals with Ad26.RSV.F and Ad.35.RSV.F once at doses of 106–108 viral particles and exposing them to RSV A2 infection 7 weeks later. All immunized animals were completely protected from virus replication in the lungs, and replication in the nares was significantly reduced in all groups compared with controls. Histopathology scores were similar in both groups and did not differ from the naive control group. The duration and cross-protective potential of the vaccine were evaluated by immunizing rats twice with homologous and heterologous prime-boost candidates taken at doses of 108 and 1010 viral particles and infecting the animals with RSV A2 or RSV B15/9 30 weeks after immunization. Challenge viruses did not replicate in the lungs in all vaccine groups, with the Ad26.RSV.F/Ad.35.RSV.F combination (1010 viral particles) better protecting animals from RSV B15/9 replication in the nares. In contrast to FI-RSV, the tested vaccines did not cause histopathologic changes in lung tissues after the challenge trial. | [54] |
|
|
| Cotton rats (i.m.). | The protective efficacy of single immunization with Ad26.RSV.preF at a dose of 105–108 viral particles and double immunization with RSV.preF protein was compared. On day 49, rats were injected with RSV A2 at a dose of 105 PFU. In the Ad26.RSV.preF group, the challenge virus did not reproduce in the noses and lungs in contrast to the RSV.preF group. A correlation was established between the production of neutralizing antibodies and protection of animals from RSV infection | [116] |
VXA-RSV f oral (Vaxart, USA) | Ad-RSVF is a type 5 adenovirus that contains a gene encoding a full-length F protein (without adjuvant) | Cotton rats (or.) | Double immunization of rats with Ad-RSVF at different doses (108-1010 PFU) protected rats from RSV A2 infection at a dose of 1 × 105 PFU on day 70: the virus did not reproduce in the noses and lungs in the group where the vaccine dose was higher (dose-dependent protective effect of immunization). The histopathology rate in the Ad-RSVF group was much lower than in the FI-RSV group. The level of induction of pro-inflammatory cytokines was minimal in the Ad-RSVF group | [117] |
ChAd155-RSV GS (GSK, UK) | Chimpanzee adenovirus containing genes encoding F-protein (deletion of transmembrane region), N, M2-1 (without adjuvant) | Calves (i.m.) | Calves were immunized with ChAd155-RSV at a dose of 5 × 1010. Challenge trial was performed shortly (4 weeks) or long (16 weeks) after immunization. In contrast ChAd155-RSV unimmunized calves showed fever, high fever, respiratory distress syndrome, high levels of pulmonary consolidation, and histopathologic changes in lung tissue after the challenge trial. When the challenge trial was performed after 4 weeks, virus replication in BAL and nasopharyngeal washings in the unimmunized was 500-1000 times and 10 times higher, respectively, than in the ChAd155-RSV group. When the challenge trial was performed at week 16, peak viral titers in BAL and nasopharyngeal washings were approximately the same in the ChAd155-RSV and PBS groups, but viral clearance occurred earlier. In order to establish the role of pre-existing immunity to RSV, calves were fed milk containing antibodies to RSV prior to immunization with ChAd155-RSV or PBS. In the ChAd155-RSV group, calves with RSV antibodies showed no signs of RSV infection (malaise, fever, respiratory rate) in contrast to the PBS group, where calves had RSV antibodies. In the ChAd155-RSV groups with RSV antibodies, the challenge virus reproduced 100 and 10 times less in the BAL and nasal cavity than in the PBS group | [118] |
SeV/RSV (NIAID, USA) | Sendai virus (parainfluenza virus type 1), which contains a gene encoding full-length F (RSV) (without adjuvant) | African green monkeys (i.n. + i.t.) | SeV/RSV was attenuated when administered at a dose of 2 × 106 EID50. The virus was not secreted in nasal washings and was 1000-fold more attenuated than b/h PIV-3 RSV F2. On day 28, monkeys were infected with RSV A2 at a dose of 1.4 × 106 EID50. Challenge virus was secreted in the upper respiratory tract to a lesser extent in the SeV/RSV group compared with controls and was not isolated from the BAL. No adverse effects were observed in the SeV/RSV group after the challenge trial. Lung slices showed small foci of lymphohistiocytic inflammation around terminal and respiratory bronchioles |
|
rBCG-N-hRSV (Catholic University of Chile, Chile) | Bacillus Calmette-Guerin (BCG) bacterium that contains the gene encoding the N protein of RSV (without adjuvant) | BALB/c mice (s.c.) | Mice were injected with rBCG-N-hRSV or BCG-WT at a dose of 3 × 105 PFU. After 3 weeks, mice were infected with RSV at a dose of 1 × 107 PFU. The BCG-WT and unimmunized mice had approximately 10% weight loss, whereas weight loss was negligible in the rBCG-N-hRSV group. The viral load in the BAL was 7- and 300-fold lower in the rBCG-N-hRSV group than in the BCG-WT and unimmunized mice groups, respectively. In histological sections of lungs, inflammation was less pronounced in the rBCG-N-hRSV group than in the groups of unimmunized mice and BCG-WT. In the long-term protection study, the challenge trial was performed 50 days after immunization. Mice in the rBCG-N-hRSV group had less weight loss compared to the other groups, the number of infiltrating cells in the BAL and the viral load were lower than in the groups of unimmunized mice and BCG-WT mice |
|
|
| BALB/c mice (s.c.) | Mice were immunized with BCG-N, BCG-M2 at a dose of 1 × 108 PFU. Mice were infected intranasally with RSV at a dose of 1 × 107 PFU. After the challenge trial, mice significantly lost weight in the control groups (BCG-WT, UV-RSV, BCG-OVA). No weight loss occurred in the BCG-N and BCG-M2 groups. Computed tomography demonstrated no evidence of pneumonia and inflammation in the lung tissue in BCG-N, BCG-M2 groups, in contrast to the group of unimmunized mice after the challenge trial. No active neutrophil infiltration in the BAL was recorded in the BCG-N, BCG-M2 groups after the challenge compared to the unimmunized mice. After the challenge trial, BCG-N and BCG-M2 groups were assigned low pulmonary histopathology scores equivalent to a naive control group that was not infected with RSV. BCG-N and BCG-M2 immunization was found to stimulate primarily T-cell immunity: T cells with a Th1-like pattern of cytokine secretion were activated |
|
|
| BALB/c mice (RAG-1 deficient) (s.c.) | Mice were immunized 2 times 10 days apart with BCG-N, BCG-M2, and BCG-WT at a dose of 1 × 108 PFU. After 21 days, mice were infected with RSV at a dose of 1 × 107 PFU. Mice in the BCG-N group actively lost weight (on par with unimmunized RSV-infected mice). After RSV infection, the BCG-N and BCG-WT groups had large numbers of infiltrating cells such as neutrophils and eosinophils in the BAL. On day 6 after challenge, the viral load in the lungs was almost the same in all groups. On day 12, a decrease in RS viral load was observed in the group of BALB/c mice (not RAG-1). These results suggest that T cells are required for RSV clearance and that the mechanisms of innate immunity are insufficient to protect against RSV infection. | [122] |
|
| Holstein calves (s.c.) | Calves were injected with rBCG-N-hRSV or WT-BCG at a dose of 106 PFU 2 times with an interval of 2 weeks. After 2 weeks, calves were infected with RSV at a dose of 104 TCID50. All calves developed signs of RSV (fever, lethargy, nasal and ocular discharge, mild dyspnea), but clinical condition scores were lower in the rBCG-N-hRSV and WT-BCG groups than in the unimmunized group. There were no differences in pulmonary lesion scores between the rBCG-N-hRSV, WT-BCG, and unimmunized calf groups after the challenge trial and no evidence of vaccine-mediated disease enhancement | [123] |
MVA-F and MVA-G (National Heart and Lung Institute, UK) (Institute of Molecular Virology, Germany) | MVA vector carrying genes encoding F- and G-proteins of RSV | BALB/c mice (i.p.) | Double immunization of mice with MVA-F and MVA-G variants at a dose of 108 infectious units resulted in the formation of pronounced humoral and T-cell immunity to RSV. Infection of immunized mice with RSV at a dose of 1.2 × 106 PFU showed accelerated virus elimination in the MVA-F and MVA-G groups compared with the group of mice immunized with the MVA vector. Signs of eosinophilia after challenge were absent in the MVA-F and wtMVA groups and were small in the MVA-G group. However, lymphocytosis was pronounced in the MVA-F group (~50%) compared with the MVA-G group (~20%). There were more polymorphonuclear cells in the MVA-G group, which was comparable to the FI-RSV group. After challenge, mice actively lost weight in the MVA-G (up to 15%), MVA-F (~12%) and FI-RSV (~10%) groups compared with the group of control animals after RSV challenge, indicating an exacerbation of the disease course after immunization and RSV infection | [124] |
