In vitro and in vivo tropism and biodistribution of recombinant simian adenovirus type 25
- Authors: Vavilova I.V.1, Ozharovskaia T.A.1, Zubkova O.V.1, Popova O.1, Voronina D.V.1, Goldovskaya P.P.1, Zrelkin D.I.1, Dzharullaeva A.S.1, Dolzhikova I.V.1, Shcheblyakov D.V.1, Logunov D.Y.1, Gintsburg A.L.1
-
Affiliations:
- Gamaleya National Research Center for Epidemiology and Microbiology
- Issue: Vol 101, No 5 (2024)
- Pages: 594-605
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18681
- DOI: https://doi.org/10.36233/0372-9311-573
- EDN: https://elibrary.ru/aspmgu
- ID: 18681
Cite item
Abstract
Introduction. Recombinant adenoviruses are widely used in the development of vaccines for a variety of infectious diseases. Despite numerous clinical studies, only a few types of human (types 5 and 26) and simian (isolate Y25) adenoviruses are currently used to produce vaccine formulations. Different types of adenoviruses vary in their cellular tropism, which plays a key role in their ability to elicit an immune response.
The aim of this study was to investigate the cellular tropism of the simian adenovirus type 25 in vitro and its biodistribution in vivo in comparison with human adenoviruses types 5 and 26.
Materials and methods. The efficiency of in vitro transduction was evaluated on 15 different cell lines using recombinant adenovirus vectors expressing the enhanced green fluorescent protein (EGFP) reporter gene. In vivo biodistribution and bioluminescence imaging were evaluated in BALB/c mice after administration of recombinant adenoviral vectors encoding the luciferase reporter gene. The acute toxicity of a recombinant simian adenovirus type 25 vector was assessed in mice and rats following intramuscular or intravenous administration.
Results. Recombinant simian adenovirus effectively transduces a wide range of cells. At the same time, a higher tropism to human glioblastoma cells (GL-6) was found compared to the other two studied adenoviruses. In vivo experiments have shown that recombinant adenoviruses are mainly localized at the injection site, and transgene expression persists for 21 days. Acute toxicity studies demonstrated that simian adenovirus type 25 vector was well-tolerated, with no animal deaths or detectable toxic effects.
Conclusion. The new platform based on the recombinant simian adenovirus type 25 is not inferior to the existing and well-established delivery systems based on human adenovirus types 5 and 26. Due to its high level of gene transfer and favorable safety profile, the use of the simian adenovirus type 25 in medicine has the potential to offer many benefits for the development of vaccines against future infectious diseases.
Full Text
Introduction
Adenoviruses (Ad) are non-enveloped DNA-containing icosahedral-shaped viruses. The Adenoviridae family consists of 6 genera, including viruses of the Mastadenovirus genus that infect mammals, including humans [1]. Human Ads are divided into 7 species (Mastadenovirus adami, Mastadenovirus blackbeardi, Mastadenovirus caesari, Mastadenovirus dominans, Mastadenovirus exoticum, Mastadenovirus faecale, Mastadenovirus russelli) depending on their morphological, virological, serological and genetic characteristics. The fundamental biological properties of Ad species Mastadenovirus caesari (formerly human adenoviruses of subgroup C) have long been widely studied, which has made them popular targets for the development of vector systems for the delivery of foreign genetic information in vivo and in vitro [2, 3].
To date, significant progress has been made in the use of Ad as vector vaccines [3-6]. Over the past 10 years, vaccines for the prevention of Ebola virus disease and COVID-19 coronavirus infection have been approved and registered in Russia [7, 8]. The experience of vaccination during the COVID-19 pandemic proved the safety and efficacy of Ad-based vector vaccines. Three Ad platforms were used for large-scale vaccination: based on human Ad of type 5 (Ad5), type 26 (Ad26) and chimpanzee adenovirus (isolate Y25) [5, 7, 9]. Despite a large number of clinical studies, only a few human and chimpanzee Ad types (Ad6, Ad35, ChAd63, ChAd3) have been studied as the basis for vector vaccines [10]. Different types have distinct characteristics in cell tropism, which may play a key role in the induction of immune response by affecting the expression and distribution of the target antigen [11]. The key characteristics in selecting alternative types of Ad are low seroprevalence in the human population and the ability to induce high levels of specific immune responses to the target antigen.
