Comparative evaluation of disinfectant efficacy against biofilm-residing microorganisms
- Authors: Fedorova L.S.1, Ilyakova A.V.1
-
Affiliations:
- Institute for Systems Biology and Medicine
- Issue: Vol 100, No 5 (2023)
- Pages: 302-309
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18476
- DOI: https://doi.org/10.36233/0372-9311-422
- EDN: https://elibrary.ru/uhrcap
- ID: 18476
Cite item
Abstract
Introduction. Bacteria in biofilms (BFs) have increased resistance to antibacterial agents, including disinfectants; however, the efficacy level varies depending on the chosen treatment. Therefore, evaluation of efficacy of main disinfectants against BF-residing microorganisms is of scientific and practical interest.
The purpose of the study was to explore the effect of disinfectants from various chemical groups on gram-positive and gram-negative bacteria residing in BFs.
Materials and methods. The effect of the following disinfectants has been evaluated: alkyldimethylbenzylammonium chloride (ADBAC), tertiary amine (TA), polyhexamethylene guanidine chloride (PHMG), hydrogen peroxide (HP), chloramine (CA), dichloroisocyanuric acid sodium salt (Na DCC), sodium hypochlorite (HC), ethyl alcohol (EA), glutaraldehyde (GA)) against Pseudomonas aeruginosa ATCC 15442 and Staphylococcus aureus ATCC 6538-P BFs. BFs were grown in 96-well plates at 37ºC for 24 hours and then exposed to biocide solutions. The efficacy of disinfectants was evaluated by the number of remaining viable cells and BF relative density.
Results. The analyzed bacterial strains formed moderate BFs; the average number of viable cells in BFs was 6.51 ± 0.19 lg. The viable bacterial cell counts in BFs reduced by more than 4 lg when exposed to HP solutions at a concentration of 6%, Na DCC solution — 0.1% (by active chlorine), HC — 1% (by active chlorine), CA – 1% (by product), PHMG — 0.05%, TA — 1.0 %. The BF density decreased by more than 70%. ADBAC solutions at concentrations of 0.1–1.0%, TA — 0.05%, HP — 3%, Na DCC solution — 0.05% (by active chlorine) caused a 2-lg reduction in viable cell counts in BFs. The efficacy of chlorine-active compounds and HP increased when 0.5% sulfonol was added. GA (0.25–1.00%) and EA (40–70%) solutions were ineffective against BF microorganisms.
Conclusion. A promising potential in combating microbial biofilms is demonstrated by disinfectants from the group of oxidizing agents (chlorine-active and oxygen-containing), TA and PHMG; using ADBAC as an individual compound is ineffective; aldehydes and alcohols are unable to destroy BFs and eliminate microorganisms in them.
Keywords
Full Text
Introduction
The ability of microorganisms to form biofilms on almost any surface is a critical problem in healthcare and other areas of human life [1–3]. Biofilms (BFs) are formed through bacterial adhesion to surfaces of objects present in the surrounding environment, being facilitated by high-humidity conditions [3]. BFs are composed of a continuous multilayer of bacterial cells attached to the surface and/or to each other, and embedded in an extracellular polymeric matrix consisting mainly of polysaccharides, proteins, and nucleic acids [3–5]. The matrix provides structural support to BF, stability and protection of BF-residing microbial cells against dehydration and other adverse environmental impacts [5–8]. Microorganisms make up approximately 10% of the BF dry mass, whereas the matrix can make up 90% [9].
Studies [10–13] have shown that bacteria in BFs are more resistant to disinfectants than their planktonic counterparts. Such resistance is mainly caused by the poor penetration of chemical compounds (disinfectants) through the extracellular matrix as well as by a lower metabolic activity and slower bacterial growth rates in BF [6, 8]. As a result, disinfection treatment guidelines developed for healthcare settings and employing planktonic cells are ineffective against BF-residing microorganisms, while no established methods used for evaluation of efficacy of disinfectants against BF microorganisms are currently available.