rVV-G и rVV-F (University of Pittsburgh School of Medicine, USA) | Cowpox viruses containing genes encoding F- or G-protein in different forms: rVVGsol (soluble, secreted form), rVVGmem (form anchored in membrane), rVVG (both forms) | BALB/c mice (i.p., scar.) | Immunization of rVVF, rVVG and rVVGmem mice resulted in the induction of high levels of antibodies, regardless of the route of administration. In the rVVGsol group, the level of RSV-specific antibodies was ~550-fold higher with i.p. administration than with immunization by scarification. When immunized mice were infected with RSV A2 strain, virus was not isolated from the lungs in the rVVF, rVVG, rVVGmem, and rVVGsol groups. When immunization by scarification was performed in the rVVG and rVVGsol groups, an increase in the number of eosinophils in the BAL after the challenge trial was observed. The rVVGmem group had 10-fold higher eosinophil counts than the rVVG and rVVGsol groups. No eosinophils were detected in the BAL during i.p. administration. The method of vaccine administration influenced the development of pulmonary pathology | [125] |
LAIV + NA/RSV and LAIV + NS1/RSV (Institute of Experimental Medicine, Russia) | Recombinant LDHV vaccine strains encoding the PCV M2-1 polyepitope T-cell cassette (70-101+114-146) | BALB/c mice (i.n.) | Mice were immunized 2 times 3 weeks apart with vaccine variants LAIV + NA/RSV or LAIV + NS1/RSV at a dose of 106 EID50 per mouse. Three weeks after the 2nd immunization, mice were infected intranasally with RSV strain A2 at a dose of 2 × 105 PFU/individual. RSV was found to be detected at insignificant levels in the LAIV + NA/RSV and LAIV + NS1/RSV groups, in contrast to the PBS and LAIV groups in lungs collected at day 5. In contrast to the FI-RSV group, vaccine variants LAIV + NA/RSV and LAIV + NS1/RSV did not induce pulmonary eosinophilia and lung pathology after RSV infection | [126] |
LAIV + NA/RSV and LAIV + NS1/RSV (Institute of Experimental Medicine, Russia) | Recombinant LDHV vaccine strains encoding the PCV M2-1 polyepitope T-cell cassette (70-101+114-146) | BALB/c mice (i.n.) | Mice were immunized 2 times with an interval of 3 weeks with vaccine variants LAIV + NA/RSV or LAIV + NS1/RSV at a dose of 106 EID50. Seven days after the 2nd immunization, lungs were harvested from mice to identify subpopulations of resident T cells. Immunization with LAIV + NA/RSV or LAIV + NS1/RSV was found to produce high levels of tissue-resident memory T cells to an embedded immunodominant CD8+ T-cell epitope. Moreover, the levels of influenza-specific memory CD8+ T-cells in the LAIV + NA/RSV and LAIV + NS1/RSV groups were higher than in the LAIV group | [43] |
LAIV-HA + G-RSV and LAIV-HA + A-RSV (Institute of Experimental Medicine, Russia) | Incorporation of the target fragment F243–294 of RSV into the HA molecule, which is the target fragment of LAIV | BALB/c mice (i.n.) | Vector vaccine candidates differed in the composition of the chimeric HA + RSV gene: the RSV insert was linked to the HA1 subunit using either AAAPGAA (A) or G4SG4S (G) linker. Mice were immunized 2 times 3 weeks apart with vaccine variants LAIV-HA + G-RSV or LAIV-HA + A-RSV at a dose of 106 EID50. Three weeks after the 2nd immunization, mice were infected intranasally with RSV strain A2 at a dose of 5 × 105 PFU/specimen. On the 5th day after infection, the extent of RSV replication in lung tissue was assessed. Immunization with LAIV-HA + G-RSV and LAIV-HA + A-RSV failed to prevent the development of RSV. Nevertheless, there was a significant difference between the virus titer values in the LAIV-HA + G-RSV group compared to PBS. Evaluation of the bronchial epithelium of mice revealed that the degree of damage was significantly lower in the LAIV-HA + G-PSV group compared to the PBS group, whereas no significant difference was found for the vaccine variant LAIV-HA + A-PSV. Histopathological evaluation of mouse lung segments after RSV infection showed a high degree of protection in the groups immunized with the vector vaccines LAIV-HA + A-RSV and LAIV-HA + G-RSV, in contrast to FI-RSV | [45] |
PR8-NS-F, PR8-sF-NS (A.A. Smorodintsev Research Institute of Influenza, Russia) | Influenza virus A/PR/8/34 truncated to 124th amino acid (NS124), which includes an immunogenic F-protein cassette (fragment F248–290 and F409–451) with or without IgGκ signal peptide (for extracellular delivery). | BALB/c mice (i.n.) | Mice were immunized once with vaccine candidates NS-2AF (contains a 2A site at the N-terminus of the RSV insert), NS-2AsF (NS-2AF, which contains IgGκ), NS-F (shortened NS1), sF-NS (NS-F, which contains IgGκ) at a dose of 6 log10 TCID50. On day 28, RSV A2 was infected at a dose of 6 log10 PFU. After challenge, viral load levels in immunized mice were 1.5 log10 PFU/mL and 2.26 log10 PFU/mL lower in the NS-2AsF and NS-2AF groups, respectively, compared with the group of non-immunized mice. In the 2nd experiment, in the sF-NS group, the viral load was 2 log10 PFU/mL less after the shuttle challenge compared with the group of unimmunized mice. In the NS-F group, the reproduction of the challenge virus was comparable to the group of unimmunized mice. Mice in the NS-2AsF group showed low levels of inflammation and minimal lymphocytic infiltration. Minimal changes in lung tissue morphology were observed in the sF-NS group | [46] |
Subunit vaccines | ||||
DS-Cav1 (NIAID, USA) | Soluble version of F protein in pre-F conformation with antigenic site Ø (poly I:C) | Mice CB6F1/J (♀BALB/cJ + ♂ C57BL/6J (i.m.) | Mice were injected with RSV pre-F in different variants (DS, Cav1, TriC, DS-Cav1) at a dose of 10 μg at an interval of 3 weeks. In all groups, the titer of neutralizing antibodies against RSV in sera was 4 times higher than in the RSV post-F group and 20 times higher than the established protective threshold | [127] |
| Soluble version of F protein in pre-F conformation with antigenic site Ø (poly I:C) | Rhesus macaques (i.m.). | Macaques were administered RSV pre-F in different variants (DS, DS-Cav1) at a dose of 50 μg with an interval of 4 weeks. In the DS and DS-Cav1 groups, the neutralizing antibody titer was 5–10 times higher in sera than in the RSV post-F group. By week 8, the neutralizing antibody titer was higher in the DS-Cav1 group than in the DS group and 60-fold higher than in the RSV post-F group | [127] |
| Soluble version of F-protein in the pre-F conformation with antigenic site Ø (nanoemulsion, Adju-Phos) | African monkeys | Monkeys were immunized three times with DS-Cav1 (125 μg) in different volumes with different adjuvants. On day 70, animals were infected with RSV at a dose of 2 × 5.5 log10 PFU. In the DS-Cav1 group (administered in a volume of 0.25 ml), the value of peak RSV titers was 3 log10 and 2 log10 lower in the lungs and nasal cavity than in the control group. A correlation was found between the protective effect in the nasal cavity and IgA stimulation, and protection in the lungs and Fc-mediated antibody activity | [128] |
| Soluble version of F protein in pre-F conformation with antigenic site Ø (Alum, Poly I :C, Poly (IC:LC), MPLA, SAS, Alum + MPLA, SAS + Carbopol, Adjuplex, AddaVax). | CB6F1/J mice (i.m.) | Mice were injected with RSV F DS-Cav1 in combination with different adjuvants 2 times with an interval of 3 weeks at a dose of 10 μg. No side effects were observed in all groups. RSV F DS-Cav1 groups with SAS + Carbopol adjuvants had the highest neutralizing antibody titers, which were 15-fold higher than in the Alum + MPLA and Alum groups, and 5-fold higher than in Poly (I:C). In the groups with MPLA, Alum + MPLA, AddaVax and Adjuplex, IgG1-immune response was recorded in sera. Vaccination in groups with a combination of SAS, SAS + Carbopol, Poly (I:C) and Poly (IC:LC) adjuvants induced IgG1- and IgG2a-immune response. The neutralizing antibody titer in the RSV F DS-Cav1 group without adjuvant was below the protective threshold value | [129] |
| Soluble version of F-protein in the pre-F conformation with antigenic site Ø (ISA 71 VG, or ISA 71 VG + Carbopol)) | Calves (i.m.) | Calves were injected with bRSV F DS-Cav1 with different adjuvants 2 times at an interval of 4 weeks at a dose of 50 µg. No difference was observed between neutralizing titers in the ISA 71 VG and ISA 71 VG + Carbopol adjuvant groups. This adjuvant combination slightly enhances the immune response in cattle |
|
DPX-RSV (Immunovaccine, USA) | The extracellular SH domain of the RSV protein SHe (subtype A) incorporated into the DepoVax oil-based platform (Pam3CSK4 and Alum) | CD-1 mice (i.m.) | Mice were injected with SHe at a dose of 25 μL mixed with alum (Alum-SHe) or as part of DPX (DPX-SHe) once or twice (3 weeks apart). Mice immunized with 1 or 2 doses of DPX-SHe had higher IgG antibody titers than the Alum-SHe group and persisted for 20 weeks after immunization. Immunization with Alum-SHe did not result in adverse reactions (decreased activity, cyanosis, decreased body temperature, and hunched posture), unlike the DPX-SHe group. A decrease in complement proteins (C3 and C4) and hypersensitivity reactions were observed in the Alum-SHe group, unlike in the DPX-SHe group | [130] |
BARS13 (Advaccine) (Biopharmaceuticals Suzhou Co, China) | BARS13 (or CSA+G consists of CSA - cyclosporine A (can induce Treg) and recombinant G-PSV (shortened) | BALB/c mice (i.m.) | Mice were injected with BARS13 at a dose of 10 μg 2 times with an interval of 2 weeks. After 2 weeks, mice were infected with RSV A2 at a dose of 5 × 107 PFU. In the BARS13 group, the viral load in the lungs after challenge was 10-fold lower than in the FI-RSV and G-protein groups. In the BARS13 group, the dynamics of weight change in mice were comparable to the group of mice that were not infected with RSV. There were no signs of histopathology in the lungs of mice from the BARS13 group. The protective effect in the BARS13 group was explained by the contribution of Treg cells, which were high in BAL and lymph nodes after the challenge trial | [131] |