Previously, a technology platform based on simian Ad type 25 (SAd25) was developed [12]. The aim of this study was to compare the tropism of Ad5, Ad26 and SAd25 in vitro and in vivo.
Materials and methods
Cell lines
15 cell lines of different origins were used in the experiments:
- human cells: HEK 293 (embryo kidney cells transformed by E1 region of Ad5), H292 (lung mucoepidermoid carcinoma cells), H460 (large cell lung carcinoma cells), H1299 (non-small cell lung cancer cells), A549 (lung adenocarcinoma cells), A431 (epidermoid carcinoma cells), GL-6 (glioblastoma cells), LHR-T (embryonic lung cells), HeLa (cervical carcinoma cells);
- monkey cells: two green monkey kidney cell lines (CV-1 and Vero E6);
- mouse cells: L929 (fibroblast cells) and EPNT-5 (glioblastoma cells);
- hamster cells: CHO (Chinese hamster ovary cells) and BHK-21 (newborn Syrian hamster kidney cells).
Recombinant adenoviruses
Recombinant Ad5- and Ad26-vectors carrying the reporter gene of the enhanced green fluorescent protein EGFP (rAd5-EGFP and rAd26-EGFP), the luciferase gene (rAd5-Luc, rAd26-Luc) were obtained earlier [7, 12, 13]. To clone the luciferase gene or the SARS-CoV-2 virus glycoprotein S gene into the rSAd25 genome, we used the technique described previously [12]. The pArms-SAd25-Luc or pArms-SAd25-S-CoV2 carrying expression cassettes with the reporter gene or antigen were linearized and added to pSAd25-EGFP containing the ΔE1/ΔE3 gene. After electroporation in E. coli BJ5183 cells, homologous recombination resulted in pSAd25-Luc or pSAd25-S-CoV2 encoding the ΔE1/ΔE3 genome of SAd25 with an expression cassette. rAd was rescued and grown in HEK 293 cells.
Laboratory animals
All animal experiments were performed in strict compliance with the recommendations of the National Standard of the Russian Federation (GOST R 53434-2009 Principles of Good Laboratory Practice), and the methods used were approved by the Biomedical Ethics Committee of N.F. Gamaleya NRCEM (Protocol No. 30 of 28.10.2022). Six-week-old female BALB/c mice (18-20 g) were obtained from the Pushchino Nursery (Russia; accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care). Outbred (mongrel) mice and rats were obtained from the Andreevka branch of the Scientific Center for Biomedical Technologies of the Federal Medical and Biological Agency of Russia. The animals had free access to water and standard rodent food.
Determination of the infectious titer of adenoviruses
The quantity of infectious Ad particles was determined by an endpoint dilution assay TCID50 (50% tissue culture infectious dose) in HEK 293 cell culture. Cells were plated into 96-well plates at 3 × 104 cells per well. Then, serial 10-fold dilutions of virus were added in 8 repeats. The plates were incubated for 12–14 days, and the result was recorded visually by the presence of cytopathic effect (CPE). The virus titer was calculated according to the Reed-Mench formula and expressed in TCID50/mL [14].
Determination of adenovirus replication
To study the kinetics of Ad replication, HEK 293 cell culture was plated into 96-well plates at the rate of 3 × 104 cells per well. Cells were then infected with rAd5-EGFP, rAd26-EGFP, and rSAd25-EGFP at a dose of 0.01 TCID50/cell. Culture plates were incubated for 6–7 days at 37ºC and 5% CO2. The results were recorded using a Lionheart FX Automated Microscope (BioTek Instruments Inc.).
Transduction of cell lines by recombinant adenoviruses
Two to three hours before transduction, cells were plated into 48-well plates at the rate of 105 cells per well. Cells were then transduced with rAd5-EGFP, rAd26-EGFP and rSAd25-EGFP at a dose of 1 TCID50/cell. Culture plates were incubated at 37ºC and 5% CO2. Fluorescence intensity was measured on a Synergy H1 Hybrid Multifunctional Reader (BioTek Instruments Inc.) using Gen5 Microplate Reader and Imager Software.