In the meantime, numerous studies have confirmed the presence of dry microbial BFs on various surfaces in healthcare settings [14–16]. Pathogenic microorganisms within BFs can survive on dry surfaces for extended periods and are periodically released as free-living planktonic cells into the environment. BFs, being a reservoir of pathogenic microorganisms, function as a source of dispersion of pathogenic bacteria in the hospital environment [17]. Studies [18–20] have confirmed the important role of microbial BFs, which are found on epidemiologically significant items in healthcare facilities, in the spread and transmission of nosocomial strains of microorganisms and occurrence of healthcare associated infections.
Currently, there are no comprehensive systematic studies on the effect of biocides on microorganisms in BFs and on the ability of the existing detergent formulations and disinfectants to destroy BFs.
Therefore, the aim of the study was to evaluate the antimicrobial activity and destructive effect of commonly used disinfectants — active chlorine and oxygen compounds, cationic surface-active agents (SAAs), alcohols, and aldehydes — against test microorganisms Pseudomonas aeruginosa ATCC 15442 and Staphylococcus aureus ATCC 6538-P living in BFs.
Materials and methods
Disinfectants based on cationic SAAs, alcohol, hydrogen peroxide, and chlorine-based agents are recommended for disinfection of surfaces in healthcare facilities. Chemical compounds selected for the study were the most typical representatives of active ingredients in disinfectant formulations:
- from the group of cationic SAAs —alkyldimethylbenzylammonium chloride (ADBAC) at concentrations of 0.1–1.0%, polyhexamethylene guanidine chloride (PHMG) — 0.02–0.05%, tertiary alkylamine (TA) – 0.1–1.0%;
- from the group of active oxygen — hydrogen peroxide (HP) at working solution concentrations of 3–6%;
- from the group of active chlorine — chloramine (CA) at working solution concentrations of 0.5–1.0% by product; dichloroisocyanuric acid sodium salt (DCCA) — 0.03–0.10% by active chlorine (AC), sodium hypochlorite (HC) — 0.5–1.0% by AC;
- from the group of alcohols – ethyl alcohol (EA) — 40–70%;
- from the group of aldehydes — glutaraldehyde (GA) – 0.25–1.00%.
In disinfection of surfaces, products based on chlorine and hydrogen peroxide are most often used with the addition of 0.5% detergent to provide additional detergent properties. Therefore, we evaluated the efficacy of chemical compounds of DCCA sodium salt, HC, and HP with the addition of 0.5% sulfonol, which is an anionic SAA.
Reference strains from the American Type Culture Collection (ATCC) — Pseudomonas aeruginosa ATCC 15442 and Staphylococcus aureus ATCC 6538-P were used as test microorganisms. These reference strains are used for evaluation of bactericidal activity against gram-negative and gram-positive bacteria in accordance with R 4.2.3676-20 – Methods of laboratory research and testing of disinfectants to assess their effectiveness and safety [21].
Bacterial BFs were grown under static conditions in 96-well flat-bottom polystyrene microtitration plates according to O'Toole et al. [22]. The overnight cultures of bacterial strains were adjusted to the 2.0 McFarland standard and diluted 1 : 100 in a tryptic soy broth. 100 μl of bacterial broth cultures were added to the wells of the 96-well flat-bottom plates, and the covered plates were incubated statically at 37ºC for 24 hours for BF formation. Then, the wells of the plates were washed three times with phosphate-buffered saline to remove planktonic cells. Then, 100 μl of detergents at different concentrations were added to the test wells, while the control wells were filled with normal saline solution (0.9% NaCl) and left for 30 min; then, 100 μl of a neutralizer containing Tween 80 (3%), saponin (0.3–3.0%), histidine (0.1%), cysteine (0.1%), sodium thiosulfate (0.1%) were added.
The efficacy of disinfectants against the formed BFs was evaluated by measuring the relative optical density of the crystal violet-stained BF matrix. For this purpose, 200 μl of 0.1% aqueous solution of crystal violet was added to the wells and left for 30 min. The BF biomass was measured by the level of extraction of the dye with ethanol, which was measured with a TECAN DESKTOP-4A7U9AL\Infinite M Plex microplate reader at the wavelength of 580 nm in optical density (OD) units.
