Enhancement of systemic and lung-localized CD4+ T-cell immune responses by truncation of NS1 protein of a seasonal live influenza vaccine strain
- Authors: Prokopenko P.I.1, Stepanova E.A.1, Matyushenko V.A.1, Rak A.Y.1, Chistyakova A.K.1, Kostromitina A.D.1, Kotomina T.S.1, Kudryavtsev I.V.1, Rubinstein A.A.1, Komlev A.S.1, Rudenko L.G.1, Isakova-Sivak I.N.1
-
Affiliations:
- Institute of Experimental Medicine
- Issue: Vol 101, No 5 (2024)
- Pages: 619-627
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18682
- DOI: https://doi.org/10.36233/0372-9311-582
- EDN: https://elibrary.ru/rwupfs
- ID: 18682
Cite item
Abstract
Introduction. There is a large variety of licensed influenza vaccines worldwide, but their common limitation is rather narrow specificity and inability to protect against antigenic-drift variants of influenza virus. Therefore, optimization of immunogenic and cross-protective properties of licensed influenza vaccines is an urgent priority of public health agenda. One such approach is to modulate the immunogenic properties of live attenuated influenza vaccine (LAIV) by truncating the open reading frame of influenza virus non-structural protein 1 (NS1). The main objective of this study is to evaluate the immunogenic properties of the H1N1 seasonal LAIV strain by truncation of the NS1 protein to 126 amino acides.
Materials and methods. Using reverse genetics technique, two H1N1 LAIV strains with full-length and truncated NS1 protein with three consecutive stop codons added after the 126th amino acid residue were obtained.C57BL/6J mice were immunized intranasally with the vaccine candidates, twice at a three-week interval. Seven days after the second immunization, cells were isolated from spleen and lung tissues and stimulated with whole wild-type H1N1 influenza virus. Levels of systemic and tissue-resident cytokine-producing CD4+ and CD8+ memory T cells were assessed by intracellular cytokine staining assay with flow cytometry. Replication of engineered vaccine strains in in vitro and in vivo systems was also evaluated.
Results. Truncation of NS1 protein of the LAIV strain significantly increased the levels of virus-specific CD4+ effector memory T cells in spleens and the levels of CD4+ tissue-resident memory T cells in lungs of mice after two-dose immunization, indicating a higher potential for protection against influenza infection of the LAIV NS126 vaccine strain compared to the classical variant of LAIV. Importantly, the LAIV NS126 strain also had a more pronounced attenuated phenotype in mice than its classical counterpart.
Full Text
Introduction
Influenza viruses pose a constant threat to the global population due to their high contagiousness and ability to cause severe epidemics, which kill up to 650,000 people annually [1]. The most effective means of fighting influenza infection remains vaccination, which is mainly aimed at preventing the development of severe cases of the disease, as well as its complications. There is a sufficiently large variety of licensed influenza vaccines, but their effectiveness in different epidemic seasons varies greatly due to the narrow specificity of the induced immune response to vaccination [2]. In this regard, the search for new approaches to improve the immunogenicity and broaden the spectrum of action of seasonal influenza vaccines is of paramount importance for global health care.
The non-structural NS1 protein of influenza A virus is multifunctional protein and participates in various stages of virus-cell interaction: it is an antagonist of the antiviral cellular response and a regulator of viral and cellular gene expression [3, 4]. In particular, the NS1 protein of influenza virus acts as an interferon (IFN) antagonist and thereby promotes productive infection by disrupting one of the most important links of antiviral immunity [5]. Furthermore, the C-terminus of NS1 protein is attributed to the function of decreasing dendritic cell activation and, consequently, impairing the stimulation of naive T cells [6]. Accordingly, the immunogenicity of live attenuated influenza vaccine (LAIV) can be enhanced by truncating the NS1 protein from the C-terminus to attenuate its anti-IFN activity. Previously, we constructed a vaccine strain of H7N9 subtype LAIV that encoded an NS1 protein shortened to 126 amino acids. Experiments in mice showed that this modification resulted in a significant enhancement of the T-cell response to the immunodominant epitope NP366 compared to immunization with full-length NS1 [7].
