Accumulated experience and future prospects of in vivo hepatitis B virus research
- Authors: Nagornykh A.M.1, Tyumentseva M.A.1, Tyumentsev A.I.1, Akimkin V.G.1
-
Affiliations:
- Central Research Institute for Epidemiology
- Issue: Vol 100, No 6 (2023)
- Pages: 495-510
- Section: REVIEWS
- URL: https://microbiol.crie.ru/jour/article/view/18489
- DOI: https://doi.org/10.36233/0372-9311-446
- EDN: https://elibrary.ru/zxggyl
- ID: 18489
Cite item
Abstract
Nowadays, an estimated more than 300 million people live with hepatitis B virus (HBV) infection globally. One of the main goals of the World Health Organization (WHO) is to eliminate viral hepatitis by the year 2030. The study of the pathogenic and immunologic properties of HBV, as well as therapeutic substances and treatment regimens, is significantly complicated by the insufficient number of susceptible biological test subjects (animal models) and the lack of zoonotic reservoirs of the virus. In this regard, researching the properties of HBV and related hepadnaviruses provides invaluable material for understanding the biology of the pathogen and the developing methods of prevention and control of this chronic infectious disease, leading to severe hepatopathies (cirrhosis and hepatocellular carcinoma).
Furthermore, prolonged HBV viremia leads to depletion of the immune system, reducing resistance against pathogens of other infections, especially those with a chronic course and socially determined spread.
The aim of this research is to evaluate existing animal models of HBV infection in the context of pathogenesis, immunologic and pathomorphological features. For the first time, the hypothesis of the possible use of certain models for the research of HBV-associated socially significant infections is considered from the point of view of the development of pathomorphological features.
To complete this review, we analyzed the information about the features of HBV infection models in vivo, published over the last 25 years in open sources (Web of Science, PubMed, Scopus, ScienceDirect, Springer). The main criteria for literature selection were the type of infecting agent, the observed immunologic features of the course of the infectious process and the availability of a description of the pathomorphological features in model organisms.
Full Text
Introduction
Hepatitis B virus (HBV) is a small enveloped hepatotropic DNA virus. HBV has a relaxed partially double-stranded circular DNA genome of approximately 3200 bases in length with 4 major overlapping open reading frames (ORF): pre-S/S, pre-C/C, X and P [1, 2]. The pre-S/S ORF encodes 3 structural envelope proteins: large (L), medium (M) and small (S). The S protein, known as HBsAg, consists of 226 amino acids. The pre-C/C ORF encodes 2 proteins: Core (core protein), also known as hepatitis B core antigen (HBcAg) forms the nucleocapsid; pre-Core (pre-core protein) is produced as a result of the translation initiation from alternate sites within the pre-C/C HBV ORF. The X ORF encodes a small regulatory X protein that is required for viral replication. Finally, the P ORF encodes a viral DNA polymerase [3], which is also a specialized reverse transcriptase required for the replication of HBV genomic DNA via an intermediate RNA [4].
HBsAg is a surface antigen and the main marker of HBV infection. In acute hepatitis, HBsAg can already be detected in the blood during the incubation period within the first 4–6 weeks from the onset of the clinical period. The presence of HBsAg for more than 6 months is considered a factor in the disease transition to the chronic stage. It should be noted that only part of HBsAg proteins formed during virus reproduction, is used for the construction of new viral particles, while the rest of the antigen enters the blood of the infected host [5].
HBcAg is a core antigen detectable only in the nuclei of hepatocytes, but is absent in the bloodstream. The detection of IgM antibodies to this antigen in the blood is of great diagnostic importance. These antibodies in acute hepatitis are detected earlier than antibodies for other viral antigens. Anti-HBcIgM are detected in 100% of patients with acute hepatitis B, while the total anti-HBc antibodies may be the only marker of HBV in the so-called "window period", when neither HBsAg, nor antibodies to it could be detected in the bloodstream. Because of this, they are detected at blood transfusion centers when testing donated blood and plasma [6].
HBcAg and HBsAg are the major structural antigens of HBV. Both antigens are potent immunogens for animal models as well as humans infected with HBV [7].
HBV protein X (HBx) is a pleiotropic regulatory protein [8]. Its role in viral replication has been demonstrated in a number of in vitro and in vivo studies. HBx was first identified as a transactivator of gene expression. HBx does not bind DNA elements directly, but attaches to proteins that are responsible for binding DNA. HBx moderately stimulates transcription driven by a wide range of promoters, including its own enhancer in the context of the entire viral genome [9]. However, the role of HBx and its induced gene transcription, as well as its impact on cytoplasmic signaling pathways during natural HBV infection, remains up for debate [10].
After the virus enters the cell, the HBV capsid is transported to the nucleus where the viral DNA is released. In the nucleus, the incomplete viral DNA genome with the help of cellular enzymes is reconstituted into covalently closed circular DNA (cccDNA), which then serves as a template for HBV gene transcription [11]. The disease is associated with the formation of infectious virions as well as overexpression of viral proteins.
HBV has a strict tropism to its host and organs. It attaches to heparan sulfate proteoglycans and is absorbed upon binding to sodium-taurocholate cotransporting polypeptide (NTCP). NTCP is a hepatocyte-specific bile acid transporter and has been identified as a high-affinity HBV receptor.
Ex vivo expression of human NTCP (hNTCP) in mouse, rat and dog hepatocytes enables HBV entry into cells, but the virus is then blocked at one or more stages of its life cycle [12]. However, hNTCP expression in the hepatocytes of pigs, cynomolgus monkeys (Macaca fascicularis) and rhesus macaques (Macaca mulatta) allows HBV to undergo a complete replication cycle. It is likely that additional host factors are required to import incomplete HBV genomes into the nucleus or to reconstitute them to a persistent form of cccDNA [13].
HBV is a member of the Hepadnaviridae family, which includes other hepatotropic enveloped DNA viruses such as Duck hepatitis B virus (DHBV), Woodchuck hepatitis virus (WHV) and Woolly monkey hepatitis B virus (WMHBV). DHBV, WHV and WMHBV are widely used as surrogate models of HBV infection. Mammalian hepadnaviruses, including HBV, are members of the Orthohepadnavirus genus and lead to the development of liver pathologies, while DHBV belongs to the Avihepadnavirus genus, though infection with this virus does not lead to the development of hepatopathies.
Surrogate animal models of HBV
Ducks
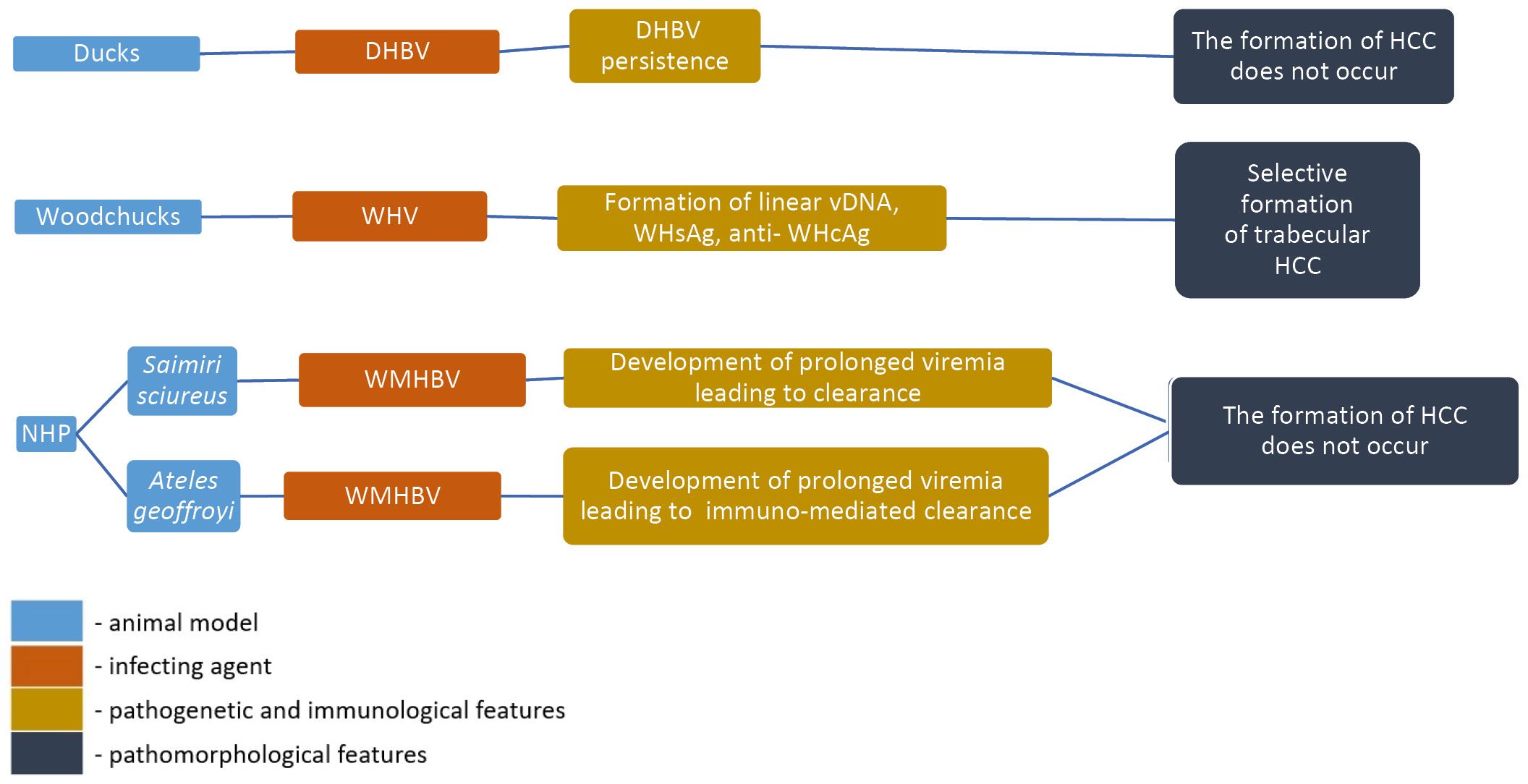
In 1980, DHBV was detected in the serum of domestic ducks and became an instrumental model for understanding the life cycle of hepadnaviruses [14]. In duck hepatocyte cultures, this model has played a key role in elucidating the mechanisms of viral replication, including viral capsid assembly, initiation of reverse transcription and finally formation of double-stranded relaxed circular DNA [15–19]. Using this model, the stages of cccDNA formation were investigated [20–22]. Duck models with persisting DHBV infection have also been used extensively to evaluate antiviral drugs, nucleocapsid assembly inhibitors and combined therapy (Fig. 1) [23–25].
