Molecular markers of acute intestinal infections in HIV-infected patients in the Chechen Republic
- Authors: Murtazaliev K.K.1, Makarova M.A.2,3, Kaftyreva L.A.2,3, Alieva E.V.4, Oshaeva A.V.1, Zhamborova M.K.2
-
Affiliations:
- Republican Clinical Center for Infectious Diseases
- Saint-Peterburg Pasteur Institute
- North-Western State Medical University named after I.I. Mechnikov
- Stavropol State Medical University
- Issue: Vol 100, No 6 (2023)
- Pages: 420-427
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18514
- DOI: https://doi.org/10.36233/0372-9311-437
- EDN: https://elibrary.ru/uwnpgo
- ID: 18514
Cite item
Abstract
Introduction. Diarrheal syndrome is the most characteristic symptom of HIV infection, which occurs in 70% of patients and is often fatal. The severity of diarrheal syndrome, irrespective of immune status, is usually determined by specific microorganisms colonising the gastrointestinal tract.
The objective of this study is to assess the prevalence of classical pathogens of acute intestinal infections in diarrheal syndrome in HIV-infected residents of the Chechen Republic (Grozny).
Materials and methods. Stool samples (n = 191) of HIV-infected patients with a history of diarrheal syndrome were studied by real-time PCR with two kits of reagents: "AmpliSens OKI screen-FL" for the detection of DNA/RNA of Shigella spp./EIEC, Salmonella spp., Campylobacter spp., Adenovirus, Rotavirus, Norovirus and Astrovirus; "AmpliSens Escherichiosis-FL" for the detection of diarrheagenic E. coli (DEC) DNA of five pathogroups: EPEC, EHEC, ETEC, EIEC, EAgEC.
Results. Genetic markers of the acute intestinal infection pathogens were detected in 20.9% of the examined individuals. In patients aged 0–7 years and 18–24 years, DNA/RNA of the tested pathogens were not detected. DNA of bacterial pathogens accounted for 93.9%, RNA of viral pathogens — 6.1%. The etiological structure of bacterial infections was represented by a significant predominance of DEC (84.8%) compared to 10.9% of Campylobacter spp. and 4.4% of Salmonella spp. The structure of viral infections included 66.7% Rotavirus and 33.3% Norovirus. Genetic markers of Adenovirus and Astrovirus have not been identified. In 77.5% of HIV-infected patients, diarrheal syndrome was caused by one pathogen (mono-infection), but in nine examined patients (22.5%) it had a combined etiology.
Conclusion. The etiology of acute intestinal infections in HIV-infected patients of the Chechen Republic includes bacterial and viral pathogens, in every fifth the cause of diarrheal disease was DEC. Due to diarrhea in HIV-infected people being a polyetiological disease, it is necessary to introduce a comprehensive, fast, reliable, and affordable method for identifying a wide range of pathogens that cause secondary infections.
Full Text
Introduction
HIV infection is a problem of great importance all over the world, including Russia, due to its pandemic prevalence and increasing rates of morbidity [1, 2]. This nosology is one of the causes of death in young patients and is therefore considered a global threat to mankind and its socio-economic progress [3]. In Russia, due to the increasing number of HIV-infected people, there is an increase in the number of opportunistic diseases and fatal cases due to secondary diseases [4].
The gastrointestinal tract (GIT) is one of the target organs of HIV infection and is involved in the pathological process at different stages of the disease [5]. HIV causes leukocytic infiltration in the mucosa, degenerative changes in intestinal crypts and atrophy of microvilli, which leads to impaired intestinal digestion and absorption, decreased intestinal colonisation resistance, dysbiosis and an infectious process prone to persistence and recurrence. As disease symptoms and morphofunctional changes in the intestinal mucosa progress, HIV-infected individuals often experience diarrhea and intestinal infections, which are a key cause of morbidity and mortality at later stages, regardless of antiretroviral therapy [6]. Diarrheal syndrome (DS) is the most characteristic symptom of HIV infection, which occurs in 70% of patients and is often fatal. In developing countries, more than 90% of HIV-infected people have DS, while in industrialized countries it is present in 50-60% of the infected. The World Health Organization's baseline scenario for 2030 assumes that mortality from HIV/AIDS and diarrheal diseases in developing countries will remain at about 1.7/1.5 million. There are many causes of DS in HIV-infected people, the most common being direct exposure to the viruses, opportunistic pathogens, long-term drug use and/or GIT tumours [7].
