A study of the safety and immunogenicity of a new vaccine for the prevention of COVID-19 based on virus-like particles in phase I clinical trials
- Authors: Grebennikova T.V.1, Zaykova O.N.1, Plotnikov A.A.1, Kostina L.V.1, Chernoryzh Y.Y.1, Eliseeva O.V.1, Latyshev O.E.1, Larichev V.F.1, Fedyakina I.T.1, Losich M.A.1, Kirillov I.M.1, Filatov I.E.1, Balandina M.V.1, Tsibezov V.V.1, Yurlov K.I.1, Lesnova E.I.1, Kondratieva V.M.1, Kozlova A.A.1, Baranets M.S.1, Gintsburg A.L.1
-
Affiliations:
- National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
- Issue: Vol 102, No 2 (2025)
- Pages: 135-149
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18822
- DOI: https://doi.org/10.36233/0372-9311-661
- EDN: https://elibrary.ru/LKOJHI
- ID: 18822
Cite item
Abstract
Introduction. One of the more promising developments in preventing the spread of infections, including COVID-19, is the production of vaccines based on virus-like particles (VLP). Currently, in the National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya of the Ministry of Health of the Russia has developed a VLP-based vaccine against COVID-19.
The aim of this study is to evaluate the tolerability, safety and immunogenicity of a new vaccine for the prevention of COVID–19 based on VLP compared with placebo for 21 days after two intramuscular injections in phase I clinical trials.
Materials and methods. A double-blind, placebo-controlled study of the tolerability, safety and immunogenicity of a vaccine for the prevention of COVID-19 based on VLP was conducted with a dose of the drug containing 40 and 80 micrograms of antigen, the placebo being 0.9% NaCl. The presence or absence of adverse events (AEs) after vaccination was noted in 180 volunteers aged 18 to 55 years; clinical and biochemical blood parameters, the intensity of humoral and cellular immunity before and after vaccination were assessed using enzyme immunoassay, neutralization reactions, lymphocyte blast transformation reactions and flow cytometry.
Results. An analysis of the tolerability and safety of the new COVID-19 VLP-vaccine showed that most adverse events were registered within the first 10 days after vaccination, mainly after the first vaccination. In the period from 11 to 21 days after vaccination, AEs were observed in isolated cases. No deaths, serious or other AEs have been reported. The administration of the studied vaccine to the volunteers had no negative effect on the basic vital signs. A comparative analysis of immunogenicity indicators in volunteers showed that the administration of a vaccine with both an antigen content of 40 µg and an antigen content of 80 µg leads to a pronounced and significant increase in the level of specific immunoglobulins, virus neutralizing antibodies and activation of a cell-mediated immune response. As part of the phase I clinical trials, a dose of 80 µg was selected as optimal.
Conclusion. It has been shown that a new vaccine for the prevention of COVID-19 based on VLP with an antigen content of 40 and 80 µg when administered intramuscularly to volunteers does not cause serious adverse events and induces a tense humoral and cellular immune response.
Keywords
Full Text
Introduction
The pandemic of a new coronavirus infection has posed serious challenges to public health services worldwide in the prevention, treatment and diagnosis of this disease. COVID-19, a severe acute respiratory infection caused by the SARS-CoV-2 virus (Coronaviridae, Orthocoronavirinae, Betacoronavirus, Sarbecovirus), is characterized by a high mortality rate, which, according to different studies, ranges from 0.5 to 15% [1–3].
The first cases of disease caused by SARS-CoV-2 virus were registered in December 2019 in China. The virus spread quite rapidly to all continents, and according to the World Health Organization (WHO), as of December 2021, there were more than 260 million confirmed cases of COVID-19 worldwide, including 5.2 million deaths1.
The most severe manifestations of coronavirus infection are interstitial pneumonia with impaired respiratory function and multi-organ failure, which were often fatal [4–6]. The duration of post-infection immunity is not fully understood [7].
According to Rospotrebnadzor, as of May 12, 2024, about 785 million cases have been detected worldwide, the most unfavorable region being the Western Pacific. In Russia from 02.03.2020 to 05.05.2024 about 24 million cases were registered in 85 subjects2. Despite WHO statements that COVID-19 has moved into the category of seasonal infections and periodically causes outbreaks of the disease along with influenza and acute respiratory viral diseases, SARS-CoV-2 virus continues to infect people and claim their lives3.
The variability of SARS-CoV-2, namely mutations in the receptor binding domain (RBD) of the S-protein, has led to the emergence of a variety of virus variants worldwide, of which Alpha (lineage B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2) and Omicron (B.1.1.529) are the most epidemically significant and of current concern to WHO [8]. The emergence of new variants of the virus, including those with reduced sensitivity to virus-neutralizing antibodies and vaccination with already available vaccines, requires regular molecular genetic monitoring of SARS-CoV-2 and the development of new highly effective vaccines that promote the formation of intense and long-lasting immunity against topical strains of the causative agent of coronavirus infection [8].
An effective vaccine against COVID-19 should be safe, non-reactogenic, and induce the formation of virus-neutralizing antibodies in titers sufficient to prevent the development of the infectious process. Furthermore, the vaccine should promote an effective immune response with the least amount of antigen used. This reduces the cost of the vaccine and makes it affordable [9].
A vaccine based on virus like particles (VLP) for COVID-19 prophylaxis has been developed in the Gamaleya Research Center for Ecological Medicine. VLPs are formed from 4 recombinant structural proteins (S, M, E, N) and are similar in structure to SARS-CoV-2 virion, but without viral RNA. The surface S-protein (S-spike) of SARS-CoV-2 is responsible for binding to specific receptors on the surface of susceptible cells. Particles containing S-protein with consensus mutations of clades 19A, Delta and Omicron are used in the vaccine formulation. Thus, it is expected that antibodies to strains of these clades will be synthesized in the body after immunization.
The structural spike and membrane proteins undergo significant mutational changes, whereas the envelope and nucleocapsid proteins are highly conserved, indicating differential selection pressure to which SARS-CoV-2 has been subjected during evolution. At the same time, the contribution of viral M, E, and N proteins to the formation of B- and T-cell immunity is equally important, which is confirmed by the development of vaccines based on these proteins [10, 11]. It was shown that the vaccine under development stimulates the T-cell immune response in golden hamsters after the 1st immunization, which also provides protection against various SARS-CoV-2 strains [12].