The aim of the present study was to evaluate the modulation of immunogenic properties of the seasonal H1N1 subtype influenza vaccine strain by truncation of the NS1 protein reading frame to 126 amino acids. The systemic and local T-cell response to all influenza virus antigens was studied by stimulating immune cells with whole live epidemic influenza H1N1 virus.
Materials and methods
Viruses
Experimental reassortant strains of the H1N1 subtype were obtained by standard reverse genetics methods based on the attenuation donor of the domestic LAIV A/Leningrad/134/17/57 (H2N2) (Len/17) [8]. The parental epidemic influenza virus was the A/Guangdong-Maonan/SWL1536/2019 (H1N1) [H1N1/wt] strain obtained from the NIBSC collection (UK). The H1N1 LAIV vaccine strain with full-length NS1 protein contained PB2, PB1, PA, NP, M and NS genes from the attenuation donor Len/17, and hemagglutinin and neuraminidase genes from the epidemic H1N1/wt virus. To obtain a recombinant influenza virus expressing a truncated NS1 protein, 3 consecutive stop codons were added after 126 amino acids of the open reading frame of the NS1 protein of Len/17 virus by site-directed mutagenesis using the Q5 Site-Directed Mutagenesis Kit (New England Biolabs) and specific primers (Evrogen Ltd.). Viruses were cultured in 10-11-day-old embryonated chicken eggs (ECE) (Siniavinskaya poultry farm) at 33ºC (for vaccine strains of LAIV) or at 37ºC (for epidemic strain H1N1/wt) and stored at –70ºC in aliquots.
Cell lines
MDCK dog kidney cells (ATCC CCL-34) and Vero green monkey kidney cells (ATCC CCL-81) were cultured in DMEM growth medium containing 10% fetal bovine serum and supplemented with an antimycotic antibiotic (Capricorn reagents listed).
Determination of the infectious activity of influenza viruses
The infectious titers of viruses were determined by the limit dilution method. To infect ECE, viruses were diluted in phosphate-salt buffer (PBS) and each dilution infected 4-6 embryos in a volume of 200 µl. The ECEs were incubated at 33ºC and 38ºC for 48 h or at 26ºC for 6 days, after which the embryos were cooled; the presence of virus in allantois fluid was determined by hemagglutination assay with chicken erythrocytes. Determination of infectious titers of viruses on cell cultures was performed by infecting daily monolayer cultures in 96-well plates with serial 10-fold dilutions of viruses. After adsorption, the inoculum was removed, cells were washed, incubated in DMEM medium containing 1 μg/mL TRSC trypsin and antibiotic-antimycotic at 33ºC for 4 days. The presence of viruses in the wells was determined by staining the cells fixed in acetone with monoclonal anti-NP-antibody conjugated with horseradish peroxidase (PPDP LLC). Illumination was performed using TMB substrate (Thermo Fisher Scientific), and optical density was measured on an xMark flatbed spectrophotometer (BioRad). Wells were considered positive at optical density values (λ = 450 nm) exceeding the values of negative control wells at least 2-fold. Virus titers in ECE and Vero and MDCK cells were calculated according to the method proposed by L.J. Reed et al. [9] and were expressed in 50% infectious doses (log10 EID50/mL and log10 TCID50/mL).
Accumulation and purification of influenza virus on a sucrose gradient
For immunologic tests, H1N1/wt influenza virus was purified on a sucrose gradient to remove nonspecific proteins from chicken embryos and to concentrate the virus. Viruses were purified on a 30%/60% density gradient using an ultracentrifuge (BeckmanCoulter) in several steps:
- clarification of the collected allantois fluid by centrifugation for 15 min at 4ºC at 3500g;
- sedimentation at 16,000g for 2 h at 4ºC and resuspension of the formed precipitate in 2 ml of FSB;
- layering of the resuspended precipitate on a 30%/60% sucrose step gradient followed by ultracentrifugation for 2 h and at 4ºC at 23,000g;
- collection of concentrated virus at the boundaries of the gradient and its washing in 10 ml of FSB by centrifugation for 1.5 h at 23,000g. In the last step, the viral precipitate was resuspended in 1 ml of PBS and stored at –70ºC in aliquots.