Fig. 1. Pathogenesis, immunological and pathological characteristics of animal models of HBV infection based on surrogate viruses.
Nevertheless, duck models based on DHBV still greatly differ from human HBV in the following aspects: DHBV is only 40% homologous to human HBV [26], DHBV uses carboxypeptidase D as an entry receptor [27], furthermore, drug toxicity may manifest differently in humans and ducks [28, 29]. Therefore, screening of candidate drugs as well as mechanism studies using this system may require further validation due to the existing differences between viruses and hosts.
Woodchucks
The discovery of WHV, which infected woodchucks (Marmota monax) at the Philadelphia Zoo [30], provided new opportunities for studying the host response to hepadnaviruses in vivo. WHV is similar to HBV not only in virological features such as length, organization, and nucleotide sequence of the genome (the similarity reaches 60-70%), but also in innate and adaptive host immune responses that occur during viral infection [31].
Currently, two species of woodchucks are used as animal models for HBV-related studies: Marmota monax [32, 33] and Marmota himalayana [34]. The course of infection, pathogenesis and disease progression in WHV-infected woodchucks are similar to HBV infection in humans. Consequently, the woodchuck model is widely used for preclinical studies of antiviral drugs as well as studies of HBV-induced hepatocellular carcinoma (HCC). As preclinical models, infected woodchucks have been tested for susceptibility to antiviral drugs such as nucleos(t)ide analogs including lamivudine [35], entecavir [36], and tenofovir [37]. Infection of newborn woodchucks usually results in chronic infection, whereas adult woodchucks usually develop acute hepatitis, indicating a similar immune response against HBV infection in humans. This has made it possible to use woodchucks as an animal model of HBV for the development of prophylactic vaccines and screening of immunotherapeutic drugs against HBV infection [15, 16, 32, 33]. In particular, the Marmota himalayana model has shown that the use of nucleoside analogs alone or in combination with DNA vaccine can induce a partial or complete immune response, preventing viremia after WHV infection [38].
Nevertheless, there are certain limitations when conducting research on the woodchuck model. Firstly, the woodchucks are a large animals and can be difficult to handle during experimental procedures. Secondly, there are a limited amount of reagents available to study the immune response of woodchucks to viral infection. Furthermore, HBV- and WHV-mediated carcinogenesis differ, as WHV DNA often integrates into the Myc proto-oncogene, resulting in the development of HCC in almost all neonatally infected woodchucks, whereas HBV prefers to integrate into the TERT, MLL4 and CTNNB1 genes [39]. Thus, the differences between HBV and WHV viruses and their effects on their respective hosts should be taken into account to evaluate the efficacy of potential therapeutic agents and vaccines against HBV.
Bats
Metagenomic analysis of tissue samples from bat populations revealed numerous hepadnavirus-like sequences showing more than 70% homology with WHV and HBV genome sequences [40]. Whole-genome sequencing of tissue samples from bats living in Panama and Gabon also revealed the presence of hepadnaviruses in these populations, but zoonotic potential was only confirmed in New World bats. This virus was capable of infecting human hepatocytes via hNTCP and cross-reacted with monoclonal antibodies against the S-domain of HBsAg. Histopathologic examination indicated a slight to moderate presence of lymphocytic as well as scant neutrophilic-eosinophilic infiltrates in the portal triads of infected animals [41]. The presence of HBx suggests the potential development of HCC in infected bats. However, the detection of antibody-positive and DNA-negative bats combined with high seroprevalence in the same research indicates that bats are possibly able to clear this infection.
The use of bats as a model organism for HBV studies is costly and time consuming due to the importance of arranging for the animal care and maintenance in an isolated vivarium. Furthermore, bat hepadnaviruses are insufficiently studied. However, the question of modeling HBV infection in bats is open to debate, given the migratory nature of these animals.
Non-human primates
Another HBV-like virus, WMHBV, infects its natural host, the woolly monkey (Lagothrix lagotricha) [42]. WMHBV has been successfully used in various studies on spider (Ateles geoffroyi) and squirrel (Saimiri sciureus) monkeys. An infectious WMHBV clone was developed for studies in spider monkey models, resulting in moderate viremia (104–105 genomic equivalent per 1 ml) 8 weeks after inoculation followed by immune-mediated clearance [28]. Woolly and spider monkeys are endangered and are unavailable for HBV-related studies for ethical reasons [43, 44]. Nevertheless, HBV models based on non-human primates may be promising. For example, squirrel monkeys infected with WMHBV have shown acute infection and in some cases chronic infection when WMHBV genomes were delivered using adeno-associated viruses [45]. It has been shown that in squirrel monkeys infected with WMHBV, viremia lasts 6-8 months, which is more than 2 times longer than the duration of viremia in other non-human primates. This fact allows us to consider them as a surrogate model for the development of therapeutic approaches for HBV treatment [45].
In 2013, a single naturally transmissible HBV strain 99% identical to the HBVgenotype D (ayw) strain was detected in a population of cynomolgus monkeys on the island of Mauritius (mcHBV), which raised some hopes of creating an HBV model based on the small Old World monkey [46]. So far, however, it has not been possible to obtain productive infection of cynomolgus monkeys with mcHBV, despite them being inoculated with high doses [47].
After hNTCP was identified as a functional HBV receptor, researchers discovered that it is a key host factor limiting HBV infection in cynomolgus monkeys and rhesus macaques [13]. hNTCP differs from primate NTCP by only five amino acid residues in the HBV binding region. However, a difference at one of these divergent amino acid residues (G158R) results in an inability to bind HBV to primate NTCP. The induction of a corresponding mutation in the primate NTCP gene resulted in HBV susceptibility [48]. In order to develop a new virus model that provides susceptibility to HBV, the R158G mutation could be induced into macaque NTCP using the CRISPR/Cas genome editing system.
Furthermore, an HBV model based on rhesus macaques transduced with a viral vector to express hNTCP in vivo was developed [47]. The addition of a viral vector followed by HBV infection resulted in sustained HBV viremia for at least 6 weeks. Humoral and antiviral cellular immunity were generated in the model animals, and cccDNA was expressed in hepatocytes of rhesus macaques during infection. However, the level of hNTCP expression in rhesus macaques remains at a low level and therefore, this model of HBV infection is not considered effective (only 0.5–1.0% of hepatocytes are HBcAg-positive, while HBsAg is not detected in blood) [47].
The above data inspire the development of other HBV models based on non-human primates which would be capable of demonstrating a higher efficiency of HBV infection.
Immunotolerant animal models of HBV
Transgenic mice
Since 1985, transgenic mouse models expressing HBV proteins (such as HBsAg [49], HBeAg [50] or HBx [51]) have been developed to study their role in pathogenesis or study the full-length HBV genomes that can produce infectious HBV virions in mouse hepatocytes [52–55]. These models have contributed immensely to the identification of oncogenic functions of HBsAg and the HBx protein that induce inflammation, alter host gene expression and ultimately lead to the development of HCC [51, 56].