BARS13 was modified by inclusion in the vaccine formulation and administered with RSV pre-F. Mice were immunized with 10 μg of BARS13 or BARS13 + pre-F 2 times with an interval of 2 weeks. After 2 weeks, mice were infected with RSV at a dose of 2 × 106 PFU. Serum IgG antibody levels were higher in the BARS13 + pre-F group than in the BARS13 group. There were high levels of Treg cells in the BARS13 + pre-F group compared with the FI-RSV and pre-F groups. Neutralizing antibody levels were 6-fold higher in the BARS13 + pre-F group than in the BARS13 group. After MS challenge trial, the pulmonary tissue burden in the BARS13 + pre-F group was 10% lower than in the BARS13 group. The degree of pulmonary inflammation in the BARS13 + pre-F group was minimal | [132] | |||
RSVPre-F3/ (GSK3844766A (GlaxoSmithKline, UK) | Peptide F stabilized in a pre-fusion conformation (AS01) | Rabbits (i.m.) | Rabbits were injected with RSVPre-F3 alone or in combination with Boostrix vaccine three times. In RSVPreF3, RSVPreF3/AS01, RSVPreF3 +Boostrix groups, IgG antibody titers to RSVPre-F3 were high. Immunization with RSVPre-F3 had no effect on body weight, vision, skin, appetite and body temperature. Clinical and pathological changes (increase in leukocytes, neutrophils, fibrinogen and C-reactive protein concentration, decrease in albumin) were recorded on the next day after vaccine administration, and the indices returned to normal within 4 weeks. Rabbits were injected with RSVPre-F3 several weeks before mating, during pregnancy and during lactation. Passive transfer of antibodies from immunized females to rabbits was observed | [133] |
Rats (i.m.) | Rats were injected with RSVPre-F3 several weeks before mating, during pregnancy and lactation. In most rats, IgG to RSVPre-F3 was detectable before mating (31/48), during pregnancy (14/31), and during lactation (16/31), and the antibodies were transmitted to the offspring. Immunization of females with RSVPre-F3 had no effect on fertility, pregnancy, lactation, survival, or changes in external signs of visceral and skeletal development in the offspring | |||
РСВ-F (NIAID, USA) | Purified RSV F protein | Cotton rats (i.m.) | Double immunization with protein at doses of 0.05-5.00 µg resulted in the formation of high levels of RSV F-specific antibodies that circulated for 6 months after vaccination, but their neutralizing activity was low. Infection of rats with RSV 3 months after immunization showed a 100-fold decrease in viral load in the lungs in the RSV-F group compared with the control group. At 6 months after immunization, protection against RSV was practically absent – in the FI-RSV and RSV-F groups. In the RSV-F group (5 μg), a vaccine-mediated disease enhancement after RSV challenge comparable to FI-RSV was observed | [134] |
Vaccines based on virus like particles | ||||
V306-SVLP (Virometix, Switzerland) | A mimetic of a peptide that binds to motavizumab V-306 (alum for 15 µg dose of V-306) | BALB/c mice (i.m.) | Mice were injected with V306-SVLP at different doses twice with an interval of 3 weeks. On day 31, mice were infected with RSV at a dose of 106 PFU. Immunization with V306-SVLP stimulated the production of neutralizing antibodies to RSV. On day 5 of the challenge trial, the virus was not reproduced in the V306-SVLP (50–300 μg) groups, in contrast to the PBS, FI-RSV, and V306-SVLP (15 μg) groups. No histopathologic changes were observed in the lungs in all V306-SVLP groups, in contrast to FI-RSV groups | [135] |
Rabbits (i.m.) | Rabbits were injected with V306-SVLP at a dose of 140 μg three times with an interval of 28 days. High levels of neutralizing antibodies to 8 different strains of RSV subtypes A and B were observed | |||
Cotton rats (i.m.) | Cotton rats were injected with 10D11 anti-V-306 monoclonal antibody preparation and palivizumab at different doses. After 1 day, rats were infected with RSV at a dose of 104 PFU. High titers of RSV in the lungs were observed in the PBS group. A dose-dependent reduction in replication was observed in the 10D11 and palivizumab groups. Vaccination with V-306 stimulated the production of antibodies with palivizumab-like properties | |||
VX-121 (Icosavax, USA) | I53-50 nanoparticle with multiple DS-Cav1 trimers (trimer F stabilized in a “pre-fusion” conformation) on the surface (AddaVax) | BALB/c mice (s.c.) | Mice were injected with DS Cav1-I53-50 or DS Cav1 at a dose of 5 μg three times with an interval of 2 weeks. DS Cav1-I53-50 induced a stronger antigen-specific and neutralizing immune response. Immunization with DS Cav1-I53-50 induced 5.3-fold higher follicular T helper formation in immunized mice than DS Cav1. Since follicular T-helpers control the proliferation of B-lymphocytes in the follicles of lymphoid organs, the number of B-cells in the lymph nodes of mice from the DS Cav1-I53-50 group was higher than in the DS Cav1 group | [136] |
I53-50 nanoparticle with multiple DS-Cav1 trimers on the surface (SWE) | Macaques (Indian) (s.c.) | Macaques were immunized twice with DS-Cav1 (50 μg) and DS-Cav1-I53-50 (96 μg) 28 days apart. DS-Cav1-I53-50 induced 5-fold stronger antigen-specific 25-fold more intense neutralizing antibody production than DS-Cav1. Specific antibodies in macaque sera were found to target the F-protein of RSV in a pre-F conformation | ||
Note. i.n. — intranasally; i.t. — intratracheally; i.m. — intramuscularly; s.c. — subcutaneously; or. — orally; i.p. — intraperitoneally; scar. — scarification
Table 2. Overview of vaccines in various stages of clinical trials
Vaccine (developer) | Clinical trial phase | Period | Test No. in the registry | Source |
Recombinant vector vaccines | ||||
RSV001 (PanAd3-RSV and MVA-RSV) (ReiThera, Italy) | Phase I (A.; E.) | 2013–2015 | NCT01805921 | |
VXA-RSV f (Vaxart, USA) | Phase I (A.) | 2016–2018 | NCT02830932 | None |
ChAd155-RSV GS (GSK, UK) | Phase I (А.) Phase I/II (141) Phase I/II (C.) Phase II (A. ♀) | 2016–2017 2016–2021 2018–2022 2015–2018 | NCT02491463 NCT02927873 NCT03636906 NCT02360475 | Yes [58] Yes [60] None Yes [138] |
MVA-BN RSV (Bavarian Nordic BN, Denmark) | Phase I (A. E.) Phase I (A.) Phase II (E.) Phase 2a (A.) Phase III (E.) | 2015–2016 2016–2018 2016–2018 2021–2021 2022–2024 | NCT02419391 NCT02864628 NCT02873286 NCT04752644 NCT05238025 | Yes [46] Yes [46] Yes [47] Yes [139] Yes [140] |
SeV/RSV (NIAID, USA) | Phase I (A.) | 2018–2019 | NCT03473002 | Yes [65] |
Ad26.RSV.preF (Janssen Vaccines & Prevention B.V., Netherlands) | Phase I (A.; E.) Phase I (C.) Phase I/2a (A.; C) | 2016–2019 2022 2017–2022 | NCT02926430 NCT03606512 NCT03303625 | Yes [141] Yes [57] Yes [57] |
Ad26.RSV.preF (Janssen Vaccines & Prevention B.V., Netherlands) | Phase II (E.) Phase II (A.; E.) | 2017–2021 2017–2021 | NCT03339713 NCT03334695 | Yes [56] Yes [142] |
rBCG-N-hRSV (Catholic University of Chile, Chile) | Phase I (A. ♂) | 2017–2018 | NCT03213405 | Yes [143] |
RSV/Flu-01E (A.A. Smorodintsev Research Institute of Influenza, Russia) | Phase I (A.; E.) Phase II (E.) | 2022–2023 2023–2024 | NCT05970744 | None None |
Ad26.RSV.FA2, Ad35.RSV.FA2 (Crucell Holland BV, Netherlands) | Phase I (A.) Phase I (A.) | 2015–2016 2015–2016 | NCT02561871 NCT02440035 | None None |
Live attenuated vaccines | ||||
MV-012-968 (Meissa, USA) | Phase I (A.) Phase I (141) | 2020–2020 2021–2023 | NCT04227210 NCT04909021 | None None |
RSV MEDI ∆M2-2 (NIAID/NIH, MedImmune (AstraZeneca), USA) | Phase I (141) | 2011–2015 | NCT01459198 | Yes [75] |
RSV cps2 (NIAID/NIH, MedImmune (AstraZeneca), USA) | Phase I (141) Phase I (141) | 2013–2015 2013–2016 | NCT01968083 NCT01852266 | Yes [73] |
RSV LID ∆M2-2, NIAID (Sanofi Pasteur, France) | Phase I (141) Phase I (141) | 2014–2016 2014–2015 | NCT02040831 NCT02237209 | [76] [76] |
RSV LID/ΔM2-2/1030s (NIAID, USA, Sanofi Pasteur, France) | Phase I (141) Phase I (141) Phase I (141) | 2020–2023 2016–2018 2015–2017 | NCT04520659 NCT02952339 NCT02794870 | None Yes [78] Yes [78] |
RSV LID cp ∆M2-2 (NIAID, USA) | Phase I (141) Phase I (141) | 2015–2018 2015–2018 | NCT02890381 NCT02948127 | Yes [144] Yes [144] |
RSV D46/NS2/N/∆M2-2-HindIII (NIAID, USA) | Phase I (141) Phase I (141) | 2017–2018 2017–2018 | NCT03102034 NCT03099291 | Yes [145] Yes [145] |
RSV ΔNS2/Δ1313/I1314L (NIAID, USА) | Phase I (141) Phase I (141) | 2013–2018 2017–2022 | NCT01893554 NCT03227029 | Yes [82] Yes [146] |
RSV 6120/∆NS2/1030s (NIAID, USA) | Phase I (141) | 2017–2022 | NCT03387137 | None |
RSV 6120/∆NS1 and RSV 6120/F1/G2/∆NS1 | Phase I (141) | 2018–2023 | NCT03596801 | None |
RSV ∆NS2/∆1313/I1314L; RSV 6120/∆NS2/1030s; RSV 276, (NIAID, USA) | Phase I and II (141) | 2019–2022 | NCT03916185 | None |
RSV ∆NS2/∆1313/I1314L; RSV 276 (NIAID, USA) | Phase I and II (141) | 2017–2020 | NCT03422237 | Yes [146] |
Vaccine (developer) | Clinical trial phase (target group) | Period | Test No. in the registry | Source |
RSV D46 cp∆M2-2, (NIAID, USA) | Phase I (141) | 2015–2019 | NCT02601612 | None |
MEDI-559, (MedImmune) (AstraZeneca, USA) | Phase I and II (141) | 2008–2011 | NCT00767416 | Yes [72] |
Subunit recombinant vaccines | ||||
GSK3888550A RSVPreF3 (GSK, UK) | Phase I (A. ♀) Phase II (A. ♀) Phase II (A. ♀) Phase III (A. ♀) | 2018–2019 2019–2022 2019–2020 2020–2023 | NCT03674177 NCT04126213 NCT04138056 NCT04605159 | Yes [147] Yes [148] None None |