Administration of recombinant adenoviruses to animals
For biodistribution studies, rAd was injected once intramuscularly at a dose of 1010 or 1011 viral particles (v.p.) per animal (n = 3). Animals in control group were injected with sterile phosphate-buffered saline (PBS) (Paneco). The research protocol was approved by the Committee on Biomedical Ethics of the Gamaleya National Research Center for Epidemiology and Microbiology, Moscow, Russia (protocol No. 30, October 28, 2022).
Determination of adenovirus DNA in animal organs and tissues
Animals were euthanized 24 h after rAd administration. Organs were harvested, weighed and homogenized in PBS. DNA was isolated using the Wizard Genomic DNA Purification Kit (Promega). Viral DNA was determined by real-time polymerase chain reaction on a CFX 96 thermocycler (Bio-Rad): primers (5’-GGCGGCGGCTGGCGGTAGA-3’ and 5’-GCAACATCTGGAACCGCGCG-3’), qPCRmix-HS SYBR mix (Eurogen). The initial denaturation step (5 min at 95ºC) was followed by 40 cycles of 15 s at 95ºC, 30 s at 61ºC and 30 s at 72ºC. The data were processed using Bio-Rad CFX Manager software.
Bioluminescence imaging in vivo
Luciferin (Promega; 2.5 mg/animal) was diluted in PBS and injected intraperitoneally into mice on days 1, 3, 7, 14 and 21 after rAd administration. Animals were anesthetized with isoflurane (Piramal Critical Care) for 5-10 min, followed by bioluminescence imaging on an IVIS Lumina Series II instrument (Caliper). Exposure times were adjusted to avoid pixel oversaturation, and flux measurements were converted to photons per second for comparative evaluation of luminescence at different time points. Luminescence image data were analyzed using the Living Image v. 4.2 software.
Methods of acute toxicity assessment
In the toxicity experiment, the effect of rSAd25-SCoV2 on experimental animals (mice and rats) was evaluated by intramuscular (for mice and rats) and intravenous (for mice) administration. For each route of administration, 4 groups of outbred mice of both sexes (10 females and 10 males per group) were formed and injected with rAd at different doses (109, 1010 and 1011 v.p. per animal). The control group was injected with PBS. Three groups of rats (10 females and 10 males per group) were formed and injected with rAd at different doses (1010 and 1011 v.p. per animal). The control group was injected with PBS. After a single injection of the drug, the animals were monitored for 14 days with daily clinical examination. Parameters of functional state, appearance, physiologic functions were recorded. On days 0, 7 and 14, the animals were weighed. On the 14th day animals were euthanized and complete necropsy was performed. At necropsy, the external state of the body, internal surfaces and passages, cranial cavity, thoracic, abdominal and pelvic cavities with organs and tissues in them, neck with organs and tissues, and skeletal-muscular system were examined.
Statistical analysis
Data were processed using the following computer programs: GraphPad 8.0 and Microsoft Excel. For quantitative results, arithmetic mean, geometric mean, standard error of the mean and standard deviation were calculated. When analyzing data from unrelated samples, Student’s test, Mann–Whitney test or Kraskell–Wallis test were used. The a-priori level of significance was taken as α = 0.05. Differences were considered statistically significant at the achieved significance level p < α.
Results
Construction of recombinant vectors based on simian adenovirus serotype 25
A recombinant replication-defective vector based on SAd25 with an EGFP reporter gene (rSAd25-EGFP) was obtained earlier [12]. Recombinant vectors carrying the luciferase gene (rSAd25-Luc) and the S-protein gene of SARS-CoV-2 coronavirus (rSAd25-S-CoV2) were obtained in a similar manner (Fig. 1, a).
Fig. 1. Schematic representation of the recombinant SAd25 genomes (a) and comparison of the reproduction efficiency of rSAd25-EGFP, rAd5-EGFP and rAd26-EGFP in HEK 293 (b).
To evaluate the reproductive efficiency of the recombinant vector based on the simian adenovirus in permissive culture, rSAd25-EGFP carrying the gene of a fluorescent protein reporter was used. rAd5-EGFP and rAd26-EGFP carrying a similar transgene served as comparison vectors. HEK 293 cells were infected with the studied Ads at a rate of approximately 1 infectious particle per 100 cells. Visual evaluation of the results was performed starting 96 h after transduction every 16 h (Fig. 1, b).