Data on preclinical trials of the drug were submitted to the Ministry of Health of the Russian Federation. Permission to conduct clinical trials was obtained from 16.02.2022 No. 115.
The aim of this study was to evaluate the tolerability, safety and immunogenicity of a new VLP-based vaccine for COVID-19 prophylaxis compared to placebo for 21 days after double intramuscular administration within the framework of phase I clinical trials.
Study objectives:
- To consistently evaluate the tolerability and safety of vaccine containing 40 and 80 µg antigen doses administered intramuscularly on the 10th day after single vaccination.
- To evaluate the tolerability and safety of vaccine containing 40 and 80 µg antigen doses administered intramuscularly on the 21st day after double vaccination.
- To evaluate the immunogenicity of the vaccine containing 40 and 80 µg antigen doses administered intramuscularly on the 21st day after double vaccination.
- To determine the optimal vaccine dosage based on the immunogenicity and safety parameters obtained for the phase II clinical trial.
Materials and methods
Double-blind randomized placebo-controlled multicenter prospective study of Phase I clinical trials with dose-ranging to assess tolerability, safety and immunogenicity of the vaccine for COVID-19 prophylaxis based on VLP (containing particles similar to SARS-CoV-2) when administered intramuscularly to volunteers aged 18–55 years was conducted from February to December 2022 on the basis of two research centers: Elektrostal Central City Hospital and N.P. Bekhtereva Institute of the Human Brain of RAS in accordance with the principles of the Declaration of Helsinki (2013), the ICH guidelines on overdose, ICH Guidelines for Good Clinical Practice (version E6, approved by SRMR/135/95), Federal Law No. 61-FL “On Circulation of Medicines” dated 12.04.2010; Order of the Ministry of Health of the Russian Federation No. 200n “On Approval of the Rules of Good Clinical Practice” dated 01.04.2016; National Standard of the Russian Federation GOST R 52379-2005 “Good Clinical Practice” approved by Order of the Federal Agency for Technical Regulation and Metrology No. 497-st. from 04.06.2014, the Rules of Good Clinical Practice of the Eurasian Economic Union” approved by the Decision of the Council of the Eurasian Economic Commission from 03.11.2016 № 79 and other applicable requirements of national legislation.
Prior to inclusion in the study, volunteers were familiarized with information about the study and signed an informed consent form. Researchers recruited to participate in the clinical trial provided signed and dated resumes describing their research activities and certificates confirming their qualifications before the start of the study.
A total of 180 male and female volunteers aged 18–55 years were included in the study. All volunteers were healthy, met the inclusion criteria (Appendix 1 on the journal's website: https://www.microbiol.crie.ru) and were divided into 3 groups:
- Group 1 — 60 volunteers who were immunized with vaccine containing 40 µg antigen twice at an interval of 21 days intramuscularly;
- Group 2 — 60 volunteers who were vaccinated with 80 µg antigen vaccine twice at an interval of 21 days intramuscularly;
- Group 3 — 60 volunteers who received placebo vaccine twice at an interval of 21 days intramuscularly.
The vaccine was purified recombinant SARS-CoV-2 virus-like particles synthesized in a baculovirus expression system. The S surface protein within the virus-like particles was represented by variants 19A, Alpha, Delta, and Omicron. The vaccine composition included a squalene-based adjuvant. The volume ratio of adjuvant to antigen was 1 : 1. Vaccine with 40 and 80 µg antigen content was investigated. A 0.9% NaCl solution was used as placebo.
Volunteers were examined at visits:
- visit 1 (hospitalization, randomization, the 1st vaccination);
- visits 2, 3 (2nd–3rd days after the 1st vaccination);
- visit 4 (10th day after the 1st vaccination);
- visit 5 (21st day after the 1st vaccination, hospitalization, 2nd vaccination);
- visits 6, 7 (2nd–3rd days after the 2nd vaccination);
- visit 8 (10th day after the 2nd vaccination);
- visit 9 (21st day after the 2nd vaccination).
We analyzed data from the electronic self-monitoring diary, performed physical examination, assessed vital signs, collected data on concomitant therapy, identified and recorded adverse events (AEs) and serious AEs, and evaluated inclusion/non-inclusion criteria. Blood was drawn to assess cellular and humoral immunity. Biochemical, clinical tests, determination of total IgE, coagulogram, and urinalysis were performed. During visits 4, 5, 8, 9, nasopharyngeal swabs were taken to determine the absence of SARS-CoV-2 virus RNA by polymerase chain reaction (PCR) method.
Lymphocyte Blast Transformation Reaction (LBTR). Blood was collected on the 10th day after the 1st and 2nd vaccinations (visits 4 and 8). Mononuclear cell fractions from peripheral blood were isolated by centrifugation on a one-step density gradient of Ficoll-Pak (PanEco), the isolated mononuclear cells were washed twice in pure RPMI-1640 medium and seeded in 96-well microculture plates at a concentration of 105 cells/well and stimulants were added in 100 μl to final concentrations. We used the medium separately (spontaneous proliferation) as a negative control; concanavalin A mitogen (5 μg/mL, PanEco) as a non-specific positive control; SARS-CoV-2 virus: PMVL-12, deposition number EPI_ISL_572398 in the Gisaid database as a specific stimulant; Congo-Crimean hemorrhagic fever antigen as a nonspecific positive control; and Seppic adjuvant as a non-specific stimulant. Cells were cultured in RPMI-1640 medium containing 20% fetal calf serum, 2 mM glutamine, 4.5 g/L glucose, 50 μg/mL gentamicin, and 0.2 units/mL insulin at 37°C in a 5% CO2 atmosphere. All of the mentioned processes were performed under sterile conditions.
Splenocyte proliferation was assessed by LBTR after 4 days using an inverted microscope (× 400). The results were expressed as proliferation stimulation index (PSI), calculated as the ratio of the mean number of lymphoblasts observed in the presence and absence of specific stimulants. A result was considered positive if the PSI > 2.