Mice immunization and organ harvesting
Female mice of the C57Bl/6J line, supplied from the Stolbovaya Branch Nursery of the National Center for Biomedical Research of the Federal Medical and Biological Agency of Russia, were used. Mice were immunized twice intranasally with an interval of 21 days with one of the recombinant strains of LAIV at a dose of 106 EID50 in a volume of 50 μl using light ether anesthesia. Control animals received an equal volume of PBS. On the 3rd day, lungs and nasal passages were collected from 4 vaccinated mice from each group and further homogenized using a TissueLyser LT automatic homogenizer (Qiagen). Lung and nasal tissue homogenates were used to determine the infectious titer of the virus in the ECE system. At 7 days after re-immunization, lung tissue and spleens were collected from 6 mice from each group for further evaluation of T-cell immunity. The research protocol was approved by the Ethics Committee of the Institute of Experimental Medicine (protocol No. 1/20, February 27, 2020).
Assessment of the T-cell immune response
Determination of systemic and memory T cells localized in the lungs was performed according to the previously described method [7] with minor modifications. Single splenocytes were isolated in CR-0 medium (RPMI-1640 supplemented with antibiotic-antimycotic, 25 mM HEPES (listed reagents from Capricorn) and 50 μM 2-mercaptoethanol (Sigma-Aldrich) using 70 μm pore size filters (BD Biosciences). Erythrocytes were then lysed using erythrocyte lysis buffer (BioLegend). For intracellular cytokine staining, 2 × 106 cells were added to 100 μL of CR-10 medium (CR-0 medium containing 10% fetal bovine serum) in sterile U-bottom microplates. Then, 100 μl of CR-10 medium containing purified whole H1N1/wt virus at a dose of 2 infectious units per cell was added to each well and incubated for 18 h at 37ºC, 5% CO2. GolgiPlug solution (BD Biosciences) was then added to the samples at a final concentration of 1 : 1000 to stop protein transport. Stimulation with phorbolmyristyl acetate (Sigma-Aldrich) was used as a positive control; unstimulated control samples and isotypic control samples were also prepared. Cells were incubated for 5 h at 37 ºC, 5% CO2, then stained for 20 min at 4ºC in the dark with ZombieAqua fluorescent live/dead cell detection dye and a mixture of the following fluorescently labeled surface antibodies: CD4-PerCP/Cy5.5, CD8-APC/Cy7, CD44-PE, and CD62L-BV421 (reagents listed are BioLegend). The Cytofix/Cytoperm kit (BD Biosciences) was used for fixation/permeabilization, after which cells were stained with antibodies to cytokines: IFN-γ — FITC, tumor necrosis factor-α (TNF-α) — APC, interleukin-2 (IL-2) — PE/Cy7 for 20 min at 4ºC in the dark. Samples were fixed with Cyto-Last buffer (antibodies and buffer – BioLegend) and analyzed using a Navios cytofluorimeter (BeckmanCoulter).
To detect tissue-resident memory T cells (TRM), lungs perfused with PBS solution were cut into small pieces with sterile scissors and treated with a mixture of DNAase I and collagenase (Sigma-Aldrich) for 40 min at 37ºC. A suspension of individual cells was then prepared using 70 μm pore size filters. Erythrocytes were lysed as described above and stimulated with whole H1N1/wt virus followed by detection of virus-specific effector memory T cells (TEM; CD44+CD62L-) expressing tissue-resident markers (CD69+CD103+). The staining kit for surface markers and intracellular cytokines included: CD4 — PerCP/Cy5.5, CD8 — APC/Cy7, CD44 — APC, CD62L — BV421, CD69-PE/Cy7, CD103 — FITC, while intracellular staining was performed for only one cytokine, IFN-γ — PE/Dazzle (listed reagents — BioLegend). The number of cytokine-positive cells in stimulated groups was counted and the level of spontaneous cytokine secretion in unstimulated control samples was subtracted.
Statistical processing of the results
Flow cytometry data were analyzed using Kaluza Analysis software (BeckmanCoulter). Statistical analysis and preparation of illustrations were performed using the GraphPad Prism v. 7.0 program. ANOVA with Tukey's correction or Mann–Whitney U-test was used to compare data; differences were considered significant at p < 0.05.