After 96 h, intense fluorescence induced by recombinant viral vectors was observed. In contrast to the comparison vectors, the formation of fluorescent focuses was detected only in wells with rSAd25-EGFP, indicating a higher rate of accumulation of viral progeny in the infected cells. Over time, the focuses increased in size, leading to cell monolayer lysis and a pronounced cytopathic effect. Thus, the rSAd25 vector has lytic potential; replication leads to significantly greater cell damage compared to rAd5 and rAd26.
An in vitro study of rSAd25-EGFP tropism
To determine the tropism of rSAd25, the transduction efficiency of different cell types (human, hamster, monkey, mouse origin) was examined in comparison with the commonly used vectors rAd5 and rAd26. The investigated replication-defective vectors contained the EGFP gene under the control of the cytomegalovirus promoter, which provides similar levels of expression after transduction. All cell lines were transfected with rSAd25-EGFP, rAd5-EGFP, and rAd26-EGFP at a dose of 1 TCID50/cell. The indicated dose of virus, on the one hand, has no cytotoxicity and, on the other hand, allows to expect that each cell will contain approximately only 1 v.p. Transduction with a higher dose leads to infection of one cell with several viral particles and, consequently, transgene expression is not linear. Transduction efficiency was assessed by the level of EGFP fluorescence in cells and expressed as relative units. The total value was determined by subtracting the fluorescence level of intact cells from the fluorescence level of transduced cells (Fig. 2).
Fig. 2. Tropism of rAd5-EGFP, rAd26-EGFP and rSAd25-EGFP to different mammalian cell cultures.
* — statistically significant difference with rSAd25-EGFP; & — statistically significant difference with rAd26-EGFP; # — statistically significant difference with rAd5-EGFP.
EGFP expression was detected in all studied cell lines. The transduction of Vero E6 monkey cells differed when different adenoviral vectors were used. The highest level of EGFP expression in Vero E6 cells was observed for rSAd25-EGFP.
The investigated adenoviruses penetrated into hamster (BHK-21, CHO) and mouse (L929, EPNT-6) cells with comparable efficiency.
The tropism of rSAd25-EGFP, rAd5-EGFP and rAd26-EGFP differed in human cells. SAd25-EGFP penetrated human glioblastoma GL-6 cells with higher efficiency. The fluorescence level of rSAd25-EGFP in human lung carcinoma H292 and H460 cells was significantly higher than that for rAd5-EGFP. Meanwhile, in another lung carcinoma cell line (H1299), rAd26-EGFP showed an advantage.
In vivo biodistribution assessment of recombinant adenoviruses
The biodistribution features of rAd5-EGFP, rAd26-EGFP and rSAd25-EGFP were studied in experiments on BALB/c mice using two methods: viral genome detection and transgene expression. 24 h after intramuscular injection of rAd at a dose of 1010 v.p., the number of Ad genome copies in tissues and organs was analyzed by real-time polymerase chain reaction (Fig. 3).
Fig. 3. Comparative analysis of adenovirus biodistribution in mice.
Each column represents the average number of genomes with a standard deviation.
Among the 14 selected organs and tissues, viral DNA was mainly detected in muscle samples from the injection site. Moreover, the copy number of rSAd25-EGFP DNA in muscle was significantly higher than that of rAd26-EGFP and rAd5-EGFP DNA, respectively. In addition to the injection site, small amounts of rAd5 genomes were detected in the lower lymph nodes as well as in the blood (rSAd25 and rAd5).