The obtained data were processed using the Prizm Graphpad v. 8.4.3 program (GraphPad Software). Statistical analysis was performed using the Statistica v. 12.6 program (StatSoft Inc.). Reliability of differences was evaluated by Student's t-criterion. A value of p < 0.05 was considered statistically significant.
Cell staining for flow cytometry. 1 million lymphocytes in a volume of 50 μl were transferred into centrifuge tubes and 5 μl each of anti-CD3, anti-CD4 and anti-CD8 monoclonal antibodies (Sorbent) were added and incubated for 45 min at 4°C. Washed twice in Hanks' solution (5 min at 200g). The supernatant was removed and cells were suspended in 200 μl of Hanks' solution and analyzed on a BD FACS Accuri C6+ flow cytometer. The obtained data were processed using the Cytoflex and Prizm Graphpad 8.0 programs. The results were analyzed using the FlowJo program (Three Star).
Humoral immunity was assessed by enzyme-linked immunosorbent assay (ELISA) and neutralization reaction (NR). Blood was collected on the 10th day after the 1st and 2nd vaccination (visits 5 and 9). ELISA was performed using a reagent kit for immunoenzymatic detection of IgG to RBD of the S surface glycoprotein S of SARS-CoV-2 coronavirus (SARS-CoV-2-RBD-ELA-Gamalei, RU No. RZN 2020/10393).
The levels of neutralizing antibodies were determined by titrating sera from 1 : 10 to 1 : 1280 against 100 TCID50 of three SARS-CoV-2 strains from the Molecular Diagnostics Laboratory collection: Wuhan, Delta (line B.1.617.2) and Omicron (variant XBB 1.5). Neutralization reactions were performed by micromethod in 96-well plates on Vero E6 green monkey kidney cell culture. Serum dilutions were incubated with viruses for 1 h at 37°C in an atmosphere of 5% CO2 and transferred to a plate with a cell monolayer. After 72 h, the reaction was counted by the presence of cytopathic action of the virus. The serum titer (the last neutralizing dilution) was considered to be the dilution at which 100% cell protection (no cytopathic effect) was ensured.
Cytokine concentrations in sera were measured by ELISA using commercial test systems (Vector-Best): Interleukin-2-ELISA-BEST “Reagent kit for immunoenzymatic determination of interleukin-2 concentration in serum”; Gamma-Interferon-ELISA-BEST “Reagent kit for immunoenzymatic determination of gamma-interferon concentration in serum”; Alpha-TNF-ELISA-BEST “Reagent kit for immunoenzymatic determination of tumor necrosis factor-alpha concentration in serum”.
Statistical processing of data was performed using Microsoft Office Excel 2007-2016 and online statistical calculators (https://math.semestr.ru, https://medstatistic.ru). Mean, standard deviations, quartiles, minimum and maximum values, as well as frequencies, depending on the nature of the data, were used as descriptive characteristics of demographic and other baseline data parameters, as well as safety parameters and immunogenicity parameters.
To analyze quantitative indicators over time in each group, repeated-measures analysis of variance or Friedman analysis was used, depending on the nature of the data distribution. For a posteriori comparisons of values at screening and follow-up visits, Dunnett's criterion was used in the case of analysis of variance and Dunn's criterion with Bonferroni correction in the case of Friedman analysis.
Comparisons of groups with each other on qualitative features were performed using the χ2 criterion or Fisher's exact test. Geometric mean titers (GMT) for each study group with 95% confidence intervals (CI) were estimated for each time point. Logarithmic transformation was applied to the raw data to analyze titers. For comparisons of the study groups with each other, analysis of variance (Bonferroni or Gates–Howell posterior comparisons if the variances were not equal) or Kraskell–Wallis analysis (Dunn posterior comparisons with Bonferroni correction) was applied to the logarithmic data, depending on the nature of the distribution. To assess the type of distribution, the Kolmogorov–Smirnov criterion was used, as well as the asymmetry and kurtosis indices.
Levene's criterion was used to test the homogeneity of dispersions, and Spearman's correlation was used to assess the relationship between neutralizing and specific antibody titers. 95% CI was calculated for the correlation coefficient. The frequencies with 95% CI calculated by the Clopper–Pearson method are given as descriptive characteristics for the efficacy parameters.
Results
In a safety and immunogenicity study of the novel VLP-based COVID-19 prophylaxis vaccine in a phase I clinical trial, screened volunteers (n = 180; 107 males and 73 females) were categorized into 3 groups, with 84% completing the study (n = 151: 52 in group 1, 53 in group 2, and 46 in group 3). Demographic data are summarized in Table 1.
Table 1. Patient demographics, M ± SD
Groups | n | Age, years | Height, cm | Body mass, kg | Body mass index, kg/m2 |
All study subjects | 180 | 29.91 ± 10.36 | 171.82 ± 8.21 | 68.02 ± 10.58 | 22.94 ± 2.50 |
Group 1 | 60 | 31.62 ± 12.00 | 172.18 ± 8.50 | 67.94 ± 9.76 | 22.84 ± 2.22 |
Group 2 | 60 | 26.58 ± 7.74 | 172.05 ± 8.82 | 66.97 ± 11.33 | 22.49 ± 2.55 |
Group 3 | 60 | 31.53 ± 10.25 | 171.22 ± 7.32 | 69.16 ± 10.66 | 23.49 ± 2.65 |
Evaluation of vaccine tolerability and safety
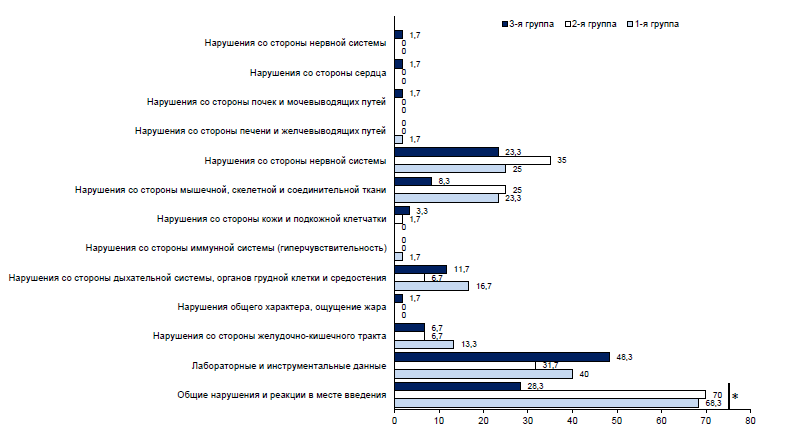
In the Appendix 2 on the journal's website: https://www.microbiol.crie.ru, all AEs by groups are presented. During the entire period of post-vaccination follow-up within 21 days after twice-daily vaccination, 572 AEs were detected in 138 (76.7%) volunteers, including 216 AEs in 47 (78.3%) volunteers in Group 1, 226 AEs in 49 (81.7%) volunteers in Group 2, and 130 AEs in 42 (70%) volunteers in Group 3 (Fig. 1). The same volunteer could experience several different reactions simultaneously.