Transgenic mice expressing HBeAg are immunologically tolerant not only to HBeAg, but also to HBcAg, however, they are capable of transplacental transmission of HBeAg. It is shown that the nonchimeric offspring of such animals after birth demonstrate a reduced T-cell response to HBcAg. All of this indicates that at the early stage of infection, HBeAg plays a crucial role in limiting the T-cell response to HBcAg and contributes to the transition of the process into a chronic course of the disease. While HBcAg expression causes no obvious pathomorphological changes in these mice, enhanced HBeAg expression inhibits its release, leading to the formation of acidophilic inclusions in the cytoplasm and causing focal nodular hyperplasia of the liver. The cells with acidophilic inclusions, in turn, resemble the ground-glass hepatocytes in HBV carriers [57].
Transgenic mice expressing HBxAg in liver tissue under the control of its native promoter have been used to establish the oncogenic potential of HBxAg. Due to HBxAg not being expressed in all hepatocytes, not all animals develop HCC. In certain mice, only moderate nuclear pleomorphism and increased mitotic hepatocytic activity of a non-inflammatory nature is observed, multifocal hepatolipidosis also occurs (Fig. 2). It is worth noting that disease progression and death are observed in males earlier than in females [58].
Fig. 2. Pathogenesis, immunological and pathological characteristics of immunotolerant animal models of HBV infection.
Transgenic mice with integrated full-length HBV genomes capable of producing HBV virions in peripheral blood have also been used to test the efficacy of antiviral drugs such as lamivudine and entecavir, as well as to evaluate the antiviral effects of therapeutic drugs based on small interfering RNA [59, 60].
Transgenic mice have an immune tolerance to HBV because the virus genome is integrated into the mouse genome [61]. Nevertheless, transgenic mice have been used to study the antiviral effects of the interferon inducer [62] and the toll-like receptor 7/8 agonist [54]. Furthermore, transplantation of immune cells from naive mice to transgenic mice can provoke the development of an immune response to HBV [63-65].
Transduced mice
The main method of transduction of mouse hepatocytes in vivo is hydrodynamic injection, which involves the rapid (6-8 s) injection of a large number of genetic constructs containing the HBV genome into the tail vein under high pressure [66]. This causes hepatocyte damage, which entails a post-injection increase in alanine transaminase levels to large values, but after transduction, HBV replication is temporary [67]. Hepatocyte transduction in vivo is accomplished using plasmid DNA, adenoviral (Adv) or adeno-associated viral vectors. Transduction with high doses of Adv has been shown to induce temporary antigenemia [68] and HBV replication for 3 months. This is followed by a strong immune response against the adenovirus itself [67]. Transduction with adeno-associated viruses results in an immune tolerant phenotype with prolonged antigenemia, minimal inflammation and liver fibrosis [69].
The use of plasmid DNA reduces the risks of strong immune responses against viral vehicles [70]. The formation of antigens, viral transcripts and DNA synthesis lasts for 14 days in immunocompetent animals. This is associated with the development of a T-cell immune response aimed at destroying infected hepatocytes. It is important to note that in immunodeficient NOD/SCID mice, the same process lasts for 81 days after transduction [70].
Optimization of the carrier containing the HBV genome extended the duration of antigenemia in immunocompetent mice up to 6 months. Due to this, HBsAg-positive mice produced antibodies against HBcAg, but not against HBsAg, resembling the pattern of the immune tolerant phase of infection in human carriers. Transfer of HBcAg-specific immune cells promoted the elimination of HBsAg from the serum of mice carriers, demonstrating the critical importance of the immune response against HBcAg in eliminating HBV persistence [71].
A crucial role in HBV persistence in vivo is played by cccDNA, which is responsible for viral recurrence in chronically infected patients. It should be noted that cccDNA is unable to infiltrate into hepatocyte nuclei of unmodified mice. Using Adv, an HBV recombinant cccDNA (rcccDNA) incapable of replication was successfully delivered to the liver of Alb-Cre transgenic mice and remained stable for more than 62 weeks, resulting in HBV persistence. The persistent non-blood inflammatory response and liver fibrosis found at the late stage of virus persistence were similar to lesions in the progressive course of chronic hepatitis B (CHB) infection in humans [72]. A similar hepatotropic rcccDNA model was established in the same mice via adeno-associated virus 8. The model was characterized by more than 72 weeks of antigenemia and cccDNA persistence for more than 51 weeks. In contrast to the previous model, minor parenchymatous inflammation and focal fibrosis were observed in the liver [73].
Thus, it is quite evident that the approach of HBV transduction of mice mediated by viral vectors and plasmid DNA is suitable for further use in HBV immunotolerance studies. Moreover, delivery of adeno-associated virus-HBV vectors to mice induced histopathologic manifestations consistent with liver fibrosis, which is characteristic of the HBV infection [74]. However, this issue has yet to be fully investigated.
Chimeric mice with humanized livers
Transgenic and transduced mice do not support a full-scale infection due to the absence of the hNTCP receptor. The Trimera model is the result of the earliest attempts of improving existing mouse models of HBV. To develop this model, fragments of infected human liver were transplanted into immunodeficient mice under the renal capsule. After engraftment, viral DNA and antigens were detectable for 2 months [75]. This model was used to evaluate antiviral drugs, despite its limited applicability to other HBV research tasks due to the relatively short time window for infection and the transient viremia. In another simple model, partially immunodeficient Nude mice were subcutaneously transplanted with HepAD38 cells, capable of inducible HBV production. Sustained viremia developed 21 days after the subcutaneous neoplasm was formed and persisted for 30 days [76].
An alternative to the Trimera model involves using mice with liver-specific expression of the urokinase-type plasminogen activator (uPA). Such animals are characterized by increased uPA concentration, hypofibrinogenemia and neonatal hemorrhages. Expression of the uPA gene induces hepatocyte necrosis in young mice and provides marked repopulation of about 87% of liver parenchyma after 9 weeks [77]. Despite sustained viremia, ground-glass hepatocytes are not detected in such animals [78]. The hu-uPA/SCID model is widely used to evaluate antiviral drugs and investigational immune therapy regimens. It is also used to study the mechanism of the innate immune response against HBV. The main disadvantage of this model is the uncontrolled expression of the uPA gene, which requires xenotransplantation of hepatocytes in the neonatal period, as well as an increased risk of extensive hemorrhage in model animals [79].
In 2018, G. Dalton et al. introduced Fah gene knockout mice. These animals significantly solved the problem of the hu-uPA/SCID model. The lack of the Fah gene causes accumulation of toxic metabolic intermediates of phenylalanine catabolism to tyrosine and induction of liver failure is regulated by administration of 2-(2-nitro-4-fluoromethylbenzoyl)-1,3-cyclohexanedione [80]. Based on this feature, several models have been established, including FRG mice carrying a genotype with the knockout of Fah, Rag2 and IL-2ry genes. These mice achieved 95% liver repopulation with human hepatocytes [81, 82]. After HBV infection, increased levels of cccDNA, vRNA and viral DNA replication intermediates were observed in hu-HEP/FRG mice. The hu-HEP/FRG model has been successfully used to study the pathogenesis of HBV [82] and evaluate the efficacy of antiviral drugs and therapeutic regimens [83].
Immunocompetent animal models of HBV
Tupaia
Tupaia (Tupaia belangeri) are small mammals that are genetically more closely related to primates than to rodents [84]. Tupaia are susceptible to HBV and HCV experimental infections, which has led them to become a non-primate animal model studied in recent decades [85, 86]. Similar to other species, tupaia have a greater propensity to develop chronic disease when infected as newborns and show similar histopathologic changes in the liver as those seen in humans infected with HBV [83]. Primary tupaia hepatocytes are widely available and used in HBV and WMHBV infections [87, 88].
Due to the primary cell culture of tupaia hepatocytes, accurate mapping of the HBV binding site became possible and NTCP was identified as its functional receptor [12]. Intraperitoneal inoculation of HBV into newborn animals leads to HBsAg persistence in the liver accompanied by short-term HBsAg- and HBeAg-antigenemia, while intrahepatic transduction of recombinant viral DNA leads to the emergence of serum antibodies against HBsAg and HBcAg and the development of chronic hepatitis ending in fibrosis in certain individuals [89]. In adult tupaia, intravenous or intraperitoneal infection leads to a rapid increase in antibody titers against HBcAg, HBsAg and HBeAg within 2-3 weeks. Histopathologic changes in the liver of chronically infected animals are similar to those observed in human HBV [89], including HCC formation at the late stage of life in 30% of animals (Fig. 3). Nonetheless, no increase was observed in the levels of serum transaminases in tupaia [90].
Fig. 3. Pathogenesis, immunological and pathological characteristics of immunocompetent animal models of HBV infection.
Nowadays, there are a number of caveats that limit the applicability of tupaia as an animal model of HBV. First, being outbread, tupaia have genetic heterogeneity that may be useful for real-world studies, but is not acceptable for large-scale animal studies. Second, only low titers of virus are detectable in vivo in tupaia. Lastly, there is an acute shortage of tools and materials to investigate HBV in this species.
Chimeric mice with humanized immune systems and livers
A significant disadvantage of chimeric mice with humanized livers is their high immunodeficiency background. To enable analysis of the human immune response to HBV, virus-induced immunopathogenesis and testing of immunomodulators for the treatment of CHB infection, protocols for the co-grafting of human hepatocytes and human immune cells into mice are constantly being updated and improved.