Results
In the present study, a strain of seasonal H1N1 subtype LAIV expressing a truncated nonstructural protein 1 truncated to 126 amino acids (LAIV H1N1 NS126) was obtained by genetic engineering methods. In in vitro experiments, it was shown that the classical vaccine strain H1N1 and the modified variant H1N1 NS1126 had similar growth characteristics in various culture systems, but the variant with NS1126 was significantly more weakly propagated at a temperature reduced to 26ºC (Table). These results are consistent with the previously obtained data on the phenotypic characteristics of vaccine strains of LAIV with NS1126 [7, 10]. Furthermore, the modified strain practically did not propagate in the upper respiratory tract of mice, in contrast to the classical strain of LAIV, which is also consistent with the previously obtained data on the enhancement of the attenuating properties of the vaccine when the reading frame of the NS1 protein is truncated.
Replicative properties of LAIV vaccine strains in vitro and in vivo systems
Vaccine strain | Virus titer in ECE, lg EID50/ml | Virus titer in cells, lg TCID50/ml | Virus titer in mouse organs, lg EID50/ml | ||||
26ºC | 33ºC | 38ºC | MDCK | Vero | lungs | nose | |
LAIV H1N1 | 5,8 ± 0,4 | 8,7 ± 0,3 | 1,9 ± 0,4 | 7,2 ± 0,3 | 6,2 ± 0,2 | ˂ 1,2 | 2,3 ± 1,2 |
LAIV H1N1 NS1126 | 4,4 ± 0,7 | 7,6 ± 0,5 | ˂ 1,2 | 5,8 ± 0,8 | 5,4 ± 0,4 | ˂ 1,2 | ˂ 1,2 |
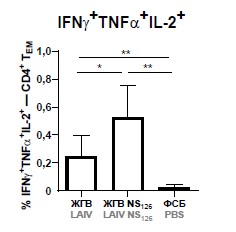
Two-dose immunization of mice with LAIV and LAIV with truncated NS126 stimulated the induction of high levels of virus-specific TEM cells with CD44+CD62L- phenotype in the spleens of mice, and truncation of NS1 protein in the vaccine strain of LAIV significantly increased the levels of polyfunctional CD4+-TEM cells secreting two (IFN-γ, TNF-α) or three (IFN-γ, TNF-α and IL-2) cytokines simultaneously (Fig. 1, а). Furthermore, only the group of mice that received LAIV NS126 showed significantly higher levels of IFN-γ-producing cytotoxic memory T cells compared to controls (Fig. 1, b). Thus, the data obtained indicate that the modified strain of LAIV NS126 may have a higher potential for protection against influenza infection than the classical LAIV variant.
Fig. 1. Number of effector memory T cells (CD44+CD62L–) with CD4+ (a) and CD8+ (b) phenotype expressing IFNγ (left panel), IFNγ and TNFα (middle panel) and IFNγ, TNFα and IL-2 (right panel) in groups of mice immunized with LAIV or LAIV with truncated NS1 protein, as well as in the control group (PBS).
Significant differences between groups (Mann–Whitney test), *p < 0.05, **p < 0.01.
Further, we investigated subpopulations of virus-specific TEM cells in the lungs with evaluation of the expression of surface markers of memory TRM cells by these cells. Evaluation of the levels of IFN-γ-producing TEM cells in the lungs of immunized mice revealed a significant enhancement of the CD4+ T-cell response in the group of animals inoculated with the prototype LAIV NS1126 compared with the classical version of the vaccine (Fig. 2, a). For cytotoxic CD8+-TEM cells in the lungs, a comparable level of immunogenicity of LAIV was shown, independent of NS1 protein modification (Fig. 2, d). At the same time, the level of expression of tissue residency markers was comparable in both vaccine groups (Fig. 2, b, c, d, e, f), indicating the localization of the identified virus-specific cells in the lung epithelium, in close proximity to the potential site of pathogen entry.
Fig. 2. Induction of virus-specific memory T cells in the lungs after immunization of mice with the study vaccine viruses.