To assess the viability of cells in BF, 100 μl of phosphate-buffered saline was added to the wells and treated with ultrasound at 37 kHz for 1 min in the Elma Ultrasonic 30S bath (Elma). The cell viability was assessed by the number of colony forming units (CFUs) after plating onto solid growth medium (tryptone soy agar) and by counting CFU/ml after the incubation at 37ºC for 24 hours.
The reliability of results was achieved by using 6 wells per 1 test sample and by calculating the average optical density of the test sample and the decimal logarithm (lg) for CFUs. Then, the average optical density of BF eluate and CFU/ml treated with disinfectants were compared with the untreated control samples. Comparisons were made using Student's t-test [23]. The obtained results were processed using the MS Excel statistical software package. The p < 0.05 value was considered statistically significant.
Disinfectants were considered effective against test microorganisms in BFs, if viable cell counts in BFs decreased more than 400 times after the exposure to disinfectants. If the optical density after exposure decreased by more than 70% compared to the control samples, the disinfectants were considered highly effective, while the decrease by 30-70% implied that they had moderate efficacy, and the decrease by less than 30% qualified them as low-effective.
Results
The tested cultures of microorganisms P. aeruginosa ATCC 15442 and S. aureus ATCC 6538-P formed BFs with a density of OD580 = 1.50 ± 0.19 after 24 hours. The average number of viable cells in BFs was 6.2 ± 0.7 lg.
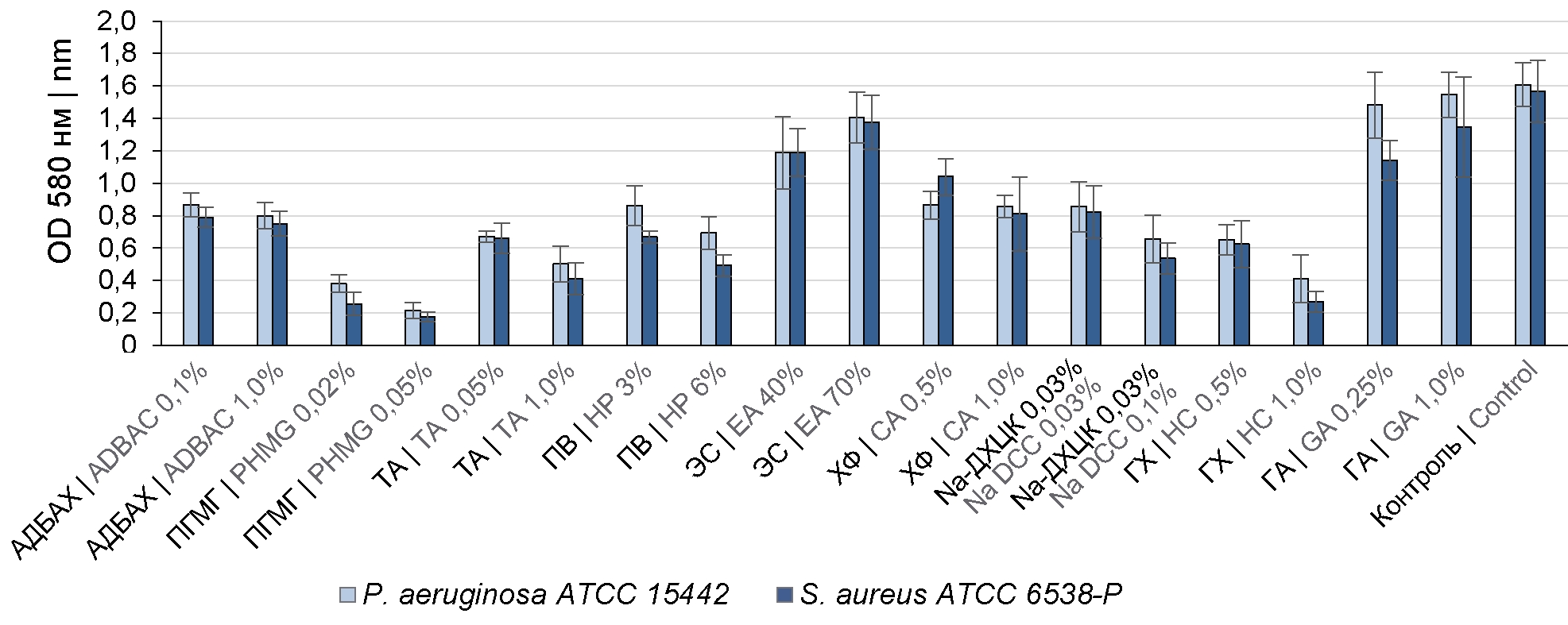
The treatment of the formed BFs with 0.1% and 1.0% ADBAC solutions decreased the BF density compared to the controls (primary BF) by 48.6 ± 9.5 and 51.2 ± 8.1% (Fig. 1) and reduced the number of viable cells in BFs by 2 and 3 lg (Fig. 2), respectively.