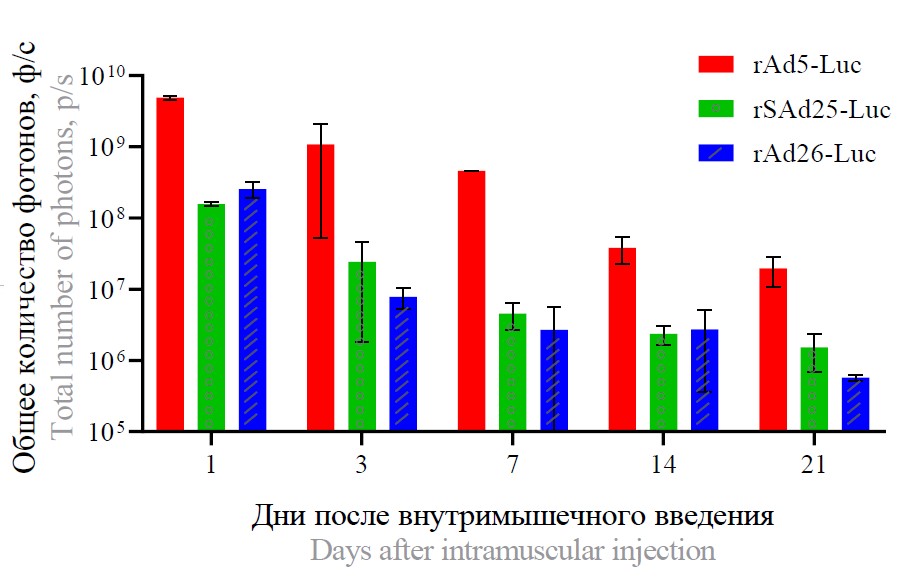
For bioluminescent imaging, we used rAds expressing the luciferase gene, which were injected into animals intramuscularly at a dose of 1011 v.p. For all Ad types, the bioluminescent signal was detected only at the injection site (Fig. 4). After a single injection of rAd5-Luc, rAd26-Luc, or rSAd25-Luc, the highest luciferase expression was detected 1 day after injection (Fig. 4, a). The highest luciferase activity was observed in mice injected with rAd5-Luc, whereas the lowest activity was observed in the group of mice with rAd26-Luc. Luciferase activity gradually decreased by day 21 for all adenoviruses (Fig. 4, b). However, the smallest drop in transgene expression level (approximately 100-fold) was observed in mice injected with rSAd25-Luc. For rAd5-Luc and rAd26-Luc, luciferase activity decreased more than 250 and 400 times, respectively (Fig. 4, c).
Fig. 4. Biodistribution of recombinant adenoviruses rAd5-Luc, rAd26-Luc, rSAd25-Luc in mice after intramuscular administration by bioluminescence imaging with averaged (a) and enhanced luminescence scale (b). c — photon flux measured in the luminescence region.
Acute toxicity study of rSAd25
The acute toxicity of rSAd25 expressing the target gene of protein S of SARS-CoV-2 virus (rSAd25-S-CoV2) was studied in mice and rats of both sexes a single intramuscular or intravenous injection. The use of rAd with the target antigen allows the most adequate assessment of toxicity of both the vector itself and the insertion.
The tested animals (mice and rats) tolerated well both intramuscular and intravenous administration of adenovirus preparation in all doses. During the observation period, no animal died and no clinical signs of intoxication were observed. Integral indices of animal condition did not differ between experimental and control groups. Positive dynamics of body weight change was observed in all groups (Fig. 5).
Fig. 5. Study of acute toxicity of rSAd25-S-CoV2 in mice and rats.
i.v. — intravenous; i.m. — intramuscular.
Macroscopic examination of mice revealed no effect of rSAd25 on the state of internal organs; no differences were found between control and experimental groups. Macroscopic examination of male rats revealed differences between experimental and control groups. In 4 out of 10 male rats injected with 1011 v.p. and in 2 out of 10 male rats injected with 1010 v.p., areas of decreased airiness of lung tissues (atelectasis) and foci of coagulation necrosis with signs of organization were observed. It is important to note that no pathomorphologic changes of lungs were detected in the studied females. The results of macroscopic examination of other internal organs of rats of experimental groups did not differ from those of the control group.
Discussion
Ad-based vectors are excellent tools for the delivery of foreign genetic information into mammalian cells due to their large packaging capacity and high functional titers. Ad vectors are widely used for the development of vector vaccines and gene therapy drugs. However, their use may be limited when target cells lack receptors involved in attachment and internalization. In this case, the use of vectors based on alternative types of Ads will be significant only if the reproductive potential of the selected vector is high.