Any pathogen that infects immunocompetent individuals is also capable of causing disease in HIV-infected individuals. The severity of DS, regardless of immune status, usually depends on the specific microorganisms colonising the GIT. However, Escherichia coli, Salmonella spp., Shigella spp., Campylobacter spp. and Rotavirus can cause particularly severe or prolonged diseases during HIV infection. According to several researchers, HIV-infected adults have 10 times higher rates of bacterial intestinal infections than the HIV-negative population. HIV-infected individuals are at a 20-100-fold increased risk of salmonellosis, associated with bacteraemia in more than 40% of cases, and the mortality rate is increased 7-fold. The average incidence of campylobacteriosis among AIDS patients is 40 times higher than in individuals not infected with HIV [8-10].
Existing data on the incidence of acute intestinal infections among immunocompromised patients are scarce, resulting in a lack of clear and definite knowledge about the etiology of HIV-infected people with DS. The aim of this study was to investigate the prevalence of classical pathogens of acute intestinal infections in HIV-infected residents of the Chechen Republic (Grozny).
Materials and methods
Fecal samples from 191 patients (124 women and 67 men) registered at the Republican AIDS Centre with a history of DS were examined at the Republican Clinical Centre for Infectious Diseases of the Ministry of Health of the Chechen Republic. The composition of the age groups of patients was as follows: 1–7 years — 1 (0.5%) patient, 18-24 years — 4 (2.1%), 25–34 years — 10 (5.2%), 35–44 years — 58 (30.4%), 45–54 years — 68 (35.6%), over 55 years — 50 (26.2%) The cohort group was formed by random sampling. All patients were informed about participation in the study and gave their voluntary consent. The study protocol was approved by the Ethics Committee of the Saint-Peterburg Pasteur Institute (protocol No.81, November 22, 2022).
The polymerase chain reaction (PCR) method with hybridisation-fluorescence detection was used, using the DT PRIME 5 thermocycler (DNA-Technology) and two reagent kits: AmpliSens OKI screen-FL (Central Research Institute of Epidemiology of Rospotrebnadzor) for detection and differentiation of DNA (RNA) of microorganisms of the Shigella spp./EIEC, Salmonella spp, Campylobacter spp. (thermophilic), Adenovirus (group F/grF), Rotavirus (group A/grA), Norovirus (2nd genotype/G II) and Astrovirus genera; AmpliSens Escherichiosis-FL (Central Research Institute of Epidemiology of Rospotrebnadzor) for detection of diarrheagenic E. coli (DEC) pathogroups EPEC, EHEC, ETEC, EIEC and EAgEC. Results were interpreted based on the fluorescence signal levels of the respective HEX, FAM or ROX channels. The identification of the strains belonging to the EPEC, ETEC, EIEC, EAgEC pathogroups was performed according to the manufacturer's instructions and methodological guidelines. Strains showing fluorescence levels above the threshold for HEX channel in PCR mix-1 were considered as shigatoxin-producing (STEC); strains showing fluorescence levels above the threshold for HEX channel in PCR mix-1 and PCR mix-2 were considered as enterohaemorrhagic (EHEC).
The obtained data were processed using Microsoft Office Excel. Fisher's exact test was used to assess the statistical significance of differences in mean values. Differences with a confidence interval of 95% (p < 0.05) were considered statistically significant.
Results
Molecular markers of the investigated pathogens of acute intestinal infections were detected in fecal samples of 40 (20.9%) of the examined patients, including 26 (21.0%) women and 14 (21.0%) men without significant statistical differences (p > 0.05) (Table 1). In patients aged 0-7 years and 18-24 years, DNA/RNA of the pathogens were not detected. No significant differences in the frequency of detection of molecular markers of acute intestinal infection pathogens in fecal samples from patients of other age groups were found (p > 0.05).