GSK3844766A RSVPreF3, Arexvy (GSK, UK) | Phase I/II (A., E) Phase I (E.) Phase II (E.) Phase III (E.) Phase III (A., E) Phase III (E.) Phase III (E.) Phase III (E.) Phase III (E.) Phase III (E.) Phase III (E.) Phase III (E.) | 2019–2021 2019–2022 2020–2021 2021–2024 2023–present 2021–present 2021–2022 2021–2022 2022–2023 2022–2023 2023–2024 2023–2024 | NCT03814590 NCT04090658 NCT04657198 NCT04886596 NCT05921903 NCT04732871 NCT04841577 NCT05059301 NCT05559476 NCT05568797 NCT05590403 NCT05879107 | Yes [91] Yes [90] Yes [92] None Yes [93] Yes [150] Yes [151] Yes [152] Yes [153] Yes [154] None |
RSVPreF3 (GSK, UK) | Phase I (A.) | 2014–2018 | NCT02298179 | Yes [155] |
DPX-RSV(A) (Immunovaccine, USA and Dalhousie University, Canada) | Phase I (A.) | 2015–2017 | NCT02472548 | Yes [89] |
RSV F DS-Cav1 (NIH/NIAID/VRC, USA) | Phase I (A.) | 2017–2020 | NCT03049488 | Yes [86] |
RSVpreF Abrysvo (Pfizer, USA) | Phase II (A. ♀) Phase I/II (A., E) Phase 2b (A. ♀) Phase II (E.) Phase III (C., A. ♀) Phase Ib (E.) Phase II (A. ♀) Phase II (A.) Phase III (E.) Phase III (A.) Phase III (E.) Phase III (A., E.) | 2019–2019 2018–2021 2019–2022 2018–2021 2020–present 2023–2023 2019–2019 2020–2021 2021–present 2021–2022 2022–2022 2023–2024 | NCT04071158 NCT03529773 NCT04032093 NCT03572062 NCT04424316 NCT05788237 NCT04071158 NCT04785612 NCT05035212 NCT05096208 NCT05301322 NCT05842967 | Yes [156] Yes [158] Yes [159] Yes [160] None Yes [156] Yes [161] None Yes [162] Yes [163] None |
RSV F vaccine (RSVpreF) (Pfizer, USA) | Phase I/II (E.) | 2018–2020 | NCT03572062 | Yes [159] |
BARS13, (Advaccine) (Suzhou) Biopharmaceuticals Co., Ltd, China) | Phase I (A.) Phase II (A., E.) Phase II (A.) | 2019–2019 2020–2023 2018–2019 | NCT04851977 NCT04681833 ACTRN12618000948291 | Yes [164] None None |
Vaccines based on virus like particles | ||||
V306-SVLP (Virometix AG, Switzerland) | Phase I (A. ♀) | 2020–2022 | NCT04519073 | Yes [105] |
SynGEM (Mucosis B.V., Netherlands) | Phase I (A. ♀♂) | 2016–2017 | NCT02958540 | Yes [104] |
RSV F (ResVax) (Novavax, USA) | Phase I (A.) Phase II (A. ♀) Phase II (A. ♀) Phase II (A. ♀) Phase II (A. ♀) | 2010–2011 2012–2013 2013–2014 2014–2016 2015–2020 | NCT01290419 NCT01704365 NCT01960686 NCT02247726 NCT02624947 | Yes [165] Yes [166] Yes [167] Yes [100] Yes [102] |
RSV F nanoparticle, (Novavax, USA) | Phase I (E.) Phase II (E.) Phase II (E.) Phase III (E.) Phase II (E.) Phase I (141) | 2012–2014 2014–2016 2015–2016 2015–2016 2017–2018 2014–2016 | NCT01709019 NCT02266628 NCT02593071 NCT02608502 NCT03026348 NCT02296463 | Yes [168] None None None None None |
Vaccine (developer) | Clinical trial phase (target group) | Period | Test No. in the registry | Source |
Vaccines based on messenger RNA | ||||
mRNA-1345 | Phase I (C.) Phase II (C., A., E.) Phase II (A. ♀) Phase II-III (E.) | 2023–present 2020–2024 2023–present 2021–present | NCT05743881 NCT04528719 NCT06143046 NCT05127434 | None Yes [35] None |
RSV mRNA LNP CL-0059; LNP CL-0137 | Phase I-II (A., E.) | 2022–present | NCT05639894 | None |
Note. C. — Children; E. — Elderly (lower threshold 50, 55 or 60 years); A. — Adults (from 18 years, upper threshold varies).
1 Innorna Announces First Participant Dosed in Phase 1 Clinical Trial of Investigational Bivalent RSV mRNA Vaccine IN006; 2024. Available at: https://innorna.com/news/330.html
2 Bavarian Nordic. Bavarian Nordic Provides Update on RSV Vaccine Program. URL: https://www.bavarian-nordic.com/investor/news/news.aspx?news=6808
3 ClinicalTrials.gov. Dose-Ranging Trial of Safety & Immunogenicity of an Oral Adenoviral-Vector Based RSV Vaccine (VXA-RSV-f). URL: https://clinicaltrials.gov/study/NCT02830932
About the authors
Tatiana S. Kotomina
Institute of Experimental Medicine
Author for correspondence.
Email: kotomina@iemspb.ru
ORCID iD: 0000-0001-9999-089X
Cand. Sci. (Biol.), senior researcher, Laboratory of immunology and prevention of viral infections, Smorodintsev virology department
Russian Federation, St. PetersburgReferences
- Shi T., McAllister D.A., O'Brien K.L., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–58. DOI: https://doi.org/10.1016/S0140-6736(17)30938-8
- Nair H., Nokes D.J., Gessner B.D., et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–55. DOI: https://doi.org/10.1016/S0140-6736(10)60206-1
- Walsh E.E., Falsey A.R. Respiratory syncytial virus infection in adult populations. Infect. Disord. Drug Targets. 2012;12(2):98–102. DOI: https://doi.org/10.2174/187152612800100116
- Hall C.B., Walsh E.E., Long C.E., Schnabel K.C. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 1991;163(4):693–8. DOI: https://doi.org/10.1093/infdis/163.4.693
- Rima B., Collins P., Easton A., et al. ICTV virus taxonomy profile: Pneumoviridae. J. Gen. Virol. 2017;98(12):2912–3. DOI: https://doi.org/10.1099/jgv.0.000959
- Kinder J.T., Moncman C.L., Barrett C., et al. Respiratory syncytial virus and human metapneumovirus infections in three-dimensional human airway tissues expose an interesting dichotomy in viral replication, spread, and inhibition by neutralizing antibodies. J. Virol. 2020;94(20):e01068-20. DOI: https://doi.org/10.1128/JVI.01068-20
- Li Y., Wang X., Blau D.M., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–64. DOI: https://doi.org/10.1016/S0140-6736(22)00478-0
- Cao D., Gao Y., Liang B. Structural insights into the respiratory syncytial virus RNA synthesis complexes. Viruses. 2021;13(5):834. DOI: https://doi.org/10.3390/v13050834
- Jeffree C.E., Rixon H.W., Brown G., et al. Distribution of the attachment (G) glycoprotein and GM1 within the envelope of mature respiratory syncytial virus filaments revealed using field emission scanning electron microscopy. Virology. 2003;306(2):254–67. DOI: https://doi.org/10.1016/s0042-6822(02)00016-8
- Rincheval V., Lelek M., Gault E., et al. Functional organization of cytoplasmic inclusion bodies in cells infected by respiratory syncytial virus. Nat. Commun. 2017;8(1):563. DOI: https://doi.org/10.1038/s41467-017-00655-9
- Shahriari S., Gordon J., Ghildyal R. Host cytoskeleton in respiratory syncytial virus assembly and budding. Virol. J. 2016;13(1):161. DOI: https://doi.org/10.1186/s12985-016-0618-z
- McLellan J.S., Ray W.C., Peeples M.E. Structure and function of RSV surface glycoproteins. Curr. Top. Microbiol. Immunol. 2013;372:83–104. DOI: https://doi.org/10.1007/978-3-642-38919-1_4
- Scudero O.B., Santiago V.F., Palmisano G., et al. The respiratory syncytial virus M2-2 protein is targeted for proteasome degradation and inhibits translation and stress granules assembly. PLoS One. 2023;18(7):e0289100. DOI: https://doi.org/10.1371/journal.pone.0289100
- Fontana J.M., Bankamp B., Rota P.A. Inhibition of interferon induction and signaling by paramyxoviruses. Immunol. Rev. 2008;225:46–67. DOI: https://doi.org/10.1111/j.1600-065X.2008.00669.x
- Wu W., Tran K.C., Teng M.N., et al. The interactome of the human respiratory syncytial virus NS1 protein highlights multiple effects on host cell biology. J. Virol. 2012;86(15):7777–89. DOI: https://doi.org/10.1128/JVI.00460-12
- Jung H.E., Kim T.H., Lee H.K. Contribution of dendritic cells in protective immunity against respiratory syncytial virus infection. Viruses. 2020;12(1):102. DOI: https://doi.org/10.3390/v12010102
- Chanock R., Finberg L. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). II. Epidemiologic aspects of infection in infants and young children. Am. J. Hyg. 1957;66(3):291–300. DOI: https://doi.org/10.1093/oxfordjournals.aje.a119902
- Connors M., Collins P.L., Firestone C.Y., Murphy B.R. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J. Virol. 1991;65(3):1634–7. DOI: https://doi.org/10.1128/JVI.65.3.1634-1637.1991
- Graham B.S. Immunological goals for respiratory syncytial virus vaccine development. Curr. Opin. Immunol. 2019;59:57–64. DOI: https://doi.org/10.1016/j.coi.2019.03.005
- Swanson K.A., Settembre E.C., Shaw C.A., et al. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc. Natl. Acad. Sci. USA. 2011;108(23):9619–24. DOI: https://doi.org/10.1073/pnas.1106536108
- Andreano E., Paciello I., Bardelli M., et al. The respiratory syncytial virus (RSV) prefusion F-protein functional antibody repertoire in adult healthy donors. EMBO Mol. Med. 2021;13(6):e14035. DOI: https://doi.org/10.15252/emmm.202114035
- Salk J.E. Studies in human subjects on active immunization against poliomyelitis. I. A preliminary report of experiments in progress. J. Am. Med. Assoc. 1953;151(13):1081–98.
- Chin J., Magoffin R.L., Shearer L.A., et al. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 1969;89(4):449–63. DOI: https://doi.org/10.1093/oxfordjournals.aje.a120957
- Kim H.W., Canchola J.G., Brandt C.D., et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969;89(4):422–34. DOI: https://doi.org/10.1093/oxfordjournals.aje.a120955
- Vissers M., Ahout I.M., de Jonge M.I., Ferwerda G. Mucosal IgG levels correlate better with respiratory syncytial virus load and inflammation than plasma IgG levels. Clin. Vaccine Immunol. 2015;23(3):243–5. DOI: https://doi.org/10.1128/CVI.00590-15
- Karron R.A., Buonagurio D.A., Georgiu A.F., et al. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc. Natl. Acad. Sci. USA. 1997;94(25):13961–6. DOI: https://doi.org/10.1073/pnas.94.25.13961
- Лещинская Н.П. СЕА. Клиника и лечение гриппа и других ОРЗ. В кн.: Сборник научных трудов ВНИИ гриппа МЗ СССР. Ленинград;1982:172–7. Leshchinskaya N.P. CEA. Clinic and treatment of influenza and other acute respiratory infections. In: Collection of Scientific Papers of the All-Russian Research Institute of Influenza of the Ministry of Health of the USSR. Leningrad;1982:172–7.