Fig. 1. Proportion of volunteers with AEs by organ system class. * — statistically significant differences.
In 50.5% of cases, the most frequently reported general abnormalities and reactions at the injection site (in 37.7% — pain at the vaccination site and in 22.5% — fatigue), which in 95% of cases were observed in the first 10 days. Also, in 20.5% of cases, clinically significant abnormalities in laboratory tests were observed equally on both the 10th and 21st day after administration of the study drug, irrespective of vaccination. In 64.5% of cases, the registered AEs had a mild degree of severity, in 31.1% — moderate, in 4.4% — severe. In the majority of cases, the relationship between the development of AEs and vaccination was considered probable. The outcome in 95% of all cases was “recovery without consequences”, while in 5% of cases the outcome was “not yet recovered”. There were no fatalities, serious AEs, or other significant AEs that were considered to be of special interest due to their clinical significance.
The results of laboratory studies confirmed that administration of the investigated vaccine to volunteers aged 18–55 years had no negative effect on the main indicators of clinical and biochemical blood tests, IgE level and indicators of general urine analysis. Deviations of laboratory parameters from the norm were registered in all groups. The majority of deviations were regarded as clinically insignificant. The greatest number of clinically significant deviations was observed in the level of creatine phosphokinase and IgE. The results of electrocardiographic study before the beginning and after the completion of the administration of the preparations allow us to conclude that there was no effect of the administration of the studied vaccines on the work of the heart muscle, all results were within normal limits. Also, no development of vaccine-related neurologic disorders was detected in vaccinated volunteers. Physical examination of the volunteers before vaccination and at each visit did not reveal any abnormalities in the health status of the vaccinated volunteers in any of the groups, except for cases associated with the manifestations of AEs.
Study of humoral immune response in the neutralization reaction
At the screening in the research centers, all volunteers were negative in the rapid test for COVID-19, but antibodies were observed in ELISA and neutralization reactions. In the placebo group, GMT values fluctuated insignificantly, and an increase in GMT was observed in groups 1 and 2 against Wuhan, Delta and Omicron strains (Fig. 2).
Fig. 2. GMT of neutralizing antibodies in all volunteers.
Fig. 3. GMT of neutralizing antibodies in volunteers with baseline low antibody titers ≤ 1/80.
Volunteers with low GMT (≤ 1/80) before vaccination showed a significant increase in antibodies in the NR after vaccination (Fig. 3). In the presence of high titers (> 1/80), lower rates of immunogenicity were observed in NR. In group 3, there was virtually no increase in antibody titers.
Study of humoral immune response by ELISA method
In the study of humoral immune response in dynamics by ELISA method it was shown that in group 1 the increase of IgG titers to S-protein SARS-CoV-2 on the 21st day after a single injection was observed in 100% of volunteers, after a 4-fold injection — in 70%, GMT amounted to 1600, and on the 21st day after a double injection GMT already amounted to 2177.26, seroconversion level — 77.8% (Fig. 4). In group 2, on the 21st day after a single vaccine administration, IgG titers increased in 100% of volunteers, and after a 4-fold vaccine administration — in 86%, the GMT was 4306.88. After the 2nd administration of the vaccine with 80 μg antigen content, the immunogenicity indices remained practically unchanged. In group 3, no increase in IgG titers was observed, and the GMT remained at the same level. The average indices of total IgA, IgM, IgG of volunteers during the dynamic observation underwent insignificant changes in all groups.
Fig. 4. Seroconversion rate at different study dates. Y axis – proportion of volunteers with seroconversion (increase in specific antibody titer ≥ 4 times), %.
On the 21st day after the 1st vaccination, specific IgG to the S-protein of SARS-CoV-2 was detected in ELISA in all volunteers of group 1, with antibody titers of 1 : 400–1 : 12,800 and an GMT of 4201.57. The percentage of volunteers who showed an increase in antibody titer was 62.5%, of which those with 4-fold seroconversion were 19.6%. On the 21st day after the 2nd vaccination, the GMT was 4306.88 and the seroconversion rate was 20.4%. The multiplicity of increase in GMT relative to screening on the 21st day after the 1st vaccination was 2.1 and after the 2nd vaccination was 2.2.
In group 2, on the 21st day after the 1st vaccination, specific IgG to S-protein SARS-CoV-2 was detected in ELISA in 100% of volunteers, with antibody titers of 1 : 1600–1 : 12 800, and the GMT was 4950.94. The proportion of volunteers with an increase in antibody titer was 57.4%, including 14.8% with 4-fold seroconversion. On the 21st day after the 2nd vaccination, the GMT was 5261.46 and the seroconversion rate was 19.6%. The multiplicity of GMT increase relative to screening on the 21st day after the 1st vaccination was 1.7, after the 2nd vaccination — 1.8.
In group 3, specific IgG in ELISA on the 21st day after the 1st administration of placebo was detected in 91.7% of volunteers, with antibody titers of 0–1 : 12,800 and an GMT of 1241.36. The proportion of volunteers who showed an increase in antibody was 18.8%, with 4-fold seroconversion in 10.4%. On the 21st day after the 2nd administration, the GMT was 2311.60, with a seroconversion rate of 15.9%. At the same time, no COVID-19 cases were detected by PCR method.