Table 1. Detection rates of molecular markers of acute intestinal infections in HIV-infected patients with diarrheal syndrome of different age in the Chechen Republic
Characteristic of examined persons | Number of samples | Positive for molecular markers | CI | |
n | % | |||
0–17 years old | 1 | 0 | 0 | 0–0,8 |
18–24 years old | 4 | 0 | 0 | 0–0,5 |
25–34 years old | 10 | 2 | 20,0 | 5,7–51,0 |
35–44 years old | 58 | 12 | 20,7 | 12,3–32,8 |
45–54 years old | 68 | 18 | 26,5 | 17,5–38,0 |
55 years and older | 50 | 8 | 16,0 | 8,3–28,5 |
Women | 124 | 26 | 21,0 | 14,73–28,95 |
Men | 67 | 14 | 21,0 | 12,9–32,1 |
Total | 191 | 40 | 20,9 | 15,8–27,3 |
The results of the molecular study are presented in Table 2. In 40 positive samples, 49 molecular markers of acute intestinal infection pathogens were detected, of which DNA of bacterial pathogens accounted for 93.9% (n = 46) and RNA of viral pathogens for 6.1% (n = 3). The differences were statistically significant, p < 0.05. The etiological structure of bacterial intestinal infections was represented by a significant predominance of diarrheagenic E. coli 84.8% (n = 39) of which: EPEC — 48.7% (n = 19), STEC — 20.5% (n = 8), EIEC — 17.9% (n = 7), EAgEC — 7.7% (n = 3), ETEC — 5.1% (n = 2) compared to 10.9% (n = 5) thermophilic Campylobacter spp. and 4.4% (n = 2) Salmonella spp. (p < 0.05). The viral pattern of acute intestinal infections included 66.7% (n = 2) Rotavirus (group A) and 33.3% (n = 1) genotype 2 Norovirus. Adenovirus grF and Astrovirus molecular markers were not detected.
Table 2. Detection rates of DNA/RNA of causative agents of acute intestinal infections in HIV-infected residents of the Chechen Republic
Pathogen | Total | Monoinfection | Combined infections | |||
n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | |
Bacterial | ||||||
Campylobacter spp. | 5 (10,9) | 4,7–23,0 | 2 (6,5) | 1,8–20,7 | 3 (20,0) | 7,1–45,2 |
Salmonella spp. | 2 (4,4) | 1,2–14,5 | 2 (6,5) | 1,8–20,8 | 0 (0) | 0–20,4 |
Diarrheagenic E. coli, including: | 39 (84,8) | 71,8–92,4 | 27 (87,1) | 71,2–94,9 | 12 (80,0) | 54,8–92,9 |
EAgEC | 3 (7,7) | 2,6–20,3 | 2 (7,4) | 2,1–23,4 | 1 (8,3) | 1,5–35,4 |
EIEC | 7 (17,9) | 9,0–32,7 | 4 (14,8) | 5,9–32,5 | 3 (25,0) | 8,9–53,2 |
ETEC | 2 (5,1) | 1,4–16,9 | 1 (3,7) | 0,7–18,3 | 1 (8,3) | 1,5–35,4 |
EPEC | 19 (48,7) | 33,9–63,8 | 14 (51,8) | 33,9–69,3 | 5 (41,7) | 19,3–68,1 |
STEC | 8 (20,5) | 10,8–35,3 | 6 (22,2) | 10,6–40,7 | 2 (16,7) | 4,7–44,8 |
Total bacterial | 46 (93,9) | 83,5–97,9 | 31 (67,4) | 52,9–79,1 | 15 (32,6) | 20,9–47,0 |
Viral | ||||||
Norovirus GII | 1 (33,3) | 6,2–79,2 | 0 (0) | 0–65,8 | 1 (100) | 20,7–100 |
Rotavirus grA | 2 (66,6) | 20,8–93,9 | 1 (50,0) | 9,5–90,6 | 1 (50,0) | 9,5–90,6 |
Total viral | 3 (6,1) | 2,1–16,5 | 1 (2,0) | 0,4–12,2 | 2 (4,1) | 1,1–13,7 |
Total | 49 (100) | 92,7–100 | 32 (65,30) | 51,3–77,1 | 17 (34,7) | 22,9–49,7 |
In 31 (77.5%) HIV-infected patients, DS was caused by a single pathogen of acute intestinal infections (monoinfection), and in 9 (22.5%) — by several. Monoinfection was significantly more often caused by bacterial pathogens: 67.4% compared to viral pathogens — 2.0 (p < 0.05). Analysis of co-infection cases showed a prevalence of bacterial (77.8%) compared to viral-bacterial coinfections (22.2%; Table 3). DEC genetic determinants were present in all cases of co-infections of acute intestinal infections.