- Topalidou X., Kalergis A.M., Papazisis G. Respiratory syncytial virus vaccines: A review of the candidates and the approved vaccines. Pathogens. 2023;12(10):1259. DOI: https://doi.org/10.3390/pathogens12101259
- Mazur N.I., Terstappen J., Baral R., et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect. Dis. 2023;23(1):e2–21. DOI: https://doi.org/10.1016/S1473-3099(22)00291-2
- Mazur N.I., Higgins D., Nunes M.C., et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect. Dis. 2018;18(10):e295–311. DOI: https://doi.org/10.1016/S1473-3099(18)30292-5
- Kelleher K., Subramaniam N., Drysdale S.B. The recent landscape of RSV vaccine research. Ther. Adv. Vaccines Immunother. 2025;13:25151355241310601. DOI: https://doi.org/10.1177/25151355241310601
- Zhang G., Zhao B., Liu J. The development of animal models for respiratory syncytial virus (RSV) infection and enhanced RSV disease. Viruses. 2024;16(11):1701. DOI: https://doi.org/10.3390/v16111701
- Drysdale S.B., Thwaites R.S., Price J., et al. What have we learned from animal studies of immune responses to respiratory syncytial virus infection? J. Clin. Virol. 2024;175:105731. DOI: https://doi.org/10.1016/j.jcv.2024.105731
- Yuan M., Han Z., Liang Y., et al. mRNA nanodelivery systems: targeting strategies and administration routes. Biomater. Res. 2023;27(1):90. DOI: https://doi.org/10.1186/s40824-023-00425-3
- Shaw C.A., Mithani R., Kapoor A., et al. Safety, tolerability, and immunogenicity of an mRNA-based respiratory syncytial virus vaccine in healthy young adults in a phase 1 clinical trial. J. Infect. Dis. 2024;230(3):e637–46. DOI: https://doi.org/10.1093/infdis/jiae035
- Corbett K.S., Edwards D.K., Leist S.R., et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586(7830):567–71. DOI: https://doi.org/10.1038/s41586-020-2622-0
- Aliprantis A.O., Shaw C.A., Griffin P., et al. A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Hum. Vaccin. Immunother. 2021;17(5):1248–61. DOI: https://doi.org/10.1080/21645515.2020.1829899
- Nussbaum J., Cao X., Railkar R.A., et al. Evaluation of a stabilized RSV pre-fusion F mRNA vaccine: Preclinical studies and Phase 1 clinical testing in healthy adults. Vaccine. 2023;41(44):6488–501. DOI: https://doi.org/10.1016/j.vaccine.2023.05.062
- Oomens A.G., Bevis K.P., Wertz G.W. The cytoplasmic tail of the human respiratory syncytial virus F protein plays critical roles in cellular localization of the F protein and infectious progeny production. J. Virol. 2006;80(21):10465–77. DOI: https://doi.org/10.1128/JVI.01439-06
- Mauger D.M., Cabral B.J., Presnyak V., et al. mRNA structure regulates protein expression through changes in functional half-life. Proc. Natl. Acad. Sci. USA. 2019;116(48):24075–83. DOI: https://doi.org/10.1073/pnas.1908052116
- Wilson E., Goswami J., Baqui A.H., et al. Efficacy and safety of an mRNA-based RSV PreF vaccine in older adults. N. Engl. J. Med. 2023;389(24):2233–44. DOI: https://doi.org/10.1056/NEJMoa2307079
- Исакова-Сивак И.Н., Кореньков Д.А., Федорова Е.А. и др. Поиск иммунодоминантных эпитопов респираторно-синцитиального вируса для конструирования векторных вакцин на основе вирусов гриппа. Бюллетень экспериментальной биологии и медицины. 2016;161(4):523–7. EDN: https://elibrary.ru/vscven. Isakova-Sivak I.N., Korenkov D.A., Fedorova E.A., et al. Analysis of immune epitopes of respiratory syncytial virus for designing of vectored vaccines based on influenza virus platform. Bulletin of Experimental Biology and Medicine. 2016;161(4):533–7. DOI: https://doi.org/10.1007/s10517-016-3454-7, EDN: https://elibrary.ru/yuxboh
- Matyushenko V., Kotomina T., Kudryavtsev I., et al. Conserved T-cell epitopes of respiratory syncytial virus (RSV) delivered by recombinant live attenuated influenza vaccine viruses efficiently induce RSV-specific lung-localized memory T cells and augment influenza-specific resident memory T-cell responses. Antiviral Res. 2020;182:104864. DOI: https://doi.org/10.1016/j.antiviral.2020.104864
- Kotomina T., Isakova-Sivak I., Stepanova E., et al. Neutralizing epitope of the fusion protein of respiratory syncytial virus embedded in the HA molecule of LAIV virus is not sufficient to prevent RS virus pulmonary replication but ameliorates lung pathology following RSV infection in mice. Open Microbiol. J. 2020;14(1):147–56. DOI: https://doi.org/10.2174/1874285802014010147
- Pulkina A., Vasilyev K., Muzhikyan A., et al. IgGκ signal peptide enhances the efficacy of an influenza vector vaccine against respiratory syncytial virus infection in mice. Int. J. Mol. Sci. 2023;24(14):11445. DOI: https://doi.org/10.3390/ijms241411445
- Samy N., Reichhardt D., Schmidt D., et al. Safety and immunogenicity of novel modified vaccinia Ankara-vectored RSV vaccine: A randomized phase I clinical trial. Vaccine. 2020;38(11):2608–19. DOI: https://doi.org/10.1016/j.vaccine.2020.01.055
- Jordan E., Lawrence S.J., Meyer T.P.H., et al. Broad antibody and cellular immune response from a phase 2 clinical trial with a novel multivalent poxvirus-based respiratory syncytial virus vaccine. J. Infect. Dis. 2021;223(6):1062–72. DOI: https://doi.org/10.1093/infdis/jiaa460
- Green C.A., Scarselli E., Voysey M., et al. Safety and immunogenicity of novel respiratory syncytial virus (RSV) vaccines based on the RSV viral proteins F, N and M2-1 encoded by simian adenovirus (PanAd3-RSV) and MVA (MVA-RSV); protocol for an open-label, dose-escalation, single-centre, phase 1 clinical trial in healthy adults. BMJ Open. 2015;5(10):e008748. DOI: https://doi.org/10.1136/bmjopen-2015-008748
- Green C.A., Scarselli E., Sande C.J., et al. Chimpanzee adenovirus- and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults. Sci. Transl. Med. 2015;7(300):300ra126. DOI: https://doi.org/10.1126/scitranslmed.aac5745
- Pierantoni A., Esposito M.L., Ammendola V., et al. Mucosal delivery of a vectored RSV vaccine is safe and elicits protective immunity in rodents and nonhuman primates. Mol. Ther. Methods Clin. Dev. 2015;2:15018. DOI: https://doi.org/10.1038/mtm.2015.18
- Shan J., Britton P.N., King C.L., Booy R. The immunogenicity and safety of respiratory syncytial virus vaccines in development: A systematic review. Influenza Other Respir. Viruses. 2021;15(4):539–51. DOI: https://doi.org/10.1111/irv.12850
- Green C.A., Sande C.J., Scarselli E., et al. Novel genetically-modified chimpanzee adenovirus and MVA-vectored respiratory syncytial virus vaccine safely boosts humoral and cellular immunity in healthy older adults. J. Infect. 2019;78(5):382–92. DOI: https://doi.org/10.1016/j.jinf.2019.02.003
- Rezaee F., Linfield D.T., Harford T.J., Piedimonte G. Ongoing developments in RSV prophylaxis: a clinician's analysis. Curr. Opin. Virol. 2017;24:70–8. DOI: https://doi.org/10.1016/j.coviro.2017.03.015
- Widjojoatmodjo M.N., Bogaert L., Meek B., et al. Recombinant low-seroprevalent adenoviral vectors Ad26 and Ad35 expressing the respiratory syncytial virus (RSV) fusion protein induce protective immunity against RSV infection in cotton rats. Vaccine. 2015;33(41):5406–14. DOI: https://doi.org/10.1016/j.vaccine.2015.08.056
- Comeaux C.A., Bart S., Bastian A.R., et al. Safety, immunogenicity, and regimen selection of Ad26.RSV.preF-based vaccine combinations: a randomized, double-blind, placebo-controlled, phase 1/2a study. J. Infect. Dis. 2024;229(1):19–29. DOI: https://doi.org/10.1093/infdis/jiad220
- Sadoff J., De Paepe E., Haazen W., et al. Safety and immunogenicity of the Ad26.RSV.preF investigational vaccine coadministered with an influenza vaccine in older adults. J. Infect. Dis. 2021;223(4):699–708. DOI: https://doi.org/10.1093/infdis/jiaa409
- Stuart A.S.V., Virta M., Williams K., et al. Phase 1/2a safety and immunogenicity of an adenovirus 26 vector respiratory syncytial virus (RSV) vaccine encoding prefusion F in adults 18–50 years and RSV-seropositive children 12–24 months. J. Infect. Dis. 2022;227(1):71–82. DOI: https://doi.org/10.1093/infdis/jiac407
- Cicconi P., Jones C., Sarkar E., et al. First-in-human randomized study to assess the safety and immunogenicity of an investigational respiratory syncytial virus (RSV) vaccine based on chimpanzee-adenovirus-155 viral vector-expressing RSV fusion, nucleocapsid, and antitermination viral proteins in healthy adults. Clin. Infect. Dis. 2020;70(10):2073–81. DOI: https://doi.org/10.1093/cid/ciz653