Assessment of cellular immunity
The studies were carried out initially (screening V), as well as on the 10th day after the 1st and 2nd vaccination. When assessing the dynamics of PSI as a result of immunization with VLP-vaccine, a significant (p < 0.05) increase in the index in the studied groups compared to the screening was observed. As the number of vaccinations increased, a gradual increase in cellular response was noted (p < 0.05). The mean PSI level reached a maximum value on the 10th day after the 2nd immunization with a vaccine containing 40 μg antigen, per dose (2.17 [1.87; 3.05]) and with a vaccine containing 80 μg antigen, per dose (2.57 [1.98; 3.17]). In LBTR, the number of stimulated lymphocytes was examined, and if they are stimulated by a specific stimulant, it may indicate a prior encounter with antigen, which is possible either post-vaccination or post-infection. In group 3, there were volunteers with very high PSI values, which may suggest post-infection stimulation, as there were no such high PSI levels post-vaccination at any of the tested doses of antigen in the vaccine. Cellular and humoral immune response are not necessarily correlated. Both the presence of antibodies without the formation of cellular reactions and the absence of antibodies but with the formation of cellular immunity are frequently observed in COVID-19 convalescents. Therefore, despite the absence of seroconversion in the group of volunteers immunized with placebo, we cannot unequivocally state the absence of asymptomatic COVID-19 infection in volunteers with high levels of PSI with cellular response. It should be noted that such a high proliferative response to a specific antigen in the placebo group is only possible after an infection has occurred.
Examination of the CD4+/CD8+-lymphocyte ratio showed no abnormalities after vaccination with the VLP vaccine containing both 40 μg and 80 μg of antigen per dose, either toward helper or cytotoxic lymphocytes. This additional information about the absence of potential immunotoxicity can be observed after vaccination (according to ICH S8) (Table 2).
Table 2. PSI data and CD4+/CD8+ ratios in the studied groups, Me [Q1; Q3]
No. | Immunization | Indicator | Group 1 | Group 2 | Group 3 |
1 | Before vaccination | n | 60 | 60 | 60 |
PSI | 1.4 [1.15; 1.69] | 1.43 [1.07; 1.83] | 1.25 [1.11; 1.59] | ||
CD4+/CD8+ | 2.15 [1.71; 2.81] | 2.44 [1.77; 2.97] | 2.7 [1.97; 3.25] | ||
2 | 10th day after | n | 54 | 52 | 49 |
PSI | 1.75 [1.31; 2.21] | 2.06 [1.51; 2.3] | 1.59 [1.22; 2.15] | ||
CD4+/CD8+ | 2.08 [1.65; 3.38] | 2.23 [1.61; 3.38] | 2.86 [2.06; 4.23] | ||
3 | 10th day after | n | 47 | 49 | 45 |
PSI | 2.17 [1.87; 3.05] | 2.57 [1.98; 3.17] | 2.17 [1.45; 2.79] | ||
CD4+/CD8+ | 3.3 [2.2; 4.37] | 3.18 [2.28; 4.42] | 4.15 [2.51; 5.09] | ||
Post-hoc analysis of PSI adjusted | p1–2 = 0.020 | p1–2 = 0.318 | p1–2 = 0.195 | ||
р1–3 < 0.0001 | р1–3 < 0.0001 | р1–3 < 0.0001 | |||
р2–3 = 0.020 | р2–3 = 0.004 | р2–3 = 0.006 | |||
To study the immune status of volunteers, the dynamics of cytokine production during double immunization was also investigated. Sera from patients were taken before immunization and on the 10th day after the 1st and 2nd immunization (Fig. 5).
Fig. 5. Dynamics of cytokine levels in blood serum of volunteers.
Authors’ contribution: Grebennikova T.V. — study concept and design, data analysis and interpretation, text editing, preparation of the final version of the article for publication; Zaykova O.N. — cell culture processing, article writing, results presentation; Plotnikov A.A. — clinical trial monitoring, results presentation; Kostina L.V. — cell culture processing, drug production and characterization; Chernoryzh Ya.Yu. — cellular immunity study, statistical analysis; Eliseeva O.V. — quality control of the obtained drug; Latyshev O.E. — drug production and characterization; Larichev V.F., Fedyakina I.T. — immunogenicity study in the neutralization reaction; Losich M.A. — cell culture processing, drug production and characterization; Kirillov I.M. — vaccine antigen purification; Filatov I.E. — immunogenicity study in the ELISA reaction (specific IgG); Balandina M.V. — cytokine production dynamics study; Tsibezov V.V. — study of immunogenicity in ELISA reaction, data interpretation; Yurlov K.I. — study of cellular immunity; Lesnova E.I., Kondratieva V.M. — study of cellular immunity; Kozlova A.A., Baranets M.S. — study of immunogenicity in neutralization reaction; Ginzburg A.L. — organizational aspect of clinical trials. Аll authors confirm that they meet the International Committee of Medical Journal Editors criteria for authorship, made a substantial contribution to the conception of the article, acquisition, analysis, interpretation of data for the article, drafting and revising the article, final approval of the version to be published.
Analysis of statistical differences showed that in group 1, for the tumor necrosis factor-α (TNF-α) concentration, there was a statistically significant difference between the screening visit (Me = 4.6) and visit 4 (Me = 7.81). No significant differences were found for the rest of the data.
The results show that the developed VLP vaccine, containing both 40 and 80 μg of antigen per dose, can be an inducer of cell-mediated immune response, in which the CD4+/CD8+-lymphocyte ratio is not changed either toward helper or cytotoxic lymphocytes. And postvaccination changes in cytokine levels are not sufficient for the development of immunopathologic conditions associated with excessive production of the proinflammatory cytokines under study.