Table 3. Characteristics of acute intestinal infections of combined etiology in HIV-infected patients in the Chechen Republic
Etiological agent | Number of cases | |||
n | % | 95% CI | p | |
Bacterial | ||||
Campylobacter spp. + EPEC | 1 | 11,1 | 2,0–43,5 | > 0,05 |
Campylobacter spp. + STEC | 1 | 11,1 | 2,0–43,5 | > 0,05 |
EPEC + EIEC | 1 | 11,1 | 2,0–43,5 | > 0,05 |
EPEC + EAgEC | 1 | 11,1 | 2,0–43,5 | > 0,05 |
EPEC + ETEC | 1 | 11,1 | 2,0–43,5 | > 0,05 |
STEC + EIEC | 1 | 11,1 | 1,99–43,5 | > 0,05 |
Total bacterial-bacterial | 7 | 77,8 | 45,3–93,7 | < 0,05 |
Viral-bacterial | ||||
Rotavirus grA + EIEC | 1 | 11,1 | 2,0–43,5 | > 0,05 |
Norovirus GII + Campylobacter spp. + EPEC | 1 | 11,1 | 2,0–43,5 | > 0,05 |
Total viral-bacterial | 2 | 22,2 | 6,3–41,7 | > 0,05 |
Total combined infections | 9 | 100,0 | 70,1–100 | |
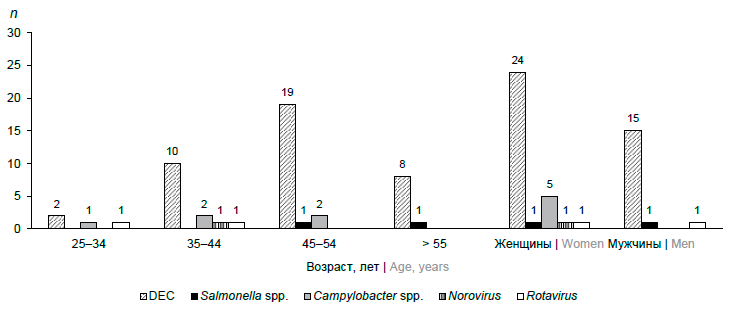
When analysing the distribution of the examined individuals by sex and age, significant differences were revealed (Figure). The main pathogens of acute intestinal infections in HIV-infected men and women were DEC. Thermophilic Campylobacter spp. were identified only in women; no significant differences were found with regard to other studied acute intestinal infection pathogens of bacterial and viral etiology Bacterial pathogens were identified in all age groups, viral pathogens — only in the groups of 25–34 and 35–44 year olds. In the age group of 25–34 years, diarrheal syndrome was caused by bacterial and viral etiology without dominance of a particular pathogen. In the age groups 35–44, 45–54 years and 55 years and older, DEC was a significantly more frequent pathogen of acute intestinal infection (p < 0.05), other bacterial and viral infections were detected without significant differences (p > 0.05).
Distribution by sex and age of HIV-infected patients with diarrheal syndrome in the Chechen Republic.
Discussion
Infectious diarrheal diseases are an urgent problem of HIV-infected people in the Chechen Republic. The overall rate of intestinal infections was 20.94 %. There were no statistical differences by sex among the HIV-infected population of the Republic. Patients aged 0–7 and 18–24 years had statistically significantly lower presence of molecular markers of classical acute intestinal infection pathogens, which may be associated with timely prescription and higher adherence to treatment with of antiretroviral and prophylactic antimicrobial drugs in these patients, which contributes to the inhibition of potential intestinal pathogens [11, 12].
The main causative agents of diarrheal diseases, not only bacterial, but also in the etiological structure of acute intestinal infections, were DEC (84.8%), most of which were represented by anthroponotic pathogens (74.4%): EPEC (48.7%), EIEC (17.9%) and EAgEC (7.7%). The results confirm that DEC are the most common microorganisms associated with diarrhea in HIV infection. The most frequent pathogroup was EPEC, which are common in HIV with diarrheal syndrome [13, 14]. Molecular markers of pathogens commonly transmitted with food: Salmonella, Campylobacter and STEC were detected in 4.4%, 10.9% and 25.5% of cases respectively [15]. Studies conducted in Austria, the Netherlands and Germany have also shown that these bacterial pathogens account for the smallest number of cases of acute gastroenteritis in HIV-infected people [16].