The standard strategy for producing recombinant replication-defective Ad vectors is to delete the E1 region of the genome. One important aspect of successful complementation of E1-deleted Ad is the functional interaction of the E1B 55K protein (produced by a trans-complementary cell line) with the E4 34K protein in the virus genome. At the same time, the development of complementary cell lines for different types of replication-defective vectors is a labor-intensive process. Therefore, the availability of non-human Ad types capable of replicating in cells such as HEK 293 (human cells transformed with E1 region of Ad5 genome) is a great advantage. Although the sequence similarity between the E1B 55K proteins of Ad5 and SAd25 is about 56%, rSAd25-EGFP was produced and accumulated in high titers in HEK 293 cell culture without additional viral genome modifications. The results obtained differ significantly from the data of other studies, which show that the replacement of open reading frame 6 in the E4 region is necessary for successful replication of the recombinant vector [15].
The nature of Ad receptor expression on the surface of cells (both primary and secondary) determines the tropism of the virus, which determines the range of vector applications. SAd25 uses CAR (coxsackie and adenovirus receptor) as a primary cellular receptor, like most other Ad types [16, 17]. The amino acids involved in the interaction with CAR are located in the loop of AB knob-domain of the fiber. The key amino acids in Ad5 are Ser408, Pro409, Lys417 and the corresponding amino acids in other types: Ser196, Pro197, Lys205 in Ad26 and Ser255, Pro256, Lys267 in SAd25 [17]. The Ad5 and SAd25 fiber sequences are 63.9% similar, and it is not surprising that both recognize the same receptor. Like Ad5 and Ad26, SAd25 uses αv-integrins as a secondary receptor for internalization due to the presence of an RGD (Arg-Glu-Asp) sequence at the base of the penton [18].
To better understand the tropism of SAd25, we analyzed the transduction efficiency of SAd25 versus Ad5 and Ad26 using replication-defective vectors expressing EGFP (SAd25-EGFP, Ad5-EGFP, Ad26-EGFP). Experimental results showed that SAd25 efficiently transduced all cell lines tested. Its broad transduction profile was attributed to its interaction with CAR and αv-integrins. However, interaction with these receptors is not the only factor determining the cellular tropism of the virus. The observed differences in Ad tropism of subgroups C, D, and E can be significantly influenced by the length of the fiber, as it is the main factor that determines the strategy of Ad attachment to the cell. Therefore, an interesting aspect of our analysis was the increased tropism of SAd25 to human neuroblastoma cells. Further studies are needed to specifically define the mechanism of virus entry into these cells.
In the present study, we showed the biodistribution of SAd25 by viral DNA detection in organs or tissues and transgene expression. It should be noted that these two indicators do not necessarily coincide with each other for several reasons: the same promoter may have variable activity in different cell types; different virus types may have different fates after entering the same cell type; degraded virus in the lysosome can no longer express the transgene, but viral DNA is still detectable.
Considering that the intramuscular route is the most commonly used vaccination strategy, a comparison of vectors based on different Ad types after a single injection was performed. Local expression of the luciferase gene continued for 3 weeks and gradually decreased. The expression level after rSAd25-Luc injection was lower than after rAd5-Luc but higher than after rAd26-Luc injection. Thus, the data showed that SAd25 could be an excellent vector for vaccine development, along with Ad5 and Ad26.
The results of acute toxicity determination demonstrated that a single intramuscular or intravenous administration of SAd25 to mice at doses exceeding the equitherapeutic dose by 100 and 1000 times was well tolerated by the animals. Death and clinical manifestations of toxic reactions were not observed. A single intramuscular administration of SAd25 to rats in the range of the studied doses did not result in significant toxic effects. Certain male rats showed pathomorphologic changes in the lungs, which were not accompanied by clinical manifestations. No other toxic reactions were detected. It is not clear whether these changes were related to Ad-vector administration or caused by other factors, and whether the changes were temporary or permanent. These factors require further investigation, including a full cycle of preclinical studies. In summary, the data obtained are consistent with the results obtained for other vectors based on different Ad vectors [19–21].
Conclusion
The new SAd25-based platform is not inferior to the existing and well-established Ad5 and Ad26-based platforms. Given the current challenges, such as the emergence of new viral infections, such as the COVID-19 pandemic, and the return of known pathogens to the population, the use of simian Ad can significantly accelerate the process of developing and introducing new vaccines. Ultimately, this will contribute to the improvement of public health, both in Russia and abroad.