Fig. 1. Comparative assessment of the effect of disinfectants on the density of the formed BFs of P. aeruginosa ATCC 15442 and S. aureus ATCC 6538-P.
Fig. 2. Comparative assessment of the effect of disinfectants on the viability of P. aeruginosa ATCC 15442 and S. aureus ATCC 6538-P cells in BFs.
The effect of TA solutions on BFs demonstrated a direct relationship between the increased concentration of disinfectants and the destruction of the BF matrix. After the formed BFs had been treated with 0.1% and 1.0% TA solutions, the BF density decreased by 58.2 ± 5.5 and 72.5 ± 8.2%, and the number of viable cells decreased by 3 and 4 lg, respectively.
The treatment of P. aeruginosa ATCC 15442 and S. aureus ATCC 6538-P BFs with 0.02% and 0.05% PHMG solutions resulted in a significant decrease in the BF density – by 78.1 ± 9.3 and 87.5 ± 5.9% and in a reduction in the number of viable cells by more than 5 lg.
The treatment with GA solutions at concentrations of 0.25 and 1.0% caused a slight decrease in the BF density – by 24.66 ± 9.6 and 10.75 ± 4.5% and an insignificant reduction in the number of microorganisms.
Ethyl alcohol at concentrations of 40% and 70% also had a weak destructive effect on BFs. The increase in concentrations of ethyl alcohol led to a decrease of its efficacy. The treatment with 40% ethyl alcohol decreased the BF density by 20–25%, while the treatment with 70% ethyl alcohol resulted in a 10-12.5% decrease (Fig. 1).
HP working solutions at a concentration of 3% decreased the BF density of P. aeruginosa ATCC 15442 and S. aureus ATCC 6538-P by 46.55 ± 9.45 and 54.45 ± 7.5%, causing a reduction in the number of viable cells by 2.8 and 3 lg, respectively (Fig. 1, 2). The increase in HP concentration to 6% resulted in increased efficacy and reduced the number of viable cells by 4.0–4.5 lg.
The treatment of BFs with active chlorine compounds demonstrated that efficacy depends on the disinfectant concentration. The best results were achieved by using 1.0% chloramine solution (by product), 0.1% DCCA sodium salt solution (by AC), and 1.0% sodium hypochlorite solution (by AC) compared to lower concentrations of these disinfectants (Fig. 1, 2).
The treatment with working solutions of 3% HP, 0.03% DCCA (by AC), and 0.5% HC (by AC) supplemented with 0.5% sulfonol detergent increased the antimicrobial effect on BF microorganisms by 40-52% (Fig. 3).
Fig. 3. Comparative assessment of the effect of disinfectants supplemented with the detergent on the BF density (a) and viability (b) of P. aeruginosa ATCC 15442 and S. aureus ATCC 6538-P
Discussion
The effect of disinfectant solutions on BFs is not completely understood. The action of disinfectants is usually limited to the interaction with the surface layer of BFs, as the matrix prevents molecules of disinfectants from unrestricted diffusion into the deeper layers. There is also evidence that some disinfectants can cause the opposite effect, leading to BF growth. Such growth promotion is associated with their destruction of surface structures, thus facilitating the inflow of nutrients into BFs [10, 11].