Rotavirus group A was detected only in 2 adult patients, although according to V.P. Bondarev et al. this pathogen is the most frecuent cause of severe gastroenteritis in children in many countries, accounting for 30–72% of hospitalised patients and 4–24% of patients with mild acute gastroenteritis not requiring hospitalisation [17]. According to the data of Form 2 "Information on infectious and parasitic diseases" for 2019-2022 and the State Report "On the state of sanitary and epidemiological well-being of the population in the Russian Federation in 2022", cases of rotavirus infection were not registered in the Republic of Ingushetia and the Chechen Republic. The absence of reported incidence may be associated with insufficient implementation of molecular biological methods in the laboratory diagnosis of acute intestinal infections. At the same time, the absence of Rotavirus in this study may be due to the absence of infants and young children in the study group, as well as the fact that the studies were conducted in the spring and summer period. Adenovirus and Astrovirus were not detected in our study, although according to L. Seid et al. they are considered potential opportunistic pathogens [18].
In 31 (77.5%) fecal samples, molecular marcers of a single acute intestinal infection pathogen were detected, and according to the results of laboratory testing, DS was identified as a monoinfection. Samples from 9 (22.5%) examined patients contained a mixture of enteropathogens (combined acute intestinal infections): in 8 (20.0%) samples markers of two acute intestinal infection pathogens were detected, in one sample (2.5%) three patogens were identified. A high prevalence of co-infections (25–53%) has been described among HIV-infected people in developing countries [14, 19]. DEC predominated in all co-infections, which is consistent with previous data [20].
In the era of effective antiretroviral therapy, the clinical picture of DEC in people living with HIV may not be comparable to their classic symptoms. The use of a comprehensive multiplex format in the laboratory diagnosis of DS in HIV-infected people is the only highly sensitive method that allows the establishment of the etiology of acute intestinal infections not only in the acute period of the disease, but also in asymptomatic carriers. A large-scale study conducted in Ethiopia using modern laboratory diagnostic methods showed that 56.3% of DS cases in HIV-infected people was caused by pathogens of bacterial, viral and/or parasitic etiology, and notably, it allowed the identification of co-infections in 35% of cases [14, 18, 21].
Conclusion
The etiology of acute intestinal infection pathogens in HIV-infected patients in the Chechen Republic includes bacterial and viral pathogens. The study showed that almost every fifth HIV-infected person was caused by DEC, which confirmed their relevance in the structure of acute intestinal infections. Laboratory diagnosis of these pathogens is possible only using molecular genetic methods. To reduce the burden of diarrheal diseases in HIV-infected people in the Chechen Republic, it is necessary to conduct targeted epidemiological and microbiological studies to identify DEC, to study the contamination of environmental objects, including water and food, and to identify risk factors. Given that DS in HIV-infected people is a polyetiological disease, it is necessary to introduce a comprehensive, rapid, reliable and available method of identifying a wide range of pathogens causing secondary diseases.
It is important to note that this is the first study conducted in the Chechen Republic, and the results obtained can be used to develop protocols for the prevention and treatment of DS in HIV-infected people at the regional and republican levels.
About the authors
Khamzat K. Murtazaliev
Republican Clinical Center for Infectious Diseases
Email: makmaria@mail.ru
ORCID iD: 0009-0003-4524-5730
Chief freelance specialist, Ministry of Health of the Chechen Republic on the problems of diagnosing and treating HIV infection; chief physician, Republican Clinical Center for Infectious Diseases
Russian Federation, GroznyMaria A. Makarova
Saint-Peterburg Pasteur Institute; North-Western State Medical University named after I.I. Mechnikov
Author for correspondence.