Reconstitution of the human immune system is achieved by intrahepatic injection of newborn NSG mice carrying the HLA-A2 allele with human hematopoietic CD34+ stem cells (hCD34+). The hCD34+ together with human liver progenitor cells repopulate mouse livers damaged by agonistic antibodies against CD95. This model achieves 25% humanization, HBV persistence with low viral load (< 5 × 105 copies/mL), and the development of an HBV-specific hCD8+ T-cell response. The accumulation of M2-like macrophages, which contribute to accelerated liver fibrosis in HBV patients, leads to the development of severe hepatitis and fibrosis in mice [91].
Another model, HIS/hu-HEP, is developed by engrafting hCD34+ cells and adult human hepatocytes into BALB/c Rag2–/–IL2ry–/– Sirpa NOD Alb-uPAtg/tg mice. HIS/hu-HEP is characterized by higher levels of HBV antigen production and the development and maintenance of productive infection for 16–20 weeks. Infection of animals of this model leads to infiltration of inflammatory cells into areas containing antigen-positive hepatocytes without subsequent formation of foci of fibrosis. At the same time, exposure to small doses of HBsAg and HBcAg antigens induces a stronger immune response [92].
The FRGS (Fah–/–Rag2–/–IL-2Rγc–/– SCID) mice are also used to develop chimeric mice with humanized immune system and liver. This is done by intrasplenial transplantation of human bone marrow mesenchymal stem cells. As a result, after 12 weeks, human bone marrow mesenchymal stem cells differentiate into functional hepatocytes directly in the liver of the mice. Furthermore, hCD45+ cells repopulate bone marrow, thymus, lymph nodes, liver and are found in the peripheral blood of model animals to varying degrees. After infection, persistent HBV infection with high viral load and antigenemia develops, attracting a large number of inflammatory cells to the liver parenchyma. The pathomorphological signs of acute lobular hepatitis with the formation of lymphoid aggregates and ductal lesions appears 12 weeks after infection, and the portal and periportal lymphocytic infiltration characteristic of HBV is detected from week 24. The formation of diffuse scars and areas of focal nodular hyperplasia in the liver, as well as elevated levels of serum γ-glutamyltranspeptidase and hyaluronic acid, persist for 36 weeks after infection. Taken together, such liver changes in animals are similar to some pathomorphological features of the CHB infection in humans. Moreover, 10% of infected animals develop cirrhosis, which resembles the natural history of CHB infection in humans [92].
Chimeric mice with humanized immune systems and livers represent a new type of animal models of HBV. Such models successfully reproduce the natural progression of HBV infection and thus offer opportunities to understand the pathophysiology of viral immunity and improve intervention strategies for HBV-related liver disease.
Primates
The chimpanzee is the only known immunocompetent model that is fully susceptible to HBV and can reproduce pathogenesis and disease progression quite accurately. It has been shown that even 1 genomic equivalent of HBV DNA is sufficient to successfully infect chimpanzees [93]. After injection of HBV obtained from patients with CHB infection, chimpanzees can develop acute and chronic disease with immune response and inflammation profiles very similar to those of HBV-infected patients [94].
In the early stages of HBV research, the chimpanzee model played an important role in evaluating the safety and efficacy of HBV vaccines under development. Chimpanzee studies were used to test the efficacy of the first-generation HBV vaccine (derived from blood plasma) and a later developed recombinant vaccine containing HBsAg produced in yeast cells [94, 95]. In recent years, the efficacy of modified recombinant vaccines, vaccines against antiviral drug-resistant mutant variants of HBV, and some therapeutic vaccination studies have also been tested using a chimpanzee model [96–98].
Chimpanzees seem to be indispensable as an immunocompetent animal model in immunity studies due to the fact that they can reproduce the same liver inflammation and cellular immune responses as in HBV-infected patients. Studies in chimpanzees have shown that non-cytopathic antiviral mechanisms mediated by pro-inflammatory cytokines can promote viral clearance during acute viral hepatitis [99]. More recently, studies in chimpanzee models of CHB infection have shown that immune modulation can enhance antiviral immunity and suppress HBV replication (e.g., through TLR-7 agonist activation) [100]. Although chimpanzees have historically been the most relevant animal species for HBV research, limited availability, high costs and serious ethical concerns have limited their use as an experimental model.
Besides chimpanzees, other primate models have been used to study HBV. For example, Barbary macaques (Macaca sylvanus) transduced with plasmid DNA containing the HBV genome develop viremia and early portal liver fibrosis, which is also characteristic of the disease in humans. At the same time, serum transaminase levels in infected macaques remain within the physiologic normal range [101]. In rhesus macaques expressing hNTCP in hepatocytes, moderate transient viremia can also be established [47].
Primate models, which are extremely important in the study of HBV, are not widely used not only for ethical reasons, but also because of their high cost and the lack of necessary materials (reagents) for such studies.
Models of socially significant HBV co-infections
HBV infection is a socially determined chronic disease and can be mixed with other infections caused by hepatitis C (HCV) and D (HDV) viruses, human immunodeficiency virus (HIV) or tuberculosis. The pathophysiologic, immunologic, and pathomorphological features of these co-infections differ significantly from those of mono-infection. Therefore, when developing therapeutic approaches, it is advisable to develop animal models that can reflect the properties of co-infections.
HBV/HCV and HBV/HDV coinfections
Hepatitis C is widespread. The WHO estimates that about 3% of the world's population is infected with HCV, with about 1.5 million new infections occurring each year. More than 250,000 people die each year from the hepatitis C sequelae (mainly cirrhosis and liver cancer).
Hepatitis D is an inflammatory liver disease caused by hepatitis D virus (HDV). HDV requires the presence of HBV for its reproduction (without HBV, even hepatitis D infection is not possible). HBV/HDV co-infection is considered the most severe form of chronic hepatitis due to the more rapid development of HCC, which is fatal.
Due to their ability to control hepatocyte apoptosis, transgenic humanized hu-uPA/SCID/beige and AFC8-hu-HSC/Hep mice can be used to mimic HBV/HCV or HBV/HDV co-infections. Virus titers in these models reach detectable serum levels 3 weeks after HBV/HDV infection. HBV viremia is slightly lower than HDV viremia because HBV/HDV co-infection is associated with induction of human type I interferon expression, in contrast to HBV mono-infection [102]. FRG and Alb-uPA mice have also been successfully used for xenotransplantation of human hepatocytes and subsequent HCV infection. Interestingly, the transmissible nature of the disease was confirmed in the course of these studies [103, 104].
Y. Amako et al. demonstrated the possibility of the infection of naive animals with serum of seropositive tupaia with subsequent development of acute hepatitis and viremia. Infection of tupaia with serum of HCV-positive humans resulted in a 2-5-fold increase in serum alanine aminotransferase level and prolonged transient viremia. At the same time, hepatolipidosis was observed in infected animals. During the next 2 years, lymphocytic infiltrates characteristic of chronic pathology was formed around the portal areas and liver sinusoids. And in the next year, the inflammation led to the development of moderate and even severe fibrosis with elements of liver cirrhosis [105].
As with HBV, the functional receptor for HDV is NTCP. At the same time, HDV requires HBV envelope proteins for release from infected hepatocytes and continuation of the infectious process [106], although it is known that HDV can persist in resting (non-dividing) woodchuck hepatocytes for at least 42 days, independently of HBV [103]. In vivo studies performed on woodchucks infected with both WHV and HDV have helped in the development of immunization strategies. However, J.L. Gerin claimed that one of the obstacles to the multilateral use of this model was the early development of WHV-induced HCC [107].
HBV/HIV co-infection
At the end of 2022, 33.1–45.7 million people worldwide are infected with HIV, according to various estimates. About 1% of people living with HBV infection (2.7 million people) are also infected with HIV. HIV-positive patients with HBV are known to have an 8-fold higher risk of liver-associated mortality due to the use of highly active antiretroviral therapy. Furthermore, HIV infection accelerates the progression of HBV infection, significantly increasing the risk of liver cirrhosis or HCC. However, HBV infection does not lead to an accelerated reduction in the amount of CD4+ T-cells [108].
Given the similar transmission routes and risk factors, cases of HBV/HIV co-infection are not uncommon. Therefore, double-humanized mouse models are used to study HBV/HIV co-infection. Such a model would be AFC8-hu-HSC/Hep mice. AFC8-hu-HSC/Hep mice are characterized by inducible hepatocyte apoptosis due to a transgene incorporated the genome (AFC8 — FK506-binding protein and caspase 8 under the control of the albumin promoter). Infection of these mice with HBV leads to the expression of human fibrogenic proteins that cause the development of liver inflammation and fibrosis correlating with the activation of hepatic stellate cells in the humanized region of the liver [109]. Humanized models of HIS/hu-HEP [91] or TK-NOG-hu-HSC/Hep [110] mice can be used in a similar manner. However, the main obstacle to the development of these models is the necessity to use tissues obtained from a single donor.