(a, d) Number of IFNγ-producing effector memory T cells (CD44+CD62L–) with CD4+ (a) and CD8+ (d) cell phenotype in groups of mice immunized with LAIV or LAIV with truncated NS126, as well as in controls. Proportion of virus-specific tissue-resident memory cells with CD69+CD103– phenotype among IFNγ+ cells in CD4+ and CD8+ T cell populations (b and f, respectively). Proportion of virus-specific tissue-resident memory cells with CD69+CD103+ phenotype among IFNγ+ cells in CD4+ and CD8+ T cell populations (c and f, respectively).
* p < 0.05, ** p < 0.01 between groups (Mann–Whitney test).
Discussion
Existing influenza vaccines induce predominantly neutralizing antibodies targeting hypervariable epitopes of the main influenza virus antigen, the hemagglutinin molecule, necessitating almost annual updates of the vaccine strain composition. Over the past decade, significant progress has been made in the development of influenza vaccines with a broader protective spectrum, targeting conserved viral antigens such as the hemagglutinin stem domain, neuraminidase, or M2e; and the development of T cell-based approaches that have the greatest potential to induce long-term cross-protective memory cell responses [11]. To date, a massive amount of evidence has accumulated on the ability of influenza viruses expressing the truncated NS1 protein to stimulate the formation of a more pronounced adaptive immune response while rendering the virus more attenuated [12-15]. However, the vast majority of studies have used a model laboratory strain A/Puerto Rico/8/34 (H1N1) or a strain based on wild-type influenza virus, which has a significant disadvantage – the probability of reverting to a virulent phenotype in case of possible reassortment with other circulating viruses. In this current study, the strain of the domestic licensed live influenza vaccine, widely used in public health practice in Russia and in a number of foreign countries, was used as the basis for our investigation [16].
We have previously shown that truncation to 126 amino acids of the NS1 protein of the H7N9 LAIV vaccine strain leads to an enhanced humoral and T-cell response in a mouse experiment [7]. In contrast to the above mentioned study, where the T-cell immune response was assessed by stimulation of cells with synthetic peptides corresponding to immunodominant CD8+ T-cell epitopes NP366, in the present study, a stimulation of immune cells of immunized mice with whole purified H1N1/wt influenza virus was performed. Such stimulation better reflects the actual clinical situation, since during influenza the organism is exposed to the circulating virus in its natural form, and infected cells present a large variety of T-cell viral epitopes on MHC-I and MHC-II complexes.
The present study demonstrated an enhanced CD4+ T-cell response in mice when they were immunized with a live influenza vaccine strain with modified NS1 protein, and this effect was expressed both at the systemic (splenocytes) and local levels (cells from lung tissues). At the same time, systemic virus-specific CD4+ T cells were characterized by a polyfunctional phenotype, producing, in addition to IFN-γ, other key proinflammatory cytokines involved in the antiviral response, such as TNF-α and IL-2. T-lymphocytes capable of secreting several cytokines simultaneously in response to antigenic stimulation are known to be more accurate predictors of the organism's ability to resist reinfection than monofunctional cells secreting only IFN-γ [17]. For systemic CD8+-TEM cells, no significant increase in the proportion of cytokine-producing T cells was detected with NS1 protein shortening, likely due to the small number of animals in the group and high dispersion. These data are in general agreement with previous results obtained for the H7N9 vaccine strain expressing a shortened variant of NS1 protein, where CD8+ T-cell response was assessed after stimulation of splenocytes with a peptide corresponding to the immunodominant epitope NP366 [7]. It is important to note that a more pronounced T-cell response formed directly in the lung tissues of mice immunized with the vaccine strain of LAIV with NS1126 indicates the potential for the development of an accelerated immune response during subsequent contact with the pathogen, since TRM cells in the lungs represent the first line of immune defense of the organism against respiratory pathogens [18, 19].
Conclusion
The present study provides evidence of enhanced systemic and lung-localized CD4+ T-cell immune response upon truncating of NS1 protein of a seasonal H1N1/wt strain. Since virus-specific T cells were detected when lymphocytes were stimulated with whole live H1N1/wt virus, it can be assumed that upon re-infection with a modern circulating virulent virus of this subtype, mice immunized with the H1N1 NS1126 variant of H1N1 LAIV will be better protected from clinical manifestations of the disease than animals that received the classical variant of LAIV.