Email: makmaria@mail.ru
ORCID iD: 0000-0003-3600-2377
D. Sci. (Med), senior researcher, Laboratory of enteric infection, Saint-Peterburg Pasteur Institute; assistant professor, Department of medical microbiology, North-Western State Medical University named after I.I. Mechnikov
Russian Federation, St. Petersburg; St. PetersburgLidiya A. Kaftyreva
Saint-Peterburg Pasteur Institute; North-Western State Medical University named after I.I. Mechnikov
Email: makmaria@mail.ru
ORCID iD: 0000-0003-0989-1404
D. Sci. (Med.), Head, Laboratory of enteric infection, Saint-Peterburg Pasteur Institute; assistant professor, Department of medical microbiology, North-Western State Medical University named after I.I. Mechnikov
Russian Federation, St. Petersburg; St. PetersburgElena V. Alieva
Stavropol State Medical University
Email: makmaria@mail.ru
ORCID iD: 0009-0007-1349-9882
D. Sci. (Med.), Associate Professor, Professor, Department of clinical laboratory diagnostics with a course of bacteriology, Stavropol State Medical University
Russian Federation, StavropolAza V. Oshaeva
Republican Clinical Center for Infectious Diseases
Email: makmaria@mail.ru
ORCID iD: 0009-0002-0771-755X
chief freelance specialist in medical microbiology, Ministry of Health of the Chechen Republic; Head, Bacteriological laboratory, Republican Clinical Center for Infectious Diseases
Russian Federation, GroznyMarina K. Zhamborova
Saint-Peterburg Pasteur Institute
Email: makmaria@mail.ru
ORCID iD: 0009-0009-9620-9784
laboratory researcher, Laboratory of enteric infection, Saint-Peterburg Pasteur Institute
Russian Federation, St. PetersburgReferences
- Радзиховская М.В., Москвичева М.Г., Брылина Н.Ю. Глобальные тенденции в развитии распространения ВИЧ-инфекции. Вестник совета молодых ученых и специалистов Челябинской области. 2018;2(2):3–12. Radzikhovskaya M.V., Moskvicheva M.G., Brylina N.Y. Global trends in the development of HIV infection. Bulletin of the Council of Young Scientists and Specialists of the Chelyabinsk Region. 2018;2(2):3–12. EDN: https://elibrary.ru/xoxqdb
- Афтаева Л.Н., Мельников В.Л., Вотолкина С.В. и др. Особенности ВИЧ-инфекции у взрослых. Сибирский научный медицинский журнал. 2020;40(4):86–90. Aftaeva L.N., Mel'nikov V.L., Votolkina S.V., et al. Features of HIV infection in adults. Siberian Scientific Medical Journal. 2020;40(4):86–90. DOI: https://doi.org/10.15372/SSMJ20200412 EDN: https://elibrary.ru/cbyvae
- Хаирова Я.Р., Скороделова М.И., Никольская М.В. Оппортунистические инфекции при ВИЧ-инфекции. Вестник Пензенского государственного университета. 2021;(2):69–72. Khairova Ya.R., Skorodelova M.I., Nikolskaya M.V. Opportunistic infections in HIV infection. Vestnik of Penza State University. 2021;(2):69–72. EDN: https://elibrary.ru/dlpqve
- Покровская А.В., Самотолкина Е.А., Матосова С.В. и др. Клинико-лабораторные особенности и прогностические факторы исхода прогрессирующей многоочаговой лейкоэнцефалопатии у ВИЧ-инфицированных пациентов. ВИЧ-инфекция и иммуносупрессии. 2023;15(1):50–9. Pokrovskaya A.V., Samotolkina E.A., Matosova S.V., et al. Clinical and laboratory features and prognostic factors for outcome of progressive multifocal leukoencephalopathy in HIV-infected patients. HIV Infection and Immunosuppressive Disorders. 2023;15(1):50–9. DOI: https://doi.org/10.22328/2077-9828-2023-15-1-50-59 EDN: https://elibrary.ru/xcrnme
- Al Anazi A.R. Gastrointestinal opportunistic infections in human immunodeficiency virus disease. Saudi J. Gastroenterol. 2009;15(2):95–9. DOI: https://doi.org/10.4103/1319-3767.48965
- Хасанова Г.М., Урунова Д.М., Ахмеджанова З.И. и др. Поражение желудочно-кишечного тракта при ВИЧ-инфекции. Тихоокеанский медицинский журнал. 2019;(3):24–8. Khasanova G.M., Urunova D.M., Akhmedzhanova Z.I., et al. Defeat of the gastrointestinal tract in HIV infection. Pacific Medical Journal. 2019;(3):24–8. DOI: https://doi.org/10.17238/PmJ1609-1175.2019.3.24-28 EDN: https://elibrary.ru/agdtdy