About the authors
Irina V. Vavilova
Gamaleya National Research Center for Epidemiology and Microbiology
Author for correspondence.
Email: vavilovairinav@yandex.ru
ORCID iD: 0009-0008-6272-0368
junior researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowTatiana A. Ozharovskaia
Gamaleya National Research Center for Epidemiology and Microbiology
Email: vavilovairinav@yandex.ru
ORCID iD: 0000-0001-7147-1553
Cand. Sci. (Biol.), senior researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowOlga V. Zubkova
Gamaleya National Research Center for Epidemiology and Microbiology
Email: vavilovairinav@yandex.ru
ORCID iD: 0000-0001-7893-8419
Cand. Sci. (Biol.), leading researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowOlga Popova
Gamaleya National Research Center for Epidemiology and Microbiology
Email: vavilovairinav@yandex.ru
ORCID iD: 0000-0003-3248-1227
junior researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowDaria V. Voronina
Gamaleya National Research Center for Epidemiology and Microbiology
Email: vavilovairinav@yandex.ru
ORCID iD: 0000-0001-6629-744X
junior researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowPolina P. Goldovskaya
Gamaleya National Research Center for Epidemiology and Microbiology
Email: vavilovairinav@yandex.ru
ORCID iD: 0009-0000-1965-0482
laboratory assistant researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowDenis I. Zrelkin
Gamaleya National Research Center for Epidemiology and Microbiology
Email: vavilovairinav@yandex.ru
ORCID iD: 0000-0003-0899-8357
junior researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowAlina Sh. Dzharullaeva
Gamaleya National Research Center for Epidemiology and Microbiology
Email: vavilovairinav@yandex.ru
ORCID iD: 0000-0003-1743-0798
Cand. Sci. (Biol.), researcher, Laboratory of cellular microbiology
Russian Federation, MoscowInna V. Dolzhikova
Gamaleya National Research Center for Epidemiology and Microbiology
Email: vavilovairinav@yandex.ru
ORCID iD: 0000-0003-2548-6142
Cand. Sci. (Biol.), Head, Laboratory of the State Virus Collection
Russian Federation, MoscowDmitry V. Shcheblyakov
Gamaleya National Research Center for Epidemiology and Microbiology
Email: vavilovairinav@yandex.ru
ORCID iD: 0000-0002-1289-3411
Cand. Sci. (Biol.), Head, Laboratory of immunobiotechnology
Russian Federation, MoscowDenis Yu. Logunov
Gamaleya National Research Center for Epidemiology and Microbiology
Email: vavilovairinav@yandex.ru
ORCID iD: 0000-0003-4035-6581
D. Sci. (Biol.), RAS Full Member, Head, Laboratory of cellular microbiology
Russian Federation, MoscowAlexander L. Gintsburg
Gamaleya National Research Center for Epidemiology and Microbiology
Email: vavilovairinav@yandex.ru
ORCID iD: 0000-0003-1769-5059
D. Sci. (Biol.), Professor, RAS Full Member, Director, National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya
Russian Federation, MoscowReferences
- Benko M., Aoki K., Arnberg N., et al. ICTV virus taxonomy profile: adenoviridae 2022. J. Gen. Virol. 2022;103:001721. DOI: https://doi.org/10.1099/jgv.0.001721
- Crystal R.G. Adenovirus: The first effective in vivo gene delivery vector. Hum. Gene Ther. 2014;25:3–11. DOI: https://doi.org/10.1089/hum.2013.2527
- Fougeroux C., Holst P.J. Future prospects for the development of cost-effective adenovirus vaccines. Int. J. Mol. Sci. 2017;18:686. DOI: https://doi.org/10.3390/ijms18040686
- Majhen D. Human adenovirus type 26 basic biology and its usage as vaccine vector. Rev. Med. Virol. 2022;32:e2338. DOI: https://doi.org/10.1002/rmv.2338