The efficacy of commercial disinfectants has been studied only in relation to planktonic forms of microorganisms, while the data on their biocidal activity against microorganisms growing within BFs are not available. Although multiple studies address the combating strategies against BFs, the disinfectants that could specifically inhibit BF formation and kill bacteria inside BFs, causing their degradation and destroying the matrix have not been found so far. The systemic study of the main disinfectants against gram-negative and gram-positive microorganisms in BFs has been conducted for the first time. The study showed that HP at a concentration of 6%, DCCA sodium salt solution at a concentration of 0.1% (by AC), sodium hypochlorite at a concentration of 1.0% (by AC), chloramine at a concentration of 1.0% (by product), PHMG at a concentration of 0.05%, and TA at a concentration of 1.0% decrease the number of microorganisms in BFs by 4 lg and more, while the BF density is decreased by more than 70%. The need to use biocides from the group of oxidizing agents at higher concentrations is most likely associated with their partial inactivation by biopolymers in BF surface layers. The BF matrix restricts the diffusion through the BF surface to deeper layers and neutralizes some disinfectants, providing the resistance of the microbial BF to the action of these compounds. Since detergents are added to active chlorine compounds to enhance their detergent properties, the effects of solutions of these compounds were studied using 0.5% sulfonol added to them. It was found that the efficacy of active chlorine compounds and HP was increased, and, most likely, this increase is associated with the increased permeability of the BF matrix due to anionic SAA — sulfonol facilitating the diffusion of disinfectants into BFs due to a reduction in the solution surface tension and, consequently, the improved wetting of the BF surface.
ADBAC demonstrated the lowest efficacy against BF bacteria among cationic SAAs, as negatively charged polysaccharides in BFs can bind positively charged ADBAC molecules, thus protecting BFs against destruction [24]. On the other hand, some studies demonstrate the possibility of using quaternary ammonium compounds for combating microbial BFs, if their effect is enhanced by synergists or enzymes in composite agents [25].
TA demonstrated good efficacy at a concentration of 1%. The TA distinctive feature is the combination of antimicrobial and detergent properties. The presence of free amino groups and the tertiary nitrogen atom generate an alkaline medium, thus contributing to improved antimicrobial activity [25].
PHMG demonstrated bactericidal benefits compared to ADBAC and TA. The significantly decreased BF density and pronounced antimicrobial effect prove that the integrity of the BF structure was significantly impaired due to the destruction of the polysaccharide matrix and the impact on bacteria. The mechanism of this effect has not been uncovered and requires further research.
Glutaraldehyde (0.25–1.00%) and ethyl alcohol (40–70%) solutions had a poor effect on BF destruction and elimination of microorganisms in BFs. Studies [26] have demonstrated that GA reacts with BF amino groups, cross-linking long molecules of biopolymers, thus causing swelling or coagulation of the BF surface layers and preventing further GA diffusion into deeper layers of microbial BFs. Ethyl alcohol owes its low efficacy to a fixing effect and poor penetration of alcohol molecules into protein-rich tissues [27]. Higher alcohol concentrations caused an increase in the BF density.
Conclusion
In combating microbial BFs, good prospects are demonstrated by disinfectants from the group of oxidizing agents (active chlorine and oxygen-containing), TA and PHMG; quaternary ammonium compounds are not effective if used individually; aldehydes and alcohols are of no use for BF destruction and elimination of BF-residing microorganisms. The method used for evaluation of efficacy of biocides can be recommended for studies and assessment of the disinfectant activity of agents for combating microbial BFs at the stage of primary selection.
About the authors
Lyudmila S. Fedorova
Institute for Systems Biology and Medicine
Author for correspondence.