- Lee L.Y., Ha do L.A., Simmons C., et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J. Clin. Invest. 2008;118(10):3478–90. DOI: https://doi.org/10.1172/JCI32460
- Díez-Domingo J., Sáez-Llorens X., Rodriguez-Weber M.A., et al. Safety and immunogenicity of a ChAd155-vectored respiratory syncytial virus (RSV) vaccine in healthy RSV-seropositive children 12–23 months of age. J. Infect. Dis. 2023;227(11):1293–302. DOI: https://doi.org/10.1093/infdis/jiac481
- Saez-Llorens X., Norero X., Mussi-Pinhata M.M., et al. Safety and immunogenicity of a ChAd155-vectored respiratory syncytial virus vaccine in infants 6-7 months of age: a phase 1/2 randomized trial. J. Infect. Dis. 2024;229(1):95–107. DOI: https://doi.org/10.1093/infdis/jiad271
- Haller A.A., Miller T., Mitiku M., Coelingh K. Expression of the surface glycoproteins of human parainfluenza virus type 3 by bovine parainfluenza virus type 3, a novel attenuated virus vaccine vector. J. Virol. 2000;74(24):11626–35. DOI: https://doi.org/10.1128/jvi.74.24.11626-11635.2000
- Gomez M., Mufson M.A., Dubovsky F., et al. Phase-I study MEDI-534, of a live, attenuated intranasal vaccine against respiratory syncytial virus and parainfluenza-3 virus in seropositive children. Pediatr. Infect. Dis. J. 2009;28(7):655–8. DOI: https://doi.org/10.1097/INF.0b013e318199c3b1
- Bernstein D.I., Malkin E., Abughali N., et al. Phase 1 study of the safety and immunogenicity of a live, attenuated respiratory syncytial virus and parainfluenza virus type 3 vaccine in seronegative children. Pediatr. Infect. Dis. J. 2012;31(2):109–14. DOI: https://doi.org/10.1097/INF.0b013e31823386f1
- Scaggs Huang F., Bernstein D.I., Slobod K.S., et al. Safety and immunogenicity of an intranasal sendai virus-based vaccine for human parainfluenza virus type I and respiratory syncytial virus (SeVRSV) in adults. Hum. Vaccin. Immunother. 2021;17(2):554–9. DOI: https://doi.org/10.1080/21645515.2020.1779517
- Wright P.F., Karron R.A., Belshe R.B., et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J. Infect. Dis. 2000; 182(5):1331–42. DOI: https://doi.org/10.1086/315859
- Karron R.A., Wright P.F., Belshe R.B., et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J. Infect. Dis. 2005;191(7):1093–104. DOI: https://doi.org/10.1086/427813
- Wright P.F., Karron R.A., Belshe R.B., et al. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine. 2007;25(42):7372–8. DOI: https://doi.org/10.1016/j.vaccine.2007.08.014
- Soto J.A., Stephens L.M., Waldstein K.A., et al. Current insights in the development of efficacious vaccines against RSV. Front. Immunol. 2020;11:1507. DOI: https://doi.org/10.3389/fimmu.2020.01507
- Karron R.A., Buchholz U.J., Collins P.L. Live-attenuated respiratory syncytial virus vaccines. Curr. Top. Microbiol. Immunol. 2013;372:259–84. DOI: https://doi.org/10.1007/978-3-642-38919-1_13
- Schickli J.H., Kaur J., Tang R.S. Nonclinical phenotypic and genotypic analyses of a Phase 1 pediatric respiratory syncytial virus vaccine candidate MEDI-559 (rA2cp248/404/1030ΔSH) at permissive and non-permissive temperatures. Virus Res. 2012;169(1):38–47. DOI: https://doi.org/10.1016/j.virusres.2012.06.027
- Malkin E., Yogev R., Abughali N., et al. Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-seronegative children 5 to 24 months of age. PLoS One. 2013;8(10):e77104. DOI: https://doi.org/10.1371/journal.pone.0077104
- Buchholz U.J., Cunningham C.K., Muresan P., et al. Live respiratory syncytial virus (RSV) vaccine candidate containing stabilized temperature-sensitivity mutations is highly attenuated in RSV-seronegative infants and children. J. Infect. Dis. 2018;217(9):1338–46. DOI: https://doi.org/10.1093/infdis/jiy066
- Bermingham A., Collins P.L. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc. Natl. Acad. Sci. USA. 1999;96(20):11259–64. DOI: https://doi.org/10.1073/pnas.96.20.11259
- Karron R.A., Luongo C., Thumar B., et al. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Sci. Transl. Med. 2015;7(312):312ra175. DOI: https://doi.org/10.1126/scitranslmed.aac8463
- McFarland E.J., Karron R.A., Muresan P., et al. Live-attenuated respiratory syncytial virus vaccine candidate with deletion of RNA synthesis regulatory protein M2-2 is highly immunogenic in children. J. Infect. Dis. 2018;217(9):1347–55. DOI: https://doi.org/10.1093/infdis/jiy040
- Bukreyev A., Belyakov I.M., Berzofsky J.A., et al. Granulocyte-macrophage colony-stimulating factor expressed by recombinant respiratory syncytial virus attenuates viral replication and increases the level of pulmonary antigen-presenting cells. J. Virol. 2001;75(24):12128–40. DOI: https://doi.org/10.1128/JVI.75.24.12128-12140.2001
- McFarland E.J., Karron R.A., Muresan P., et al. Live respiratory syncytial virus attenuated by M2-2 deletion and stabilized temperature sensitivity mutation 1030s is a promising vaccine candidate in children. J. Infect. Dis. 2020;221(4):534–43. DOI: https://doi.org/10.1093/infdis/jiz603
- Stobart C.C., Rostad C.A., Ke Z., et al. A live RSV vaccine with engineered thermostability is immunogenic in cotton rats despite high attenuation. Nat. Commun. 2016;7:13916. DOI: https://doi.org/10.1038/ncomms13916
- Mirzaei H., Faghihloo E. Viruses as key modulators of the TGF‐β pathway; a double‐edged sword involved in cancer. Rev. Med. Virol. 2018;28(2):e1967. DOI: https://doi.org/10.1002/rmv.1967
- Bakre A., Wu W., Hiscox J., et al. Human respiratory syncytial virus non-structural protein NS1 modifies miR-24 expression via transforming growth factor-β. J. Gen. Virol. 2015;96(11):3179–91. DOI: https://doi.org/10.1099/jgv.0.000261
- Karron R.A., Luongo C., Mateo J.S., et al. Safety and immunogenicity of the respiratory syncytial virus vaccine RSV/ΔNS2/Δ1313/I1314L in RSV-seronegative children. J. Infect. Dis. 2020;222(1):82–91. DOI: https://doi.org/10.1093/infdis/jiz408
- Rossey I., Saelens X. Vaccines against human respiratory syncytial virus in clinical trials, where are we now? Expert. Rev. Vaccines. 2019;18(10):1053–67. DOI: https://doi.org/10.1080/14760584.2019.1675520
- Brito L.A., O'Hagan D.T. Designing and building the next generation of improved vaccine adjuvants. J. Control Release. 2014;190:563–79. DOI: https://doi.org/10.1016/j.jconrel.2014.06.027
- Delgado M.F., Coviello S., Monsalvo A.C., et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009;15(1):34–41. DOI: https://doi.org/10.1038/nm.1894
- Graham B.S. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol. Rev. 2011;239(1):149–66. DOI: https://doi.org/10.1111/j.1600-065X.2010.00972.x
- Crank M.C., Ruckwardt T.J., Chen M., et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science. 2019;365(6452):505–9. DOI: https://doi.org/10.1126/science.aav9033
- Ruckwardt T.J., Morabito K.M., Phung E., et al. Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: a phase 1, randomised, open-label, dose-escalation clinical trial. Lancet Respir. Med. 2021;9(10):1111–20. DOI: https://doi.org/10.1016/S2213-2600(21)00098-9
- Langley J.M., MacDonald L.D., Weir G.M., et al. A respiratory syncytial virus vaccine based on the small hydrophobic protein ectodomain presented with a novel lipid-based formulation is highly immunogenic and safe in adults: a first-in-humans study. J. Infect. Dis. 2018;218(3):378–87. DOI: https://doi.org/10.1093/infdis/jiy177
- Kotb S., Haranaka M., Folschweiller N., et al. Safety and immunogenicity of a respiratory syncytial virus prefusion F protein (RSVPreF3) candidate vaccine in older Japanese adults: A phase I, randomized, observer-blind clinical trial. Respir. Investig. 2023;61(2):261–9. DOI: https://doi.org/10.1016/j.resinv.2022.11.003
- Leroux-Roels I., Davis M.G., Steenackers K., et al. Safety and immunogenicity of a respiratory syncytial virus prefusion F (RSVPreF3) candidate vaccine in older adults: phase 1/2 randomized clinical trial. J. Infect. Dis. 2023;227(6):761–72. DOI: https://doi.org/10.1093/infdis/jiac327