- Hall V.P. Common gastrointestinal complications associated with human immunodeficiency virus/AIDS: An overview. Crit. Care Nurs. Clin. North Am. 2018;30(1):101–7. DOI: https://doi.org/10.1016/j.cnc.2017.10.009
- Тимченко В.Н., Архипова Ю.А. Поражение желудочно-кишечного тракта при ВИЧ-инфекции у детей. Детские инфекции. 2010;9(3):22–6. Timchenko V.N., Arkhipova Yu.A. Gastrointestinal manifestations of pediatric HIV-infection. Children Infections. 2010;9(3):22–6. EDN: https://elibrary.ru/musaep
- Shah S., Kongre V., Kumar V., Bharadwaj R. A study of parasitic and bacterial pathogens associated with diarrhea in HIV-positive patients. Cureus. 2016;8(9):e807. DOI: https://doi.org/10.7759/cureus.807
- Belay A., Ashagrie M., Seyoum B., et al. Prevalence of enteric pathogens, intestinal parasites and resistance profile of bacterial isolates among HIV infected and non-infected diarrheic patients in Dessie Town, Northeast Ethiopia. PloS One. 2020;15(12):e0243479. DOI: https://doi.org/10.1371/journal.pone.0243479
- Carlucci J.G, Liu Y., Clouse K., Vermund S.H. Attrition of HIV-positive children from HIV services in low and middle-income countries. AIDS. 2019;33(15):2375–86. DOI: https://doi.org/10.1097/QAD.0000000000002366
- Adetokunboh O.O., Uthman O.A., Wiysonge C.S. Non-specific effects of childhood vaccines on acute childhood morbidity among HIV-exposed children in sub-Saharan Africa: a multilevel analysis. Hum. Vaccin. Immunother. 2018;14(10):2382–90. DOI: https://doi.org/10.1080/21645515.2018.1516490
- Basile F.W., Fedele M.C., Lo Vecchio A. Gastrointestinal diseases in children living with HIV. Microorganisms. 2021;9(8):1572. https://doi.org/10.3390/microorganisms9081572
- Montalvo-Otivo R., Vilcapoma P., Murillo A., et al. Evaluation of chronic diarrhea in patients newly diagnosed with HIV infection through the FilmArray® gastrointestinal panel. Rev. Gastroenterol. Mex. (Engl. Ed.). 2023. DOI: https://doi.org/10.1016/j.rgmxen.2023.02.002
- Feasey N.A., Healey P., Gordon M.A. Review article: the aetiology, investigation and management of diarrhoea in the HIV-positive patient. Aliment. Pharmacol. Ther. 2011;34(6):587–603. DOI: https://doi.org/10.1111/j.1365-2036.2011.04781.x
- Newman K.L., Newman G.S., Cybulski R.J., Fang F.C. Gastroenteritis in men who have sex with men in Seattle, Washington, 2017-2018. Clin. Infect. Dis. 2020;71(1):109–15. DOI: https://doi.org/10.1093/cid/ciz783
- Бондарев В.П., Шевцов В.А., Индикова И.Н. и др. Эпидемиология ротавирусной инфекции и тактика вакцинопрофилактики. БИОпрепараты. Профилактика, диагностика, лечение. 2019;19(2):81–7. Bondarev V.P., Shevtsov V.A., Indikova I.N., et al. Rotavirus Epidemiology and Vaccination Tactics. Biological Products. Prevention, Diagnosis, Treatment. 2019;19(2):81–7. DOI: https://doi.org/10.30895/2221-996X-2019-19-2-81-87 EDN: https://elibrary.ru/wgiruv
- Seid L., Stokes W., Bayih A.G., et al. Molecular detection of Enteropathogens from diarrheic stool of HIV positive patients in Gondar, Ethiopia. BMC Infect. Dis. 2018;18(1):354. DOI: https://doi.org/10.1186/s12879-018-3265-8
- Bejide O.S., Odebode M.A., Ogunbosi B.O., et al. Diarrhoeal pathogens in the stools of children living with HIV in Ibadan, Nigeria. Front. Cell. Infect. Microbiol. 2023;13:1108923. DOI: https://doi.org/10.3389/fcimb.2023.1108923
- Masiga F., Kigozi E., Najjuka C.F., et al. Diarrhoeagenic Escherichia coli isolated from children with acute diarrhoea at Rakai hospital, Southern Uganda. Afr. Health Sci. 2022;22(1):581–8. DOI: https://doi.org/10.4314/ahs.v22i1.67
- Ayele A.A., Tadesse D., Manilal A., et al. Prevalence of enteric bacteria and enteroparasites in human immunodeficiency virus-infected individuals with diarrhoea attending antiretroviral treatment clinic, Arba Minch General Hospital, southern Ethiopia. New Microbes New Infect. 2020;38:100789. DOI: https://doi.org/10.1016/j.nmni.2020.100789
Supplementary files