Email: fedorova-ls@yandex.ru
ORCID iD: 0000-0003-2663-0273
D. Sci. (Med.), Professor, Head, Laboratory of overcoming microbial resistance, Institute for Systems Biology and Medicine
Russian Federation, MoscowAnastasia V. Ilyakova
Institute for Systems Biology and Medicine
Email: fedorova-ls@yandex.ru
ORCID iD: 0000-0002-1867-3495
Researcher, Laboratory of overcoming microbial resistance, Institute for Systems Biology and Medicine
Russian Federation, MoscowReferences
- Algburi A., Comito N., Kashtanov D., et al. Control of biofilm formation: antibiotics and beyond. Appl. Environ. Microbiol. 2017;83(3):e02508–16. DOI: https://doi.org/10.1128/aem.02508-16
- Charron R., Boulanger M., Briandet R., Bridier A. Biofilms as protective cocoons against biocides: from bacterial adaptation to One Health issues. Microbiology (Reading). 2023;169(6): 001340. DOI: https://doi.org/10.1099/mic.0.001340
- Vieira-da-Silva B., Castanho M.A.R.B. The structure and matrix dynamics of bacterial biofilms as revealed by antimicrobial peptides’ diffusion. J. Pept. Sci. 2023;29(6):e3470. DOI: https://doi.org/10.1002/psc.3470
- Тутельян А.В., Юшина Ю.К., Соколова О.В. и др. Образование биологических плёнок микроорганизмов на пищевых производствах. Вопросы питания. 2019;88(3)32–43. Tutelyan A.V., Yushina Yu.K., Sokolova O.V., et al. Formation of biological films by microororganisms in food productions. Problems of Nutrition. 2019;88(3)32–43. DOI: https://doi.org/10.24411/0042-8833-2019-10027 EDN: https://elibrary.ru/tredji
- Hobley L., Harkins C., MacPhee C.E., Stanley-Wall N.R. Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiol. Rev. 2015;39(5):649–69. DOI: https://doi.org/10.1093/femsre/fuv015
- Akinbobola A.B., Sherrya L., Mckay W.G., et al. Tolerance of Pseudomonas aeruginosa in in-vitro biofilms to high-level peracetic acid disinfection. J. Hosp. Infect. 2017;97(2):162–8. DOI: https://doi.org/10.1016/j.jhin.2017.06.024
- Donlan R.M., Costerton J.W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002;15(2):167–93. DOI: https://doi.org/10.1128/CMR.15.2.167-193.2002
- Hung C., Zhou Y., Pinkner J.S., et al. Escherichia coli biofilms have an organized and complex extracellular matrix structure. mBio. 2013;4(5):e00645-13. DOI: https://doi.org/10.1128/mBio.00645-13
- Günther F., Scherrer M., Kaiser S.J., et al. Comparative testing of disinfectant efficacy on planktonic bacteria and bacterial biofilms using a new assay based on kinetic analysis of metabolic activity. J. Appl. Microbiol. 2017;122(3):625–33. DOI: https://doi.org/10.1111/jam.13358
- Cochran W.L., McFeters G.A., Stewart P.S. Reduced susceptibility of thin Pseudomonas aeruginosa biofilms to hydrogen peroxide and monochloramine. J. Appl. Microbiol. 2000;88(1): 22–30. DOI: https://doi.org/10.1046/j.1365-2672.2000.00825.x
- Lineback C.B., Nkemngong C.A., Wu S.T., et al. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob. Resist. Infect. Control. 2018;7:154. DOI: https://doi.org/10.1186/s13756-018-0447-5
- Bae Y.M., Baek S.Y., Lee S.Y. Resistance of pathogenic bacteria on the surface of stainless steel depending on attachment form and efficacy of chemical sanitizers. Int. J. Food Microbiol. 2012;153(3):465–73. DOI: https://doi.org/10.1016/j.ijfoodmicro.2011.12.017
- Chowdhury D., Tahir S., Legge M., et al. Transfer of dry surface biofilm in the healthcare environment: the role of healthcare workers’ hands as vehicles. J. Hosp. Infect. 2018;100(3):e85–e90. DOI: https://doi.org/10.1016/j.jhin.2018.06.021