Discussion
Inactivated and live vaccines are the most widely used for the prevention of infectious diseases. However, the emergence and spread of new infections, the variability of their causative agents and the recent pandemic that affected everyone show the need to develop and improve means of specific therapy and prophylaxis. In parallel with the development of mRNA-based, viral vector-based, and subunit vaccine production technology, the technology of VLP production is gaining popularity, which represents an alternative platform for vaccine development. The advantage of such a platform is the possibility of creating multivalent vaccines that are capable of inducing humoral and cellular immune response with the production of virus-neutralizing antibodies of a broad spectrum. This is important in the development of vaccines for the prevention of infections caused by viruses with high genetic variability, such as SARS-CoV-2. At the same time, the absence of genetic material in VLP-vaccines can guarantee an increased level of safety, which is confirmed by preclinical studies [13–15].
In the present study, the tolerability, safety and immunogenicity of a new vaccine against coronavirus infection caused by SARS-CoV-2 were investigated in phase I clinical trials in healthy volunteers. The drug is purified recombinant SARS-CoV-2 VLPs that are synthesized in a baculovirus expression system. The surface S-protein within the VLP is represented by variants 19A, Alpha, Delta and Omicron. The vaccine contains a squalene-based adjuvant.
The main objectives were to assess the tolerability, safety and immunogenicity of the vaccine compared to placebo for 21 days after double intramuscular administration, as well as to determine the optimal dose of antigen for further study of the safety and efficacy of the drug in phase II clinical trials.
When developing vaccines, including those for COVID-19 prophylaxis, special attention is paid to studies of safety, non-reactogenicity and tolerability of new vaccines, as these indicators directly affect the possibility of widespread use of these drugs and the level of public confidence in vaccination. In particular, studies of injectable forms of a number of mRNA vaccines from COVID-19 have revealed both local reactions, such as pain at the injection site, and serious adverse reactions, and some publications on vaccine safety have been withdrawn by journal editors despite the authors' objections. VLP-based vaccines have proven to be highly effective and safe, as demonstrated by vaccines against human papillomavirus (Gardasil, Gardasil9, Cervarix), hepatitis E (Hecolin) hepatitis B (Sci-B-Vac) and malaria (Mosquirix) [16–18].
According to the results of the presented study, AEs were observed both in volunteers vaccinated with the vaccine with 40 and 80 µg of antigen per dose and in the group immunized with placebo. At the same time, the majority of AEs were registered during the first 10 days after vaccination, mainly after the 1st vaccination. In the period from the 11th to the 21st day after vaccination, there were only a few cases of AEs.
The majority of the reported AEs with a “probable” and “possible” relationship were related to expected adverse events after immunization to the effect of the vaccine preparation. AEs of particular interest associated with COVID-19 vaccination (Guillain–Barré syndrome, generalized seizures, anaphylaxis, thrombocytopenia, coagulopathy, etc.) were not reported in any volunteers.
The detected fluctuations in the mean values of clinical blood and urine indices before vaccination and at different periods after vaccination do not allow us to speak about the influence of vaccination on these indices and can be explained by random factors, restructuring of the immune system of the body of the vaccinated in response to the introduction of antigen.
The average values of vital function indices in the study group were within the normal range, and the changes in these parameters according to the results of measurements after the beginning of the drug administration, compared to the initial values, were insignificant and within the reference values.
Significant changes in individual laboratory parameters relative to screening were observed in all study groups. Importantly, these abnormalities occurred both in volunteers who received the study vaccine at both doses and in the group of volunteers who received placebo. The absence of serious vaccine-related AEs or deaths during the study suggests good tolerability and safety of the vaccine with both 40 mcg and 80 mcg of antigen per dose.
Researchers at Radbaud University Medical Center in Nijmegen, the Netherlands, conducted a single-center clinical trial with dose-matched adjuvant ABNCoV2 vaccine based on VLP or capsid-like particles (cVLP). The RBD of the spike glycoprotein SARS-CoV-2 glycoprotein was covalently attached to the cVLP carrier. Forty-five healthy volunteers, aged 18-55 years, who were immunized intramuscularly, twice, were studied. Participants had a total of 249 possibly vaccine-related NIs within a week of vaccination (185 grade 1; 63 grade 2; 1 grade 3). Two serious events occurred; one was classified as a possible adverse reaction [19].
VLPs of the developed vaccine are capable of exerting a strong immunostimulatory effect on the organism, activating T- and B-lymphocytes, as they contain the main immunogenic proteins SARS-CoV-2 in native conformation. They easily penetrate into lymph nodes and are taken up by antigen-presenting cells, in particular dendritic cells, with subsequent antigen presentation by molecules of the major histocompatibility complex class II [19, 20].
Studies of immunogenicity of the developed vaccine against SARS-CoV-2 in the framework of phase I clinical trials showed that administration of the vaccine containing 40 and 80 μg of antigen to volunteers induced a significant increase in GMT compared to placebo. At the same time, the immune response was stronger in volunteers who initially had low antibody titers (NR — titer ≤ 1/80, ELISA — titer ≤ 1/800). When volunteers had high antibody titers (1/1600–1/3200 and ≥ 1/6400 in ELISA) at screening, these immunogenicity rates were lower and no seroconversion was observed. Of note, virally neutralizing antibodies were produced to various SARS-CoV-2 strains, including clades 19A, Delta, and Omicron. In group 3, an increase in antibody titers was noted in a number of volunteers, but no 4-fold increase was observed. It should be noted that COVID-19 was not detected in those included in the study.
In group 2, the immunogenicity indices in ELISA were insignificantly but superior to both the values in the general population and in volunteers with initially low and high antibody titers. Indicators of immune response intensity, antibody titers increase, including 4-fold, in NR were also higher in group 2, especially for the current and predominant Omicron strain. Thus, in this group the GMT was 275.24, a multiple of 3.1, while in group 1 the GMT was 160, a multiple of 2.0.
High indices of immune response in group 3 most likely indicate a COVID-19 disease in a latent form, which did not manifest itself clinically and was not confirmed by PCR-test. It is important to note that this study was conducted at the height of the epidemic, and the main infectious agent at that time was the Omicron strain.
The insufficient sensitivity of some commercial ELISA test systems for detecting antibodies to SARS-CoV-2 should also be noted [21]. For example, in influenza, antibodies appear in 50–80% of unvaccinated adults without signs of disease [22]. A number of researchers note that up to 80% of COVID-19 infections may be asymptomatic [23, 24].