- Patel R., Kaki M., Potluri V.S., et al. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccin. Immunother. 2022;18:2002083. DOI: https://doi.org/10.1080/21645515.2021.2002083
- Ledgerwood J.E., Sullivan N.J., Graham B.S. Chimpanzee adenovirus vector Ebola vaccine – preliminary report. N. Engl. J. Med. 2015;373:776. DOI: https://doi.org/10.1056/NEJMc1505499
- Logunov D.Y., Dolzhikova I.V., Zubkova O.V., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–97. DOI: https://doi.org/10.1016/S0140-6736(20)31866-3
- Dolzhikova I.V., Zubkova O.V., Tukhvatulin A.I., et al. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: An open phase I/II trial in healthy adults in Russia. Hum. Vaccin. Immunother. 2017;13(3):613–20. DOI: https://doi.org/10.1080/21645515.2016.1238535
- Zhu F.C., Guan X.H., Li Y.H., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–88. DOI: https://doi.org/10.1016/S0140-6736(20)31605-6
- Trivedi P.D., Byrne B.J., Corti M. Evolving horizons: adenovirus vectors’ timeless influence on cancer, gene therapy and vaccines. Viruses. 2023;15(12):2378. DOI: https://doi.org/10.3390/v15122378
- Marquez-Martinez S., Vijayan A., Khan S., Zahn R. Cell entry and innate sensing shape adaptive immune responses to adenovirus-based vaccines. Curr. Opin. Immunol. 2023;80:102282. DOI: https://doi.org/10.1016/j.coi.2023.102282
- Ожаровская Т.А., Попова О., Зубкова О.В. и др. Разработка и характеристика векторной системы на основе аденовируса обезьян 25-го серотипа. Вестник РГМУ. 2023;(1):4–11. Ozharovskaia T.A., Popova O., Zubkova O.V., et al. Development and characterization of a vector system based on the simian adenovirus type 25. Bulletin of RSMU. 2023;(1):4–11. DOI: https://doi.org/10.24075/brsmu.2023.006
- Logunov D.Y., Zubkova O.V., Karyagina-Zhulina A.S., et al. Identification of HI-like loop in CELO adenovirus fiber for incorporation of receptor binding motifs. J. Virol. 2007;81(18):9641–52. DOI: https://doi.org/10.1128/JVI.00534-07
- Ramakrishnan M.A. Determination of 50% endpoint titer using a simple formula. World J. Virol. 2016;5(2):85–6. DOI: https://doi.org/10.5501/wjv.v5.i2.85
- Lan W., Quan L., Li Y., et al. Isolation of novel simian adenoviruses from macaques for development of a vector for human gene therapy and vaccines. J. Virol. 2023;97(10):e0101423. DOI: https://doi.org/10.1128/jvi.01014-23
- Roelvink P.W., Lizonova A., Lee J.G., et al. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 1998;72(10):7909–15. DOI: https://doi.org/10.1128/JVI.72.10.7909-7915.1998
- Law L.K., Davidson B.L. What does it take to bind CAR? Mol. Ther. 2005;12(4):599–609. DOI: https://doi.org/10.1016/j.ymthe.2005.05.017
- Zhang Y., Bergelson J.M. Adenovirus receptors. Journal of Virology. 2005;79(19):12125–31. DOI: https://doi.org/10.1128/jvi.79.19.12125-12131.2005
- Tandon M., Sharma A., Vemula S.V., et al. Sequential administration of bovine and human adenovirus vectors to overcome vector immunity in an immunocompetent mouse model of breast cancer. Virus Res. 2012;163(1):202–11. DOI: https://doi.org/10.1016/j.virusres.2011.09.031
- Lichtenstein D.L., Spencer J.F., Doronin K., et al. An acute toxicology study with INGN 007, an oncolytic adenovirus vector, in mice and permissive Syrian hamsters; comparisons with wild-type Ad5 and a replication-defective adenovirus vector. Cancer Gene. Ther. 2009;16(8):644–54. DOI: https://doi.org/10.1038/cgt.2009.5
- Hassan P.M., Ali T., Saber E., et al. Potency, toxicity and protection evaluation of PastoCoAd candidate vaccines: Novel preclinical mix and match rAd5 S, rAd5 RBD-N and SOBERANA dimeric-RBD protein. Vaccine. 2022;40(20):2856–68. DOI: https://doi.org/10.1016/j.vaccine.2022.03.066
Supplementary files