About the authors
Polina I. Prokopenko
Institute of Experimental Medicine
Author for correspondence.
Email: pi.prokopenko@gmail.com
ORCID iD: 0000-0002-7247-979X
junior researcher, A.A. Smorodintsev department of virology
Russian Federation, St. PetersburgEkaterina A. Stepanova
Institute of Experimental Medicine
Email: pi.prokopenko@gmail.com
ORCID iD: 0000-0002-8670-8645
Cand. Sci. (Biol.), leading researcher, A.A. Smorodintsev department of virology
Russian Federation, St. PetersburgVictoria A. Matyushenko
Institute of Experimental Medicine
Email: pi.prokopenko@gmail.com
ORCID iD: 0000-0002-4698-6085
researcher, A.A. Smorodintsev Department of virology
Russian Federation, St. PetersburgAlexandra Ya. Rak
Institute of Experimental Medicine
Email: pi.prokopenko@gmail.com
ORCID iD: 0000-0001-5552-9874
Cand. Sci. (Biol.), leading researcher, A.A. Smorodintsev department of virology
Russian Federation, St. PetersburgAnna K. Chistyakova
Institute of Experimental Medicine
Email: pi.prokopenko@gmail.com
ORCID iD: 0000-0001-9541-5636
laboratory assistant, A.A. Smorodintsev department of virology
Russian Federation, St. PetersburgArina D. Kostromitina
Institute of Experimental Medicine
Email: pi.prokopenko@gmail.com
ORCID iD: 0000-0001-5432-0171
junior research assistant, A.A. Smorodintsev department of virology
Russian Federation, St. PetersburgTatyana S. Kotomina
Institute of Experimental Medicine
Email: pi.prokopenko@gmail.com
ORCID iD: 0000-0001-9999-089X
Cand. Sci. (Biol.), leading researcher, A.A. Smorodintsev department of virology
Russian Federation, St. PetersburgIgor V. Kudryavtsev
Institute of Experimental Medicine
Email: pi.prokopenko@gmail.com
ORCID iD: 0000-0001-7204-7850
Cand. Sci. (Biol.), Head, Laboratory of cellular immunology, Department of immunology
Russian Federation, St. PetersburgArtem A. Rubinstein
Institute of Experimental Medicine
Email: pi.prokopenko@gmail.com
ORCID iD: 0000-0002-8493-5211
laboratory assistant, Department of immunology
Russian Federation, St. PetersburgAlexey S. Komlev
Institute of Experimental Medicine
Email: pi.prokopenko@gmail.com
ORCID iD: 0000-0001-9111-0755
junior researcher, Department of general pathology and pathophysiology
Russian Federation, St. PetersburgLarisa G. Rudenko
Institute of Experimental Medicine
Email: pi.prokopenko@gmail.com
ORCID iD: 0000-0002-0107-9959
D. Sci. (Med.), Professor, Head, A.A. Smorodintsev department of virology
Russian Federation, St. PetersburgIrina N. Isakova-Sivak
Institute of Experimental Medicine
Email: pi.prokopenko@gmail.com
ORCID iD: 0000-0002-2801-1508
D. Sci. (Biol.), RAS Corresponding Member, Head, Laboratory of immunology and prevention of viral infections, A.A. Smorodintsev department of virology
Russian Federation, St. PetersburgReferences
- Iuliano A.D., Roguski K.M., Chang H.H., et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–300. DOI: https://doi.org/10.1016/s0140-6736(17)33293-2
- Osterholm M.T., Kelley N.S., Sommer A., Belongia E.A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12(1):36–44. DOI: https://doi.org/10.1016/s1473-3099(11)70295-x
- Vasin A.V., Temkina O.A., Egorov V.V., et al. Molecular mechanisms enhancing the proteome of influenza A viruses: an overview of recently discovered proteins. Virus Res. 2014;185:53–63. DOI: https://doi.org/10.1016/j.virusres.2014.03.015
- Marc D. Influenza virus non-structural protein NS1: interferon antagonism and beyond. J. Gen. Virol. 2014;95(Pt. 12):2594–611. DOI: https://doi.org/10.1099/vir.0.069542-0
- García-Sastre A., Egorov A., Matassov D., et al. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252(2):324–30. DOI: https://doi.org/10.1006/viro.1998.9508