- Leroux-Roels I., Van Ranst M., Vandermeulen C., et al. Safety and immunogenicity of a revaccination with a respiratory syncytial virus prefusion F vaccine in older adults: a phase 2b study. J. Infect. Dis. 2024;229(2):355–66. DOI: https://doi.org/10.1093/infdis/jiad321
- Schwarz T.F., Hwang S.J., Ylisastigui P., et al. Immunogenicity and safety following 1 dose of AS01E-adjuvanted respiratory syncytial virus prefusion F protein vaccine in older adults: a phase 3 trial. J. Infect. Dis. 2024;230(1):e102–10. DOI: https://doi.org/10.1093/infdis/jiad546
- Ison M.G., Papi A., Athan E., et al. Efficacy and safety of respiratory syncytial virus (RSV) prefusion F protein vaccine (RSVPreF3 OA) in older adults over 2 RSV seasons. Clin. Infect. Dis. 2024;78(6):1732–44. DOI: https://doi.org/10.1093/cid/ciae010
- Walsh E.E., Falsey A.R., Scott D.A., et al. A Randomized phase 1/2 study of a respiratory syncytial virus prefusion F vaccine. J. Infect. Dis. 2022;225(8):1357–66. DOI: https://doi.org/10.1093/infdis/jiab612
- Kampmann B., Madhi S.A., Munjal I., et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N. Engl. J. Med. 2023;388(16):1451–64. DOI: https://doi.org/10.1056/NEJMoa2216480
- Walsh E.E., Perez Marc G., Zareba A.M., et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N. Engl. J. Med. 2023;388(16):1465–77. DOI: https://doi.org/10.1056/NEJMoa2213836
- Davis M., Towner W., DeHaan E., et al. Bivalent RSVpreF vaccine in adults 18 to <60 years old with high-risk conditions. Clin. Infect. Dis. 2024;ciae550. DOI: https://doi.org/10.1093/cid/ciae550
- Jeong H., Seong B.L. Exploiting virus-like particles as innovative vaccines against emerging viral infections. J. Microbiol. 2017;55(3):220–30. DOI: https://doi.org/10.1007/s12275-017-7058-3
- Muňoz F.M., Swamy G.K., Hickman S.P., et al. Safety and immunogenicity of a respiratory syncytial virus fusion (F) protein nanoparticle vaccine in healthy third-trimester pregnant women and their infants. J. Infect. Dis. 2019;220(11):1802–15. DOI: https://doi.org/10.1093/infdis/jiz390
- Madhi S.A., Polack F.P., Piedra P.A., et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N. Engl. J. Med. 2020;383(5):426–39. DOI: https://doi.org/10.1056/NEJMoa1908380
- Van Braeckel-Budimir N., Haijema B.J., Leenhouts K. Bacterium-like particles for efficient immune stimulation of existing vaccines and new subunit vaccines in mucosal applications. Front. Immunol. 2013;4:282. DOI: https://doi.org/10.3389/fimmu.2013.00282
- Rigter A., Widjaja I., Versantvoort H., et al. A protective and safe intranasal RSV vaccine based on a recombinant prefusion-like form of the F protein bound to bacterium-like particles. PLoS One. 2013;8(8):e71072. DOI: https://doi.org/10.1371/journal.pone.0071072
- Ascough S., Vlachantoni I., Kalyan M., et al. Local and systemic immunity against respiratory syncytial virus induced by a novel intranasal vaccine. A randomized, double-blind, placebo-controlled clinical trial. Am. J. Respir. Crit. Care Med. 2019;200(4):481–92. DOI: https://doi.org/10.1164/rccm.201810-1921OC
- Leroux-Roels I., Bruhwyler J., Stergiou L., et al. Double-blind, placebo-controlled, dose-escalating study evaluating the safety and immunogenicity of an epitope-specific chemically defined nanoparticle RSV vaccine. Vaccines (Basel). 2023;11(2):367. DOI: https://doi.org/10.3390/vaccines11020367
- Noble M., Khan R.A., Walker B., et al. Respiratory syncytial virus-associated hospitalisation in children aged https://doi.org/10.1183/23120541.00593-2021
- Coultas J.A., Smyth R., Openshaw P.J. Respiratory syncytial virus (RSV): a scourge from infancy to old age. Thorax. 2019;74(10):986–93. DOI: https://doi.org/10.1136/thoraxjnl-2018-212212
- Shahabi A., Peneva D., Incerti D., et al. Assessing variation in the cost of palivizumab for respiratory syncytial virus prevention in preterm infants. Pharmacoecon. Open. 2018;2(1):53–61. DOI: https://doi.org/10.1007/s41669-017-0042-3
- Keam S.J. Nirsevimab: first approval. Drugs. 2023;83(2):181–7. DOI: https://doi.org/10.1007/s40265-022-01829-6
- Mueller S., Stauft C.B., Kalkeri R., et al. A codon-pair deoptimized live-attenuated vaccine against respiratory syncytial virus is immunogenic and efficacious in non-human primates. Vaccine. 2020;38(14):2943–8. DOI: https://doi.org/10.1016/j.vaccine.2020.02.056
- Widjojoatmodjo M.N., Boes J., van Bers M., et al. A highly attenuated recombinant human respiratory syncytial virus lacking the G protein induces long-lasting protection in cotton rats. Virol. J. 2010;7:114. DOI: https://doi.org/10.1186/1743-422X-7-114
- Luongo C., Winter C.C., Collins P.L., Buchholz U.J. Increased genetic and phenotypic stability of a promising live-attenuated respiratory syncytial virus vaccine candidate by reverse genetics. J. Virol. 2012;86(19):10792–804. DOI: https://doi.org/10.1128/JVI.01227-12
- Luongo C., Winter C.C., Collins P.L., Buchholz U.J. Respiratory syncytial virus modified by deletions of the NS2 gene and amino acid S1313 of the L polymerase protein is a temperature-sensitive, live-attenuated vaccine candidate that is phenotypically stable at physiological temperature. J. Virol. 2013;87(4):1985–96. DOI: https://doi.org/10.1128/JVI.02769-12
- Endt K., Wollmann Y., Haug J., et al. A recombinant MVA-based RSV vaccine induces T-cell and antibody responses that cooperate in the protection against RSV infection. Front. Immunol. 2022;13:841471. DOI: https://doi.org/10.3389/fimmu.2022.841471
- Taylor G., Thom M., Capone S., et al. Efficacy of a virus-vectored vaccine against human and bovine respiratory syncytial virus infections. Sci. Transl. Med. 2015;7(300):300ra127. DOI: https://doi.org/10.1126/scitranslmed.aac5757
- Saeland E., van der Fits L., Bolder R., et al. Immunogenicity and protective efficacy of adenoviral and subunit RSV vaccines based on stabilized prefusion F protein in pre-clinical models. Vaccine. 2022;40(6):934–44. DOI: https://doi.org/10.1016/j.vaccine.2021.12.043
- Joyce C., Scallan C.D., Mateo R., et al. Orally administered adenoviral-based vaccine induces respiratory mucosal memory and protection against RSV infection in cotton rats. Vaccine. 2018;36(29):4265–77. DOI: https://doi.org/10.1016/j.vaccine.2018.05.112
- de Jong R., Stockhofe-Zurwieden N., Bonsing J., et al. ChAd155-RSV vaccine is immunogenic and efficacious against bovine RSV infection-induced disease in young calves. Nat. Commun. 2022;13(1):6142. DOI: https://doi.org/10.1038/s41467-022-33649-3
- Jones B.G., Sealy R.E., Rudraraju R., et al. Sendai virus-based RSV vaccine protects African green monkeys from RSV infection. Vaccine. 2012;30(5):959–68. DOI: https://doi.org/10.1016/j.vaccine.2011.11.046
- Cespedes P.F., Rey-Jurado E., Espinoza J.A., et al. A single, low dose of a cGMP recombinant BCG vaccine elicits protective T cell immunity against the human respiratory syncytial virus infection and prevents lung pathology in mice. Vaccine. 2017;35(5):757–66. DOI: https://doi.org/10.1016/j.vaccine.2016.12.048
- Bueno S.M., Gonzalez P.A., Cautivo K.M., et al. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc. Natl. Acad. Sci. USA. 2008;105(52):20822–7. DOI: https://doi.org/10.1073/pnas.0806244105
- Cautivo K.M., Bueno S.M., Cortes C.M., et al. Efficient lung recruitment of respiratory syncytial virus-specific Th1 cells induced by recombinant bacillus Calmette-Guerin promotes virus clearance and protects from infection. J. Immunol. 2010;185(12):7633–45. DOI: https://doi.org/10.4049/jimmunol.0903452
- Diaz F.E., Guerra-Maupome M., McDonald P.O., et al. A recombinant BCG vaccine is safe and immunogenic in neonatal calves and reduces the clinical disease caused by the respiratory syncytial virus. Front. Immunol. 2021;12:664212. DOI: https://doi.org/10.3389/fimmu.2021.664212
- Olszewska W., Suezer Y., Sutter G., Openshaw P.J. Protective and disease-enhancing immune responses induced by recombinant modified vaccinia Ankara (MVA) expressing respiratory syncytial virus proteins. Vaccine. 2004;23(2):215–21. DOI: https://doi.org/10.1016/j.vaccine.2004.05.015
- Bembridge G.P., Garcia-Beato R., Lopez J.A., et al. Subcellular site of expression and route of vaccination influence pulmonary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. J. Immunol. 1998;161(5):2473–80.