- Johani K., Abualsaud D., Costa D.M., et al. Characterization of microbial community composition, antimicrobial resistance and biofilm on intensive care surfaces. J. Infect. Public Health. 2017;11(3):418–24. DOI: https://doi.org/10.1016/j.jiph.2017.10.005
- Hu H., Johani K., Gosbell I.B., et al. Intensive care unit environmental surfaces are contaminated by multidrug-resistant bacteria in biofilms: combined results of conventional culture, pyrosequencing, scanning electron microscopy, and confocal laser microscopy. J. Hosp. Infect. 2015;91(1):35–44. DOI: https://doi.org/10.1016/j.jhin.2015.05.016
- Ledwoch K., Vickery K., Maillard J.Y. Dry surface biofilms: what you need to know. Br. J. Hosp. Med. (Lond). 2022;83(8): 1–3. DOI: https://doi.org/10.12968/hmed.2022.0274
- Otter J.A., Vickery K., Walker J.T., et al. Surface-attached cells, biofilms and biocide susceptibility: implications for hospital cleaning and disinfection. J. Hosp. Infect. 2015;89(1):16–27. DOI: https://doi.org/10.1016/j.jhin.2014.09.008
- Ledwoch K., Dancer S.J., Otter J.A., et al. Beware biofilm! Dry biofilms containing bacterial pathogens on multiple healthcare surfaces; a multi-centre study. J. Hosp. Infect. 2018;100(3):e47–e56. DOI: https://doi.org/10.1016/j.jhin.2018.06.028
- Costa D.M., Johani K., Melo D.S., et al. Biofilm contamination of high-touched surfaces in intensive care units: epidemiology and potential impacts. Lett. Appl. Microbiol. 2019;68(4):269–76. DOI: https://doi.org/10.1111/lam.13127
- Руководство Р 4. 2. 3 6 7 6- 2 0. Методы лабораторных исследований и испытаний дезинфекционных средств для оценки их эффективности и безопасности. М.; 2021.
- O’Toolе G.A., Kaplan H.B., Kolter R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54:49–79. DOI: https://doi.org/10.1146/annurev.micro.54.1.49
- Платонов А.Е. Статистический анализ в медицине и биологии: задачи, терминология, логика, компьютерные методы. М.; 2001. Platonov A.Ye. Statistical Analysis in Medicine and Biology: Tasks, Terminology, Logic, Computer Methods. Moscow; 2001. EDN: https://elibrary.ru/pbdijn
- Campanac C., Pineau L., Payard A., et al. Interaction between biocide cationic agents and bacterial biofilm. Antimicrob. Agents Chemother. 2002;46(5):1469–74. DOI: https://doi.org/10.1128/aac.46.5.1469-1474.2002
- Диденко Л.В., Смирнова Т.А., Толордава Э.Р. и др. Влияние третичных алкиламинов на биопленки, образованные Escherichia coli и Staphylococcus aureus (бактериологическое и электронно-микроскопическое исследование). Дезинфекционное дело. 2014;(2): 40–5. Didenko L.V., Smirnova T.A., Tolordava E.R., et al. Influence of tertiary alkylamines to biofilms, which were created by Escherichia coli and Staphylococcus aureus (bacteriological and electron microscopical investigation). Disinfection Affairs. 2014;(2):40–5. EDN: https://elibrary.ru/sgfmhl
- Simons C., Walsh S.E., Maillard J.Y., Russel A.D. A note: ortho-phthalaldehyde: proposed mechanism of action of a new antimicrobial agent. Lett. Appl. Microbiol. 2000;31(4):299–302. DOI: https://doi.org/10.1046/j.1472-765x.2000.00817.x
- Silveira M.G., Baumgartner M., Rombouts F.M., Abee T. Effect of adaptation to ethanol on cytoplasmic and membrane protein profiles of Oenococcus oeni. Appl. Environ. Microbiol. 2004;70(5):2748–55. DOI: https://doi.org/10.1128/aem.70.5.2748-2755.2004
Supplementary files