Researchers from the Netherlands studied the immune response of 45 volunteers who were immunized with ABNCoV2 vaccine based on cVLP with different amounts of antigen: 6, 12, 25, 50 or 70 μg [19]. A dose-dependent antibody formation was observed after the 2nd vaccination when immunized with vaccines containing 25–70 μg of antigen. Antibodies neutralized the major SARS-CoV-2 variants, but viral neutralizing activity was lower with the Omicron variant (BA.1), specific interferon-γ (IFN-γ)+CD4+ T cells were activated. The researchers' overall conclusion: immunization with the vaccine was well-tolerated, safe and resulted in a functional immune response.
SARS-CoV-2 infection induces immune responses that may have important implications for the development of vaccination strategies. T-cell immunity plays a central role in the control of SARS-CoV-2 infection. Antigen-specific CD4+ and CD8+ T cells and neutralizing antibodies play a protective role against SARS-CoV-2, whereas impairment of the adaptive immune response, namely a lack of naive T cells, can lead to adverse disease outcomes.
In the study of the immune response, it is important to assess the degree of cytokine imbalance and immune cell activation. Antigen mimicry between viral and human proteins can lead to the development of immune-mediated hemolysis, decreased leukocyte counts, cytokine storm, procoagulant state, and macrophage activation [25]. The synthesis of cytokines IFN-γ, interleukin-2, and TNF-α accounts for the Th1-type immune response [26]. IFN-γ and interleukin-2 activate macrophages, natural killer cells, and cytotoxic lymphocytes, which are crucial for virus elimination. IFN-γ is the most potent factor in macrophage activation. Full activation of macrophages can be achieved by low levels of IFN-γ. In vaccine development, it is important to avoid toxicity associated with its over-activation.
Abnormal stimulation of T cells and antigen-presenting cells (dendritic cells, macrophages and B cells) can lead to the development of a cytokine storm generated by suppressive release of cytokines, particularly TNF-α, which promotes migration of neutrophils from vessels and activation of clotting pathways. Hyperinflammatory reactions correlate with increased levels of serum interleukin-2, -6 and -7 [27, 28].
The developed VLP-vaccine can be an inducer of cell-mediated immune response, in which the CD4+/CD8+-lymphocyte ratio does not change either toward helper or cytotoxic lymphocytes. At the same time, the post-vaccination change in cytokine levels is not sufficient for the development of immunopathologic conditions associated with excessive production of the proinflammatory cytokines under study.
When the developed vaccine was used, antibody formation and cellular response increased markedly as the number of immunizations increased. However, the immunogenicity parameters obtained after immunization with the vaccine containing 80 µg antigen per dose were superior to those obtained after immunization with the vaccine containing 40 µg antigen per dose (especially for the Omicron strain); therefore, this dose was selected as the optimal dose for phase I clinical trials.
Conclusion
The tolerability, safety and immunogenicity of a new vaccine for COVID-19 prophylaxis based on VLP within the framework of phase I clinical trials on 180 volunteers were evaluated. It has been shown that vaccination with preparations containing 40 and 80 µg of antigen in the dose intramuscularly twice with an interval of 21 days does not cause serious AEs and induces humoral and cellular immune response. The CD4+/CD8+-lymphocyte ratio does not change either toward helper or cytotoxic lymphocytes. Post-vaccination changes in cytokine levels are not sufficient for the development of immunopathologic conditions associated with excessive production of the proinflammatory cytokines under study.
1 WHO Director-General's opening remarks at the media briefing on COVID-19. 11 March 2020. URL: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
WHO. COVID-19 Epidemiological Update. 06.11.2024. URL: https://www.who.int/docs/default-source/coronaviruse/situation-reports/covid-19_epi_update_173.pdf?Sfvrsn= 457952e6_4&download=true
2 The epidemiological situation and the spread of COVID-19 in the world as of 8 Moscow time on 05/12/2024 /FCUN ROSNIPCHI "Microbe". Federal Service for Consumer Rights Protection and Human Welfare. (In Russ.) URL: https://www.rospotrebnadzor.ru/12.05.2024%20г.%20Информация%20о%20случаях%20заболевания.docx
3 COVID-19 Cases, World / WHO Coronavirus (COVID-19) dashboard. URL: https://data.who.int/dashboards/covid19/cases
About the authors
Tatiana V. Grebennikova
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Author for correspondence.