The mouse model of HIL, obtained by transplantation of embryonic hCD34+ cells into the liver parenchyma of immunodeficient mice, is more simple to use. With this method, it is possible to obtain mice with double humanization and minimized risk of graft-versus-host reaction. After infection, HIL mice exhibited nodular liver fibrosis progressing to diffuse fibrosis with multiple septa. The diffuse fibrosis led to the development of severe scarring and nodular cirrhosis as a result of inflammation and activation of hepatic stellate cells. At the same time, a threefold increase in the number of hCD45+ cells and improved regulation of both human and murine fibrogenic genes were observed in the liver parenchyma of animals [111].
HBV/Mycobacterium tuberculosis co-infection
Tuberculosis, like infections caused by HCV, HDV and HIV, is a socially significant disease. In 2021, an estimated 10.6 million people worldwide had tuberculosis. Co-infections of HBV and Mycobacterium tuberculosis (MTB) are not uncommon.
It is a known fact that antitubercular drugs are primarily used in the therapy of HBV and MTB co-infection, which against the background of hepatic tuberculosis can carry a huge risk of liver damage [112]. Rhesus macaques and cynomolgus monkeys are susceptible to MTB infection by the natural route of infection, and they develop granulomas, including in the liver, with a highly ordered architecture with central necrosis surrounded by a peripheral rim [113].
The development of an aerosolized method of infection mice made it possible to increase the virulence of mycobacteria relative to the intravenous method [114]. The BLT model obtained by transplantation of fetal tissues of human bone marrow, liver and thymus under the kidney capsule of immunodeficient mice is susceptible to infection with mycobacteria. 3-4 weeks after MTB inoculation, multifocal inflammation of liver parenchyma developed. The bacteria were localized in the areas of inflammation, but no ductal obstruction was observed in the liver 4 weeks after infection [115]. The main difficulty of the BLT model lies in the xenotransplantation procedure, which requires manipulations with extreme precision. Furthermore, in the BLT model, the developing human liver tissue does not reconstitute the full structure of the organ.
Highly immunodeficient TK-NOG mice, suitable for xenotransplantation of both liver tissue and hematopoietic stem cells, may become a universal model for some infections. Their humanization may make it possible to mimic not only HBV infection combined with HIV [110], but also make these mice a promising model for future studies of HBV/MTB co-infection.
L. Zhan et al. reported the susceptibility of tupaia to experimental intravenous infection with high and low doses of MTB. Given that tupaia are outbred, the observed clinical signs of disease varied among animals even within the same experimental group. Pathomorphological examination showed the presence of tuberculous granulomas in the lungs and spleen with central caseous necrosis and a large number of inflammatory cells in the periphery. Focal necrosis with inflammatory cells was common in the adrenal cortex and capsule and cerebellum. Infiltrated with inflammatory cells, MTB-containing lesion foci were found in the liver, kidney and extensive necrotic foci in muscle. It is important to note thatanimals reached humane endpoints by week 11 [116]. Taking all of this into account, tupaia can also be considered a promising model for future HBV/MTB co-infection studies.
Conclusion
DHBV is only 40% homologous to HBV [26] and uses carboxypeptidase D as an entry receptor [27]. Therefore, ducks have low relevance as models for evaluating the efficacy of antiviral therapy [117]. Similar to ducks, woodchucks, bats, and primates are not inbred, and immunologic reagents for comprehensive studies of hepadnavirus infections are not available. Furthermore, many natural models of infection are simply endangered.
Mice with immunotolerance can only maintain HBV replication in the context of mouse morphologic and physiologic features. In this case, the life cycle of the virus is incomplete due to the absence of the stages of virus entry, cccDNA formation and infection dissemination. The relevance of the results obtained on chimeric mice possessing a humanized liver cannot be translated to humans due to their immunodeficiency and, consequently, the absence of pathomorphological changes caused by the immune response against infected cells.
Tupaia can be infected with HBV, but they are also outbred and their use in vivo as models requires preliminary studies. Double-humanized mice represent a promising area for further research. However, the stability of viremia is directly dependent on the degree of humanization of mouse liver with administered human hepatocytes [102]. Furthermore, transplantation (engraftment) of xenogeneic immune system and liver induces mismatches in the major histocompatibility complex system, which leaves open the question of the relevance of recognition mechanisms and immune responses against the virus in such models.
Given the inaccessibility of chimpanzees for HBV research, as well as the similarity of the immune system and the relative availability of some reagents, non-human primates possibly provide the most reliable immune data for translational research [46]. However, these results need to be carefully interpreted by cross-checking in other biological systems.
A new trend is the development of animal models of HBV-associated socially significant infections. Immunodeficiency of some models would possibly become their advantage, as in the case of simulated HBV/HIV co-infection. On the other hand, simultaneous infection with two pathogens carries the risk of a significant reduction in the quality of life of the animal. Therefore, the design of such studies should be reviewed by ethical committees in the strictest manner.
During the study of a pathogen such as HBV, there are no models that are of no practical value. In conclusion, models that can demonstrate both the long-term development of hepatopathy and the immunologic features of the infection process in vivo will become more widespread in future studies of both hepatotropic viruses and co-infections. Furthermore, the identification of novel natural reservoirs of hepadnavirus infections for the purpose of expanding the selection of available models will shed light on the origin and evolution of HBV.
About the authors
Aleksey M. Nagornykh
Central Research Institute for Epidemiology
Author for correspondence.
Email: nagornih@cmd.su
ORCID iD: 0000-0002-8320-2999
Cand. Sci. (Vet.), senior researcher, Scientific group of experimental models’ development, Laboratory of experimental pharmacology, Department of molecular diagnostics and epidemiology
Russian Federation, MoscowMarina A. Tyumentseva
Central Research Institute for Epidemiology
Email: tyumentseva@cmd.su
ORCID iD: 0000-0002-3145-3702
Cand. Sci. (Biol.), Head, Laboratory of genome editing, Department of molecular diagnostics and epidemiology
Russian Federation, MoscowAleksandr I. Tyumentsev
Central Research Institute for Epidemiology
Email: tymencev@cmd.su
ORCID iD: 0000-0003-0537-2586
Cand. Sci. (Biol.), Head, Laboratory of experimental pharmacology, Department of molecular diagnostics and epidemiology
Russian Federation, MoscowVasily G. Akimkin
Central Research Institute for Epidemiology
Email: vgakimkin@yandex.ru
ORCID iD: 0000-0003-4228-9044
D. Sci. (Med.), Professor, Full Member of the Russian Academy of Sciences, Director
Russian Federation, MoscowReferences
- Miller R.H., Kaneko S., Chung C.T., et al. Compact organization of the hepatitis B virus genome. Hepatology. 1989;9(2):322–7. DOI: https://doi.org/10.1002/hep.1840090226
- Song H., Xu F., Xiao Q., Tan G. Hepatitis B virus X protein and its host partners. Cell. Mol. Immunol. 2021;18(5):1345–6. DOI: https://doi.org/10.1038/s41423-021-00674-z
- Seeger C., Mason W.S. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 2000;64(1):51–68. DOI: https://doi.org/10.1128/mmbr.64.1.51-68.2000
- Summers J., Mason W.S. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell. 1982;29(2):403–15. DOI: https://doi.org/10.1016/0092-8674(82)90157-x
- Gerlich W.H. The enigma of concurrent hepatitis B surface antigen (HBsAg) and antibodies to HBsAg. Clin. Infect. Dis. 2007;44(9):1170–2. DOI: https://doi.org/10.1086/513296
- Tupoleva T.A., Ignatova E.N., Gulyaeva A.A., et al. The screening of donor blood on antibodies to nuclear antigen of hepatitis B virus as a tool of increasing of safety of transfusion for patients with diseases of blood system. Klin. Lab. Diagn. 2016;61(5):311–6. DOI: https://doi.org/10.18821/0869-2084-2016-61-5-311-316
- Aguilar J.C., Lobaina Y., Muzio V., et al. Development of a nasal vaccine for chronic hepatitis B infection that uses the ability of hepatitis B core antigen to stimulate a strong Th1 response against hepatitis B surface antigen. Immunol. Cell Biol. 2004;82(5):539–46. DOI: https://doi.org/10.1111/j.0818-9641.2004.01278.x
- Bouchard M.J., Schneider R.J. The enigmatic X gene of hepatitis B virus. J. Virol. 2004;78(23):12725–34. DOI: https://doi.org/10.1128/jvi.78.23.12725-12734.2004
- Syed G.H., Wyles D.L., Siddiqui A. Hepatitis viruses. In: Reference Module in Biomedical Sciences. Elsevier;2014.
- Warner N., Locarnini S. Replication of hepatitis B virus. In: Zakim and Boyer’s Hepatology. Elsevier;2012:86–96.