- Kotomina T., Isakova-Sivak I., Matyushenko V., et al. Recombinant live attenuated influenza vaccine viruses carrying CD8 T-cell epitopes of respiratory syncytial virus protect mice against both pathogens without inflammatory disease. Antiviral Res. 2019;168:9–17. DOI: https://doi.org/10.1016/j.antiviral.2019.05.001
- McLellan J.S., Chen M.., Joyce MG., et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342(6158):592–8. DOI: https://doi.org/10.1126/science.1243283
- Zohar T., Hsiao J.C., Mehta N., et al. Upper and lower respiratory tract correlates of protection against respiratory syncytial virus following vaccination of nonhuman primates. Cell Host Microbe. 2022;30(1):41–52.e5. DOI: https://doi.org/10.1016/j.chom.2021.11.006
- Sastry M., Zhang B., Chen M., et al. Adjuvants and the vaccine response to the DS-Cav1-stabilized fusion glycoprotein of respiratory syncytial virus. PLoS One. 2017;12(10):e0186854. DOI: https://doi.org/10.1371/journal.pone.0186854
- MacDonald L.D., MacKay A., Kaliaperumal V., et al. Type III hypersensitivity reactions to a B cell epitope antigen are abrogated using a depot forming vaccine platform. Hum. Vaccin. Immunother. 2018;14(1):59–66. DOI: https://doi.org/10.1080/21645515.2017.1375637
- Li C., Zhou X., Zhong Y., et al. A recombinant G protein plus cyclosporine A-based respiratory syncytial virus vaccine elicits humoral and regulatory T cell responses against infection without vaccine-enhanced disease. J. Immunol. 2016;196(4):1721–31. DOI: https://doi.org/10.4049/jimmunol.1502103
- Su C., Zhong Y., Zhao G., et al. RSV pre-fusion F protein enhances the G protein antibody and anti-infectious responses. NPJ Vaccines. 2022;7(1):168. DOI: https://doi.org/10.1038/s41541-022-00591-w
- Stokes A.H., Franklin K., Fisher D.E., et al. Repeated dose toxicity study and developmental and reproductive toxicology studies of a respiratory syncytial virus candidate vaccine in rabbits and rats. Int. J. Toxicol. 2021;40(2):125–42. DOI: https://doi.org/10.1177/1091581820985782
- Murphy B.R., Sotnikov A.V., Lawrence L.A., et al. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3-6 months after immunization. Vaccine. 1990;8(5):497–502. DOI: https://doi.org/10.1016/0264-410x(90)90253-i
- Zuniga A., Rassek O., Vrohlings M., et al. An epitope-specific chemically defined nanoparticle vaccine for respiratory syncytial virus. NPJ Vaccines. 2021;6(1):85. DOI: https://doi.org/10.1038/s41541-021-00347-y
- Marcandalli J., Fiala B., Ols S., et al. Induction of potent neutralizing antibody responses by a designed protein nanoparticle vaccine for respiratory syncytial virus. Cell. 2019;176(6):1420-31.e17. DOI: https://doi.org/10.1016/j.cell.2019.01.046
- Петухова Г.Д., Найхин А.Н., Баранцева И.Б. и др. Локальный гумморальный и клеточный иммунный ответ мышей при гриппозной инфекции и вакцинации. Медицинская иммунология. 2006;8(4):511–6. Petukhova G.D., Naikhin A.N., Barantseva I.B., et al. Local antibody and cellular immune responses to influenza infection and vaccination. Medical Immunology (Russia). 2006;8(4):511–6. EDN: https://elibrary.ru/hspxqz
- Beran J., Lickliter J.D., Schwarz T.F., et al. Safety and immunogenicity of 3 formulations of an investigational respiratory syncytial virus vaccine in nonpregnant women: results from 2 phase 2 trials. J. Infect. Dis. 2018;217(10):1616–25. DOI: https://doi.org/10.1093/infdis/jiy065
- Jordan E., Kabir G., Schultz S., et al. Decreased viral load, symptom reduction, and prevention of respiratory syncytial virus infection with MVA-BN-RSV vaccine. 2022. medRxiv. Preprint. DOI: https://doi.org/10.1101/2022.12.02.22283030
- Jordan E., Kabir G., Schultz S., et al. Reduced respiratory syncytial virus load, symptoms, and infections: a human challenge trial of MVA-BN-RSV vaccine. J. Infect. Dis. 2023;228(8):999–1011. DOI: https://doi.org/10.1093/infdis/jiad108
- Williams K., Bastian A.R., Feldman R.A., et al. Phase 1 safety and immunogenicity study of a respiratory syncytial virus vaccine with an adenovirus 26 vector encoding prefusion F (Ad26.RSV.preF) in adults aged ≥60 years. J. Infect. Dis. 2020;222(6):979–88. DOI: https://doi.org/10.1093/infdis/jiaa193
- Sadoff J., De Paepe E., DeVincenzo J., et al. Prevention of respiratory syncytial virus infection in healthy adults by a single immunization of Ad26.RSV.preF in a human challenge study. J. Infect. Dis. 2022;226(3):396–406. DOI: https://doi.org/10.1093/infdis/jiab003
- Abarca K., Rey-Jurado E., Munoz-Durango N., et al. Safety and immunogenicity evaluation of recombinant BCG vaccine against respiratory syncytial virus in a randomized, double-blind, placebo-controlled phase I clinical trial. EClinicalMedicine. 2020;27:100517. DOI: https://doi.org/10.1016/j.eclinm.2020.100517
- Cunningham C.K., Karron R., Muresan P., et al. Live-attenuated respiratory syncytial virus vaccine with deletion of RNA synthesis regulatory protein M2-2 and cold passage mutations is overattenuated. Open Forum Infect. Dis. 2019;6(6):ofz212. DOI: https://doi.org/10.1093/ofid/ofz212
- McFarland E.J., Karron R.A., Muresan P., et al. Live-attenuated respiratory syncytial virus vaccine with M2-2 deletion and with small hydrophobic noncoding region is highly immunogenic in children. J. Infect. Dis. 2020;221(12):2050–9. DOI: https://doi.org/10.1093/infdis/jiaa049
- Cunningham C.K., Karron R.A., Muresan P., et al. Evaluation of recombinant live-attenuated respiratory syncytial virus (RSV) vaccines RSV/DeltaNS2/Delta1313/I1314L and RSV/276 in RSV-seronegative children. J. Infect. Dis. 2022;226(12):2069–78. DOI: https://doi.org/10.1093/infdis/jiac253
- Schwarz T.F., Johnson C., Grigat C., et al. Three dose levels of a maternal respiratory syncytial virus vaccine candidate are well tolerated and immunogenic in a randomized trial in nonpregnant women. J. Infect. Dis. 2022;225(12):2067–76. DOI: https://doi.org/10.1093/infdis/jiab317
- Bebia Z., Reyes O., Jeanfreau R., et al. Safety and immunogenicity of an investigational respiratory syncytial virus vaccine (RSVPreF3) in mothers and their infants: a phase 2 randomized trial. J. Infect. Dis. 2023;228(3):299–310. DOI: https://doi.org/10.1093/infdis/jiad024
- Papi A., Ison M.G., Langley J.M., et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N. Engl. J. Med. 2023;388(7):595–608. DOI: https://doi.org/10.1056/NEJMoa2209604
- Chandler R., Montenegro N., Llorach C., et al. Immunogenicity, reactogenicity, and safety of AS01E-adjuvanted RSV prefusion F protein-based candidate vaccine (RSVPreF3 OA) when co-administered with a seasonal quadrivalent influenza vaccine in older adults: results of a phase 3, open-label, randomized controlled trial. Clin. Infect. Dis. 2024;ciad786. DOI: https://doi.org/10.1093/cid/ciad786
- Ferguson M., Murray A., Pliamm L., et al. Lot-to-lot immunogenicity consistency of the respiratory syncytial virus prefusion F protein vaccine in older adults. Vaccine X. 2024;18:100494. DOI: https://doi.org/10.1016/j.jvacx.2024.100494
- Buynak R., Cannon K., DeAtkine D., et al. Randomized, open-label phase 3 study evaluating immunogenicity, safety, and reactogenicity of RSVPreF3 OA coadministered with FLU-QIV-HD in adults aged ≥65. Infect. Dis. Ther. 2024;13(8):1789–805. DOI: https://doi.org/10.1007/s40121-024-00985-4
- Clark R., Davies S., Labrador J., et al. Safety and immunogenicity of respiratory syncytial virus prefusion F protein vaccine when co-administered with adjuvanted seasonal quadrivalent influenza vaccine in older adults: a phase 3 randomized trial. Clin. Infect. Dis. 2024;79(4):1088–98. DOI: https://doi.org/10.1093/cid/ciae365
- Ferguson M., Schwarz T.F., Nunez S.A., et al. Noninferior immunogenicity and consistent safety of respiratory syncytial virus prefusion F protein vaccine in adults 50-59 years compared to ≥60 years of age. Clin. Infect. Dis. 2024;79(4):1074–84. DOI: https://doi.org/10.1093/cid/ciae364
- Leroux-Roels G., De Boever F., Maes C., et al. Safety and immunogenicity of a respiratory syncytial virus fusion glycoprotein F subunit vaccine in healthy adults: Results of a phase 1, randomized, observer-blind, controlled, dosage-escalation study. Vaccine. 2019;37(20):2694–703. DOI: https://doi.org/10.1016/j.vaccine.2019.04.011
- Peterson J.T., Zareba A.M., Fitz-Patrick D., et al. Safety and immunogenicity of a respiratory syncytial virus prefusion F vaccine when coadministered with a tetanus, diphtheria, and acellular pertussis vaccine. J. Infect. Dis. 2022;225(12):2077–86. DOI: https://doi.org/10.1093/infdis/jiab505
- Falsey A.R., Walsh E.E., Scott D.A., et al. Phase 1/2 randomized study of the immunogenicity, safety, and tolerability of a respiratory syncytial virus prefusion F vaccine in adults with concomitant inactivated influenza vaccine. J. Infect. Dis. 2022;225(12):2056–66. DOI: https://doi.org/10.1093/infdis/jiab611
- Simões E.A.F., Center K.J., Tita A.T.N., et al. Prefusion F protein-based respiratory syncytial virus immunization in pregnancy. N. Engl. J. Med. 2022;386(17):1615–26. DOI: https://doi.org/10.1056/NEJMoa2106062
- Baber J., Arya M., Moodley Y., et al. A phase 1/2 study of a respiratory syncytial virus prefusion F vaccine with and without adjuvant in healthy older adults. J. Infect. Dis. 2022;226(12):2054–63. DOI: https://doi.org/10.1093/infdis/jiac189
- Ginsburg A.S., Srikantiah P. Respiratory syncytial virus: promising progress against a leading cause of pneumonia. Lancet Glob. Health. 2021;9(12):e1644–5. DOI: https://doi.org/10.1016/S2214-109X(21)00455-1
- Schmoele-Thoma B., Zareba A.M., Jiang Q., et al. Vaccine efficacy in adults in a respiratory syncytial virus challenge study. N. Engl. J. Med. 2022;386(25):2377–86. DOI: https://doi.org/10.1056/NEJMoa2116154
- Baker J., Aliabadi N., Munjal I., et al. Equivalent immunogenicity across three RSVpreF vaccine lots in healthy adults 18-49 years of age: Results of a randomized phase 3 study. Vaccine. 2024;42(13):3172–9. DOI: https://doi.org/10.1016/j.vaccine.2024.03.070
- Athan E., Baber J., Quan K., et al. Safety and immunogenicity of bivalent RSVpreF vaccine coadministered with seasonal inactivated influenza vaccine in older adults. Clin. Infect. Dis. 2024;78(5):1360–8. DOI: https://doi.org/10.1093/cid/ciad707
- Cheng X., Zhao G., Dong A., et al. A First-in-Human Trial to Evaluate the Safety and Immunogenicity of a G Protein-Based Recombinant Respiratory Syncytial Virus Vaccine in Healthy Adults 18–45 Years of Age. Vaccines (Basel). 2023;11(5):999. DOI: https://doi.org/10.3390/vaccines11050999
- Glenn G.M., Smith G., Fries L., et al. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine. 2013;31(3):524–32. DOI: https://doi.org/10.1016/j.vaccine.2012.11.009
- Glenn G.M., Fries L.F., Thomas D.N., et al. A randomized, blinded, controlled, dose-ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. J. Infect. Dis. 2016;213(3):411–22. DOI: https://doi.org/10.1093/infdis/jiv406
- August A., Glenn G.M., Kpamegan E., et al. A phase 2 randomized, observer-blind, placebo-controlled, dose-ranging trial of aluminum-adjuvanted respiratory syncytial virus F particle vaccine formulations in healthy women of childbearing age. Vaccine. 2017;35(30):3749–59. DOI: https://doi.org/10.1016/j.vaccine.2017.05.045
- Fries L., Shinde V., Stoddard J.J., et al. Immunogenicity and safety of a respiratory syncytial virus fusion protein (RSV F) nanoparticle vaccine in older adults. Immun. Ageing. 2017;14:8. DOI: https://doi.org/10.1186/s12979-017-0090-7
- Goswami J., Baqui A.H., Doreski P.A., et al. Humoral immunogenicity of mRNA-1345 RSV vaccine in older adults. J. Infect. Dis. 2024;230(5):e996–1006. DOI: https://doi.org/10.1093/infdis/jiae316
Supplementary files