- Haye K., Burmakina S., Moran T., et al. The NS1 protein of a human influenza virus inhibits type I interferon production and the induction of antiviral responses in primary human dendritic and respiratory epithelial cells. J. Virol. 2009;83(13):6849–62. DOI: https://doi.org/10.1128/jvi.02323-08
- Prokopenko P., Matyushenko V., Rak A., et al. Truncation of NS1 protein enhances T cell-mediated cross-protection of a live attenuated influenza vaccine virus expressing wild-type nucleoprotein. Vaccines (Basel). 2023;11(3):501. DOI: https://doi.org/10.3390/vaccines11030501
- Rekstin A., Isakova-Sivak I., Petukhova G., et al. Immunogenicity and cross protection in mice afforded by pandemic H1N1 live attenuated influenza vaccine containing wild-type nucleoprotein. Biomed. Res. Int. 2017;2017(1):9359276. DOI: https://doi.org/10.1155/2017/9359276
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938;27:493–7. DOI: https://doi.org/10.1093/oxfordjournals.aje.a118408
- Kotomina T., Isakova-Sivak I., Matyushenko V., et al. Recombinant live attenuated influenza vaccine viruses carrying CD8 T-cell epitopes of respiratory syncytial virus protect mice against both pathogens without inflammatory disease. Antiviral. Res. 2019;168:9–17. DOI: https://doi.org/10.1016/j.antiviral.2019.05.001
- Isakova-Sivak I., Stepanova E., Mezhenskaya D., et al. Influenza vaccine: progress in a vaccine that elicits a broad immune response. Expert. Rev. Vaccines. 2021;20(9):1097–112. DOI: https://doi.org/10.1080/14760584.2021.1964961
- Pica N., Langlois R.A., Krammer F., et al. NS1-truncated live attenuated virus vaccine provides robust protection to aged mice from viral challenge. J. Virol. 2012;86(19):10293–301. DOI: https://doi.org/10.1128/jvi.01131-12
- Baskin C.R., Bielefeldt-Ohmann H., García-Sastre A., et al. Functional genomic and serological analysis of the protective immune response resulting from vaccination of macaques with an NS1-truncated influenza virus. J. Virol. 2007;81(21):11817–27. DOI: https://doi.org/10.1128/jvi.00590-07
- Vasilyev K.A., Yukhneva M.A., Shurygina A.P.S., et al. Enhancement of the immunogenicity of influenza A virus by the inhibition of immunosuppressive function of NS1 protein. Microbiology Independent Research Journal. 2018;(5):48–58. DOI: https://doi.org/10.18527/2500-2236-2018-5-1-48-58 EDN: https://elibrary.ru/ytgzsp
- Vasilyev K., Shurygina A.P., Sergeeva M., et al. Intranasal immunization with the influenza A virus encoding truncated NS1 protein protects mice from heterologous challenge by restraining the inflammatory response in the lungs. Microorganisms. 2021;9(4):690. DOI: https://doi.org/10.3390/microorganisms9040690 EDN: https://elibrary.ru/zfpdqm
- Rudenko L., Yeolekar L., Kiseleva I., Isakova-Sivak I. Development and approval of live attenuated influenza vaccines based on Russian master donor viruses: process challenges and success stories. Vaccine. 2016;34(45):5436–41. DOI: https://doi.org/10.1016/j.vaccine.2016.08.018
- Makedonas G., Betts M.R. Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin. Immunopathol. 2006;28(3):209–19. DOI: https://doi.org/10.1007/s00281-006-0025-4
- Takamura S. Persistence in temporary lung niches: a survival strategy of lung-resident memory CD8+ T cells. Viral. Immunol. 2017;30(6):438–50. DOI: https://doi.org/10.1089/vim.2017.0016
- Topham D.J., Reilly E.C. Tissue-resident memory CD8+ T cells: from phenotype to function. Front. Immunol. 2018;9:515. DOI: https://doi.org/10.3389/fimmu.2018.00515
Supplementary files