- Yan H., Peng B., Liu Y., et al. Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide. J. Virol. 2014;88(6):3273–84. DOI: https://doi.org/10.1128/jvi.03478-13
- Schieck A., Schulze A., Gähler C., et al. Hepatitis B virus hepatotropism is mediated by specific receptor recognition in the liver and not restricted to susceptible hosts. Hepatology. 2013;58(1):43–53. DOI: https://doi.org/10.1002/hep.26211
- Lempp F.A., Wiedtke E., Qu B., et al. Sodium taurocholate cotransporting polypeptide is the limiting host factor of hepatitis B virus infection in macaque and pig hepatocytes. Hepatology. 2017;66(3):703–16. DOI: https://doi.org/10.1002/hep.29112
- Mason W.S., Seal G., Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J. Virol. 1980;36(3):829–36. DOI: https://doi.org/10.1128/jvi.36.3.829-836.1980
- Tavis J.E., Massey B., Gong Y. The duck hepatitis B virus polymerase is activated by its RNA packaging signal, epsilon. J. Virol. 1998;72(7):5789–96. DOI: https://doi.org/10.1128/jvi.72.7.5789-5796.1998
- Tavis J.E., Ganem D. Evidence for activation of the hepatitis B virus polymerase by binding of its RNA template. J. Virol. 1996;70(9):5741–50. DOI: https://doi.org/10.1128/jvi.70.9.5741-5750.1996
- Beck J. dNTP versus NTP discrimination by phenylalanine 451 in duck hepatitis B virus P protein indicates a common structure of the dNTP-binding pocket with other reverse transcriptases. Nucleic Acids Res. 2002;30(7):1679–87. DOI: https://doi.org/10.1093/nar/30.7.1679
- Junker-Niepmann M., Bartenschlager R., Schaller H. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 1990;9(10):3389–96. DOI: https://doi.org/10.1002/j.1460-2075.1990.tb07540.x
- Tavis J.E., Ganem D. RNA sequences controlling the initiation and transfer of duck hepatitis B virus minus-strand DNA. J. Virol. 1995;69(7):4283–91. DOI: https://doi.org/10.1128/jvi.69.7.4283-4291.1995
- Tuttleman J.S., Pourcel C., Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47(3):451–60. DOI: https://doi.org/10.1016/0092-8674(86)90602-1
- Wu T.T., Coates L., Aldrics C.E., et al. In hepatocytes infected with duck hepatitis B virus, the template for viral RNA synthesis is amplified by an intracellular pathway. Virology. 1990;175(1):255–61. DOI: https://doi.org/10.1016/0042-6822(90)90206-7
- Summers J., Smith P.M., Horwich A.L. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J. Virol. 1990;64(6):2819–24. DOI: https://doi.org/10.1128/jvi.64.6.2819-2824.1990
- Foster W.K., Miller D.S., Scougall C.A., et al. Effect of antiviral treatment with Entecavir on age- and dose-related outcomes of duck hepatitis B virus infection. J. Virol. 2005;79(9):5819–32. DOI: https://doi.org/10.1128/jvi.79.9.5819-5832.2005
- Colledge D., Civitico G., Locarnini S., Shaw T. In vitro antihepadnaviral activities of combinations of Penciclovir, lamivudine, and adefovir. Antimicrob. Agents Chemother. 2000;44(3):551–60. DOI: https://doi.org/10.1128/aac.44.3.551-560.2000
- Campagna M.R., Liu F., Mao R., et al. Sulfamoylbenzamide derivatives inhibit the assembly of hepatitis B virus nucleocapsids. J. Virol. 2013;87(12):6931–42. DOI: https://doi.org/10.1128/jvi.00582-13
- Schaefer S. Hepatitis B virus taxonomy and hepatitis B virus genotypes. World J. Gastroenterol. 2007;13(1):14. DOI: https://doi.org/10.3748/wjg.v13.i1.14
- Tong S., Li J., Wands J.R. Carboxypeptidase D is an avian hepatitis B virus receptor. J. Virol. 1999;73(10):8696–702. DOI: https://doi.org/10.1128/jvi.73.10.8696-8702.1999
- Lanford R.E., Chavez D., Barrera A., Brasky K.M. An infectious clone of woolly monkey hepatitis B virus. J. Virol. 2003;77(14):7814–9. DOI: https://doi.org/10.1128/jvi.77.14.7814-7819.2003
- Mason W.S., Cullen J., Saputelli J., et al. Characterization of the antiviral effects of 2′ carbodeoxyguanosine in ducks chronically infected with duck hepatitis B virus. Hepatology. 1994;19(2):398–411. DOI: https://doi.org/10.1002/hep.1840190219
- Summers J., Smolec J.M., Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc. Natl Acad. Sci. USA. 1978;75(9):4533–7. DOI: https://doi.org/10.1073/pnas.75.9.4533
- Mulrooney-Cousins P.M., Michalak T.I. Asymptomatic hepadnaviral persistence and its consequences in the woodchuck model of occult hepatitis B virus infection. J. Clin. Transl. Hepatol. 2015;3(3):211–9. DOI: https://doi.org/10.14218/jcth.2015.00020
- Menne S., Tumas D.B., Liu K.H., et al. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B. J. Hepatol. 2015;62(6):1237–45. DOI: https://doi.org/10.1016/j.jhep.2014.12.026
- Cheng H.R., Kao J.H., Wu H.L., et al. Clinical and virological features of occult hepatitis B in patients with HBsAg seroclearance post‐treatment or spontaneously. Liver. Int. 2014;34(6):e71-9. DOI: https://doi.org/10.1111/liv.12324
- Liu Y., Wang B., Wang L., et al. Transcriptome analysis and comparison of Marmota monax and Marmota himalayana. PLoS One. 2016;11(11):e0165875. DOI: https://doi.org/10.1371/journal.pone.0165875
- Zhou T., Saputelli J., Aldrich C.E., et al. Emergence of drug-resistant populations of woodchuck hepatitis virus in woodchucks treated with the antiviral nucleoside lamivudine. Antimicrob. Agents. Chemother. 1999;43(8):1947–54. DOI: https://doi.org/10.1128/aac.43.8.1947
- Colonno R.J., Genovesi E.V., Medina I., et al. Long‐term entecavir treatment results in sustained antiviral efficacy and prolonged life span in the woodchuck model of chronic hepatitis infection. J. Infect. Dis. 2001;184(10):1236–45. DOI: https://doi.org/10.1086/324003
- Fairman J., Liu K.H., Menne S. Prevention of liver tumor formation in woodchucks with established hepatocellular carcinoma by treatment with cationic liposome-DNA complexes. BMC Cancer. 2017;17(1):172. DOI: https://doi.org/10.1186/s12885-017-3163-2
- Wang B., Zhu Z., Zhu B., et al. Nucleoside analogues alone or combined with vaccination prevent hepadnavirus viremia and induce protective immunity: Alternative strategy for hepatitis B virus post-exposure prophylaxis. Antiviral. Res. 2014;105:118–25. DOI: https://doi.org/10.1016/j.antiviral.2014.02.016
- Li X., Zhang J., Yang Z., et al. The function of targeted host genes determines the oncogenicity of HBV integration in hepatocellular carcinoma. J. Hepatol. 2014;60(5):975–84. DOI: https://doi.org/10.1016/j.jhep.2013.12.014
- He B., Li Z., Yang F., et al. Virome profiling of bats from Myanmar by metagenomic analysis of tissue samples reveals more novel mammalian viruses. PLoS One. 2013;8(4):e61950. DOI: https://doi.org/10.1371/journal.pone.0061950
- Drexler J.F., Geipel A., König A., et al. Bats carry pathogenic hepadnaviruses antigenically related to hepatitis B virus and capable of infecting human hepatocytes. Proc. Natl Acad. Sci. USA. 2013;110(40):16151–6. DOI: https://doi.org/10.1073/pnas.1308049110
- Lanford R.E., Chavez D., Brasky K.M., et al. Isolation of a hepadnavirus from the woolly monkey, a New World primate. Proc. Natl Acad. Sci. USA. 1998;95(10):5757–61. DOI: https://doi.org/10.1073/pnas.95.10.5757
- Ange-van Heugten K.D., Burns R., Verstegen M.W.A., et al. Evaluation of diabetes determinants in woolly monkeys (Lagothrix lagotricha). J. Anim. Physiol. Anim. Nutr. (Berl.). 2007;91(11-12):481–91. DOI: https://doi.org/10.1111/j.1439-0396.2007.00679.x
- Hagell S., Whipple A.V., Chambers C.L. Population genetic patterns among social groups of the endangered Central American spider monkey (Ateles geoffroyi) in a human‐dominated landscape. Ecol. Evol. 2013;3(5):1388–99. DOI: https://doi.org/10.1002/ece3.547
- Chen C.Y., Winer B.Y., Chavez D., et al. Woolly monkey-HBV infection in squirrel monkeys as a surrogate nonhuman primate model of HBV infection. Hepatol. Commun. 2020;4(3):371–86. DOI: https://doi.org/10.1002/hep4.1471
- Dupinay T., Gheit T., Roques P., et al. Discovery of naturally occurring transmissible chronic hepatitis B virus infection among Macaca fascicularis from Mauritius island: Hepatology. Hepatology. 2013;58(5):1610–20. DOI: https://doi.org/10.1002/hep.26428
- Burwitz B.J., Wettengel J.M., Mück-Häusl M.A., et al. Hepatocytic expression of human sodium-taurocholate cotransporting polypeptide enables hepatitis B virus infection of macaques. Nat. Commun. 2017;8(1):2146. DOI: https://doi.org/10.1038/s41467-017-01953-y
- Takeuchi J.S., Fukano K., Iwamoto M., et al. A single adaptive mutation in sodium taurocholate cotransporting polypeptide induced by hepadnaviruses determines virus species specificity. J. Virol. 2019;93(5):e01432-18. DOI: https://doi.org/10.1128/jvi.01432-18
- Chisari F.V., Pinkert C.A., Milich D.R., et al. A transgenic mouse model of the chronic hepatitis B surface antigen carrier state. Science. 1985;230(4730):1157–60. DOI: https://doi.org/10.1126/science.3865369
- Milich D.R., Jones J.E., Hughes J.L., et al. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc. Natl Acad. Sci. USA. 1990;87(17):6599–603. DOI: https://doi.org/10.1073/pnas.87.17.6599
- Kim C.M., Koike K., Saito I., et al. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991; 351(6324):317–20. DOI: https://doi.org/10.1038/351317a0
- Winer B.Y., Shirvani-Dastgerdi E., Bram Y., et al. Preclinical assessment of antiviral combination therapy in a genetically humanized mouse model for hepatitis delta virus infection. Sci. Transl. Med. 2018;10(447):eaap9328. DOI: https://doi.org/10.1126/scitranslmed.aap9328
- Guidotti L.G., Matzke B., Schaller H., Chisari F.V. High-level hepatitis B virus replication in transgenic mice. J. Virol. 1995;69(10):6158–69. DOI: https://doi.org/10.1128/jvi.69.10.6158-6169.1995
- Wang Y., Chen K., Wu Z., et al. Immunizations with hepatitis B viral antigens and a TLR7/8 agonist adjuvant induce antigen-specific immune responses in HBV-transgenic mice. Int. J. Infect. Dis. 2014;29:31–6. DOI: https://doi.org/10.1016/j.ijid.2014.07.015
- Moriyama T., Guilhot S., Klopchin K., et al. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science. 1990;248(4953):361–4. DOI: https://doi.org/10.1126/science.1691527
- Chisari F.V., Filippi P., McLachlan A., et al. Expression of hepatitis B virus large envelope polypeptide inhibits hepatitis B surface antigen secretion in transgenic mice. J. Virol. 1986;60(3):880–7. DOI: https://doi.org/10.1128/jvi.60.3.880-887.1986
- Tian Y., Kuo C.F., Akbari O., Ou J.H.J. Maternal-derived hepatitis B virus e antigen alters macrophage function in offspring to drive viral persistence after vertical transmission. Immunity. 2016;44(5):1204–14. DOI: https://doi.org/10.1016/j.immuni.2016.04.008
- Zhu H., Wang Y., Chen J., et al. Transgenic mice expressing hepatitis B virus X protein are more susceptible to carcinogen induced hepatocarcinogenesis. Exp. Mol. Pathol. 2004;76(1):44–50. DOI: https://doi.org/10.1016/j.yexmp.2003.09.001
- McCaffrey A.P., Nakai H., Pandey K., et al. Inhibition of hepatitis B virus in mice by RNA interference. Nat. Biotechnol. 2003;21(6):639–44. DOI: https://doi.org/10.1038/nbt824
- Julander J.G., Colonno R.J., Sidwell R.W., Morrey J.D. Characterization of antiviral activity of entecavir in transgenic mice expressing hepatitis B virus. Antiviral. Res. 2003;59(3):155–61. DOI: https://doi.org/10.1016/s0166-3542(03)00109-8
- Hong M., Bertoletti A. Tolerance and immunity to pathogens in early life: insights from HBV infection. Semin. Immunopathol. 2017;39(6):643–52. DOI: https://doi.org/10.1007/s00281-017-0641-1
- Wieland S.F., Guidotti L.G., Chisari F.V. Intrahepatic induction of alpha/beta interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J. Virol. 2000;74(9):4165–73. DOI: https://doi.org/10.1128/jvi.74.9.4165-4173.2000
- Guidotti L.G., Ishikawa T., Hobbs M.V., et al. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4(1):25–36. DOI: https://doi.org/10.1016/s1074-7613(00)80295-2
- Baron J.L., Gardiner L., Nishimura S., et al. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002;16(4):583–94. DOI: https://doi.org/10.1016/s1074-7613(02)00305-9
- Publicover J., Goodsell A., Nishimura S., et al. IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J. Clin. Invest. 2011;121(3):1154–62. DOI: https://doi.org/10.1172/jci44198
- Yang P.L., Althage A., Chung J., et al. Immune effectors required for hepatitis B virus clearance. Proc. Natl Acad. Sci. USA. 2010;107(2):798–802. DOI: https://doi.org/10.1073/pnas.0913498107
- Huang L.R., Gäbel Y.A., Graf S., et al. Transfer of HBV genomes using low doses of Adenovirus vectors leads to persistent infection in immune competent mice. Gastroenterology. 2012;142(7):1447–50.e3. DOI: https://doi.org/10.1053/j.gastro.2012.03.006
- John von Freyend M., Untergasser A., Arzberger S., et al. Sequential control of hepatitis B virus in a mouse model of acute, self-resolving hepatitis B: Control of HBV infection in mice. J. Viral. Hepat. 2011;18(3):216–26. DOI: https://doi.org/10.1111/j.1365-2893.2010.01302.x
- Yang D., Liu L., Zhu D., et al. A mouse model for HBV immunotolerance and immunotherapy. Cell. Mol. Immunol. 2014;11(1):71–8. DOI: https://doi.org/10.1038/cmi.2013.43
- Yang P.L., Althage A., Chung J., Chisari F.V. Hydrodynamic injection of viral DNA: A mouse model of acute hepatitis B virus infection. Proc. Natl Acad. Sci. USA. 2002;99(21):13825–30. DOI: https://doi.org/10.1073/pnas.202398599
- Huang L.R., Wu H.L., Chen P.J., Chen D.S. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc. Natl Acad. Sci. USA. 2006;103(47):17862–7. DOI: https://doi.org/10.1073/pnas.0608578103
- Li G., Zhu Y., Shao D., et al. Recombinant covalently closed circular DNA of hepatitis B virus induces long‐term viral persistence with chronic hepatitis in a mouse model. Hepatology. 2018;67(1):56–70. DOI: https://doi.org/10.1002/hep.29406
- Wu M., Wang C., Shi B., et al. A novel recombinant cccDNA-based mouse model with long term maintenance of rcccDNA and antigenemia. Antiviral. Res. 2020;180:104826. DOI: https://doi.org/10.1016/j.antiviral.2020.104826
- Ye L., Yu H., Li C., et al. Adeno-associated virus vector mediated delivery of the HBV genome induces chronic hepatitis B virus infection and liver fibrosis in mice. PLoS One. 2015;10(6):e0130052. DOI: https://doi.org/10.1371/journal.pone.0130052
- Vuyyuru R., Herzog-Hauff S., Tavakoli S., et al. Vaccination with dendritic cells induces strong HBV specific Th cell and CTL responses in HBV trimera mice. Z. Gastroenterol. 2007;45(01):A4_24. DOI: https://doi.org/10.1055/s-2007-967882
- Schinazi R.F., Bassit L., Clayton M.M., et al. Evaluation of single and combination therapies with tenofovir disoproxil fumarate and emtricitabine in vitro and in a robust mouse model supporting high levels of hepatitis B virus replication. Antimicrob. Agents Chemother. 2012;56(12):6186–91. DOI: https://doi.org/10.1128/aac.01483-12
- Tsuge M., Hiraga N., Takaishi H., et al. Infection of human hepatocyte chimeric mouse with genetically engineered hepatitis B virus. Hepatology. 2005;42(5):1046–54. DOI: https://doi.org/10.1002/hep.20892
- Meuleman P., Libbrecht L., De Vos R., et al. Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology. 2005;41(4):847–56. DOI: https://doi.org/10.1002/hep.20657
- Meuleman P., Lerouxroels G. The human liver-uPA-SCID mouse: A model for the evaluation of antiviral compounds against HBV and HCV. Antiviral. Res. 2008;80(3):231–8. DOI: https://doi.org/10.1016/j.antiviral.2008.07.006
- Dalton G., Oh S.H., Premont R., et al. Hedgehog signaling directly regulates metabolism and proliferation in hepatocytes and is critical for normal liver regeneration following partial hepatectomy in mice. Gastroenterology. 2018;154(6):S-166. DOI: https://doi.org/10.1016/s0016-5085(18)30966-1
- Bissig K.D., Wieland S.F., Tran P., et al. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J. Clin. Invest. 2010;120(3):924–30. DOI: https://doi.org/10.1172/jci40094
- Long K.R., Lomonosova E., Li Q., et al. Efficacy of hepatitis B virus ribonuclease H inhibitors, a new class of replication antagonists, in FRG human liver chimeric mice. Antiviral. Res. 2018;149:41–7. DOI: https://doi.org/10.1016/j.antiviral.2017.11.008
- Ruan P., Yang C., Su J., et al. Histopathological changes in the liver of tree shrew (Tupaia belangeri chinensis) persistently infected with hepatitis B virus. Virol. J. 2013;10(1):333. DOI: https://doi.org/10.1186/1743-422x-10-333
- Fan Y., Huang Z.Y., Cao C.C., et al. Genome of the Chinese tree shrew. Nat. Commun. 2013;4(1):1426. DOI: https://doi.org/10.1038/ncomms2416
- Walter E., Keist R., Niederöst B., et al. Hepatitis B virus infection of tupaia hepatocytes in vitro and in vivo: Hepatitis B virus infection of tupaia hepatocytes in vitro and in vivo. Hepatology. 1996;24(1):1–5. DOI: https://doi.org/10.1002/hep.510240101
- Winer B.Y., Ding Q., Gaska J.M., Ploss A. In vivo models of hepatitis B and C virus infection. FEBS Lett. 2016;590(13): 1987–99. DOI: https://doi.org/10.1002/1873-3468.12157
- Yan H., Zhong G., Xu G., et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. DOI: https://doi.org/10.7554/elife.00049
- Köck J., Nassal M., MacNelly S., et al. Efficient infection of primary Tupaia hepatocytes with purified human and woolly monkey hepatitis B virus. J. Virol. 2001;75(11):5084–9. DOI: https://doi.org/10.1128/jvi.75.11.5084-5089.2001
- Yang C., Ruan P., Ou C., et al. Chronic hepatitis B virus infection and occurrence of hepatocellular carcinoma in tree shrews (Tupaia belangeri chinensis). Virol. J. 2015;12(1):26. DOI: https://doi.org/10.1186/s12985-015-0256-x
- Bility M.T., Cheng L., Zhang Z., et al. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: Induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014;10(3):e1004032. DOI: https://doi.org/10.1371/journal.ppat.1004032
- Dusséaux M., Masse-Ranson G., Darche S., et al. Viral load affects the immune response to HBV in mice with humanized immune system and liver. Gastroenterology. 2017;153(6):1647–61.e9. DOI: https://doi.org/10.1053/j.gastro.2017.08.034
- Yuan L., Jiang J., Liu X., et al. HBV infection-induced liver cirrhosis development in dual-humanised mice with human bone mesenchymal stem cell transplantation. Gut. 2019;68(11):2044–56. DOI: https://doi.org/10.1136/gutjnl-2018-316091
- Asabe S., Wieland S.F., Chattopadhyay P.K., et al. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J. Virol. 2009;83(19):9652–62. DOI: https://doi.org/10.1128/jvi.00867-09
- Wieland S.F. The chimpanzee model for hepatitis B virus infection. Cold Spring Harb. Perspect. Med. 2015;5(6):a021469. DOI: https://doi.org/10.1101/cshperspect.a021469
- Gerety R.J., Tabor E., Purcell R.H., Tyeryar F.J. Summary of an international workshop on hepatitis B vaccines. J. Infect. Dis. 1979;140(4):642–8. DOI: https://doi.org/10.1093/infdis/140.4.642
- Payette P.J., Ma X., Weeratna R.D., et al. Testing of CpG-optimized protein and DNA vaccines against the hepatitis B virus in chimpanzees for immunogenicity and protection from challenge. Intervirology. 2006;49(3):144–51. DOI: https://doi.org/10.1159/000089375
- Shata M.T.M., Pfahler W., Brotman B., et al. Attempted therapeutic immunization in a chimpanzee chronic HBV carrier with a high viral load. J. Med. Primatol. 2006;35(3):165–71. DOI: https://doi.org/10.1111/j.1600-0684.2006.00152.x
- Kamili S., Sozzi V., Thompson G., et al. Efficacy of hepatitis B vaccine against antiviral drug-resistant hepatitis B virus mutants in the chimpanzee model. Hepatology. 2009;49(5):1483–91. DOI: https://doi.org/10.1002/hep.22796
- Guidotti L.G., Rochford R., Chung J., et al. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284(5415):825–9. DOI: https://doi.org/10.1126/science.284.5415.825
- Lanford R.E., Guerra B., Chavez D., et al. GS-9620, an oral agonist of toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144(7):1508–17. DOI: https://doi.org/10.1053/j.gastro.2013.02.003
- Gheit T., Sekkat S., Cova L., et al. Experimental transfection of Macaca sylvanus with cloned human hepatitis B virus. J. Gen. Virol. 2002;83(7):1645–9. DOI: https://doi.org/10.1099/0022-1317-83-7-1645
- Tham C.Y.L., Kah J., Tan A.T., et al. Hepatitis delta virus acts as an immunogenic adjuvant in hepatitis B virus-infected hepatocytes. Cell Rep. Med. 2020;1(4):100060. DOI: https://doi.org/10.1016/j.xcrm.2020.100060
- Netter H.J., Gerin J.L., Tennant B.C., Taylor J.M. Apparent helper-independent infection of woodchucks by hepatitis delta virus and subsequent rescue with woodchuck hepatitis virus. J. Virol. 1994;68(9):5344–50. DOI: https://doi.org/10.1128/jvi.68.9.5344-5350.1994
- Mercer D.F., Schiller D.E., Elliott J.F., et al. Hepatitis C virus replication in mice with chimeric human livers. Nat. Med. 2001;7(8):927–33. DOI: https://doi.org/10.1038/90968
- Amako Y., Tsukiyama-Kohara K., Katsume A., et al. Pathogenesis of hepatitis C virus infection in Tupaia belangeri. J. Virol. 2010;84(1):303–11. DOI: https://doi.org/10.1128/jvi.01448-09
- Sureau C. The role of the HBV envelope proteins in the HDV replication cycle. Curr. Top. Microbiol. Immunol. 2006;307: 113–31. DOI: https://doi.org/10.1007/3-540-29802-9_6
- Gerin J.L. Animal models of hepatitis delta virus infection and disease. ILAR J. 2001;42(2):103–6. DOI: https://doi.org/10.1093/ilar.42.2.103
- Porras-Ramírez A., Rico-Mendoza A. Coinfection of hepatitis B and C in HIV patients: а review of the state of the art. In: Hepatitis B and C. IntechOpen; 2020. DOI: https://doi.org/10.5772/intechopen.83704
- Washburn M.L., Bility M.T., Zhang L., et al. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 2011;140(4): 1334–44. DOI: https://doi.org/10.1053/j.gastro.2011.01.001
- Dagur R.S., Wang W., Makarov E., et al. Establishment of the dual humanized TK-NOG mouse model for HIV-associated liver pathogenesis. J. Vis. Exp. 2019;(151):10.3791/58645. DOI: https://doi.org/10.3791/58645
- Keng C.T., Sze C.W., Zheng D., et al. Characterisation of liver pathogenesis, human immune responses and drug testing in a humanised mouse model of HCV infection. Gut. 2016;65(10):1744–53. DOI: https://doi.org/10.1136/gutjnl-2014-307856
- Arora U., Garg P., Agarwal S., et al. Complexities in the treatment of coinfection with HIV, hepatitis B, hepatitis C, and tuberculosis. Lancet Infect. Dis. 2021;21(12):e399–406. DOI: https://doi.org/10.1016/s1473-3099(20)30765-9
- Mehra S., Alvarez X., Didier P.J., et al. Granuloma correlates of protection against tuberculosis and mechanisms of immune modulation by Mycobacterium tuberculosis. J. Infect. Dis. 2013; 207(7):1115–27. DOI: https://doi.org/10.1093/infdis/jis778
- North R.J. Mycobacterium tuberculosis is strikingly more virulent for mice when given via the respiratory than via the intravenous route. J. Infect. Dis. 1995;172(6):1550–3. DOI: https://doi.org/10.1093/infdis/172.6.1550
- Calderon V.E., Valbuena G., Goez Y., et al. A humanized mouse model of tuberculosis. PLoS One. 2013;8(5):e63331. DOI: https://doi.org/10.1371/journal.pone.0063331
- Zhan L., Ding H., Lin S., et al. Experimental Mycobacterium tuberculosis infection in the Chinese tree shrew. FEMS Microbiol. Lett. 2014;360(1):23–32. DOI: https://doi.org/10.1111/1574-6968.12524
- Quinet J., Jamard C., Burtin M., et al. Nucleic acid polymer REP 2139 and nucleos(T)ide analogues act synergistically against chronic hepadnaviral infection in vivo in Pekin ducks. Hepatology. 2018;67(6):2127–40. DOI: https://doi.org/10.1002/hep.29737
Supplementary files