Email: t_grebennikova@mail.ru
ORCID iD: 0000-0002-6141-9361
Dr. Sci. (Biol.), Professor, Corresponding Member of RAS, Head, Laboratory of molecular diagnostics, Head, Deputy Director for scientific work, D.I. Ivanovsky Institute of Virology, Head, Testing Center
Россия, MoscowOlga N. Zaykova
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: zaykova_o_n@mail.ru
ORCID iD: 0000-0003-4708-2069
Cand. Sci. (Biol.), senior researcher, Laboratory of molecular diagnostics, researcher, Diagnostic and Prevention Research institute for Human and Animal Diseases
Россия, MoscowAlexey A. Plotnikov
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: alesp@ya.ru
ORCID iD: 0009-0009-1253-1152
postgraduate student, junior researcher, Laboratory of molecular diagnostics
Россия, MoscowLyudmila V. Kostina
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: lvkostina@mail.ru
ORCID iD: 0000-0002-9556-1454
Cand. Sci. (Biol.), senior researcher, Laboratory of molecular diagnostics, researcher, Diagnostic and Prevention Research institute for Human and Animal Diseases
Россия, MoscowYana Yu. Chernoryzh
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: revengeful_w@mail.ru
ORCID iD: 0000-0001-9848-8515
Cand. Sci. (Med.), researcher, Laboratory of molecular diagnostics
Россия, MoscowOlesia V. Eliseeva
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: olesenka80@mail.ru
ORCID iD: 0000-0002-0723-9749
Cand. Sci. (Biol.), senior researcher, Laboratory of molecular diagnostics
Россия, MoscowOleg E. Latyshev
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: oleglat80@mail.ru
ORCID iD: 0000-0002-5757-3809
Cand. Sci. (Biol.), senior researcher, Laboratory of molecular diagnostics
Россия, MoscowViktor F. Larichev
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: vlaritchev@mail.ru
ORCID iD: 0000-0001-8262-5650
Dr. Sci. (Med.), leading researcher, Laboratory of biology and indication of arboviruses
Россия, MoscowIrina T. Fedyakina
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: irfed2@mail.ru
ORCID iD: 0000-0001-6421-9632
Cand. Sci. (Biol.), Head, Laboratory of virus ecology, leading researcher, Department of virus ecology
Россия, Moscow
Milana A. Losich
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: mkohnovich@rambler.ru
ORCID iD: 0000-0002-5618-1918
Cand. Sci. (Biol.), senior researcher, Laboratory of molecular diagnostics, Group of comparative virology
Россия, MoscowIlya M. Kirillov
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: iliyakirillov@yandex.ru
ORCID iD: 0000-0002-4933-850X
senior researcher, Laboratory of virus ecology
Россия, MoscowIlya E. Filatov
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: filat69rus@yandex.ru
ORCID iD: 0000-0001-5274-224X
junior researcher, Laboratory of molecular diagnostics
Россия, Moscow
Marina V. Balandina
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: mbalandina77@mail.ru
ORCID iD: 0009-0002-8179-1379
Cand. Sci. (Biol.), senior researcher, Laboratory of molecular diagnostics
Россия, MoscowValery V. Tsibezov
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: tsibezov@yandex.ru
ORCID iD: 0000-0003-2150-5764
Cand. Sci. (Biol.), leading researcher, Laboratory of specific means of prevention of viral diseases
Россия, MoscowKirill I. Yurlov
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: kir34292@yandex.ru
ORCID iD: 0000-0002-4694-2445
researcher, Laboratory of cellular engineering
Россия, MoscowEkaterina I. Lesnova
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: wolf252006@yandex.ru
ORCID iD: 0000-0002-2801-6843
researcher, Laboratory of molecular diagnostics
Россия, MoscowValeria M. Kondratieva
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: 1999valeriak@mail.ru
ORCID iD: 0000-0001-9163-4516
postgraduate student, Laboratory of molecular diagnostics
Россия, MoscowAlina A. Kozlova
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: malinkakozlova88@gmail.com
ORCID iD: 0000-0003-2749-3258
Cand. Sci. (Biol.), researcher, Laboratory of molecular diagnostics
Россия, MoscowMarina S. Baranets
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: shizotorex@mai.ru
ORCID iD: 0000-0002-3466-3588
Cand. Sci. (Med.), researcher, Laboratory of molecular diagnostics
Россия, MoscowAleksandr L. Gintsburg
National Research Center for Epidemiology and Microbiology named after N.F. Gamaleya
Email: gintsburg@gamaleya.org
ORCID iD: 0000-0003-1769-5059
Dr. Sci. (Biol.), Professor, Academician RAS, Director
Россия, MoscowReferences
- Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13. DOI: https://doi.org/10.1016/s0140-6736(20)30211-7
- Усков А.Н., Лобзин Ю.В., Рычкова С.В. и др. Течение новой коронавирусной инфекции у детей: некоторые аспекты мониторинга заболеваемости и анализа летальности. Журнал инфектологии. 2020;12(3):12–20. Uskov A.N., Lobzin Yu.V., Rychkova S.V., et al. Course of a new coronavirus infection in children: some aspects of monitoring and analysis of mortality. Journal Infectology. 2020;12(3):12–20. DOI: https://doi.org/10.22625/2072-6732-2020-12-3-12-20 EDN: https://elibrary.ru/jyuxsy
- Гарафутдинов Р.Р., Мавзютов А.Р., Никоноров Ю.М. и др. Бетакоронавирус SARS-CoV-2, его геном, разнообразие генотипов и молекулярно-биологические меры борьбы с ним. Биомика. 2020;12(2):242–71. Garafutdinov R.R., Mavzyutov A.R., Nikonorov Yu.M., et al. Betacoronavirus SARS-CoV-2, its genome, variety of genotypes and molecular-biological approaches to combat it. Biomics. 2020;12(2):242–71. DOI: https://doi.org/10.31301/2221-6197.bmcs.2020-15 EDN: https://elibrary.ru/dhderx
- Горенков Д.В., Хантимирова Л.М., Шевцов В.А. и др. Вспышка нового инфекционного заболевания COVID-19: β-коронавирусы как угроза глобальному здравоохранению. БИОпрепараты. Профилактика, диагностика, лечение. 2020;20(1):6–20. Gorenkov D.V., Khantimirova L.M., Shevtsov V.A., et al. An outbreak of a new infectious disease COVID-19: β-coronaviruses as a threat to global healthcare. Biological Products. Prevention, Diagnosis, Treatment. 2020;20(1):6–20. DOI: https://doi.org/10.30895/2221-996X-2020-20-1-6-20 EDN: https://elibrary.ru/euulmy
- Романов Б.К. Коронавирусная инфекция COVID-2019. Безопасность и риск фармакотерапии. 2020;8(1):3–8. Romanov B.K. Coronavirus disease COVID-2019. Safety and Risk of Pharmacotherapy. 2020;8(1):3–8. DOI: https://doi.org/10.30895/2312-7821-2020-8-1-3-8EDN: https://elibrary.ru/vzvbrk
- Никифоров В.В., Суранова Т.Г., Чернобровкина Т.Я. и др. Новая коронавирусная инфекция (COVID-19): клинико-эпидемиологические аспекты. Архивъ внутренней медицины. 2020;10(2):87–93. Nikiforov V.V., Suranova T.G., Chernobrovkina T.Ya., et al. New coronavirus infection (COVID-19): Clinical and epidemiological aspects. Archive of Internal Medicine. 2020;10(2):87–93. DOI: https://doi.org/10.20514/2226-6704-2020-10-2-87-93 EDN: https://elibrary.ru/melbop
- Tregoning J.S., Brown E.S., Cheeseman H.M., et al. Vaccines for COVID-19. Clin. Exp. Immunol. 2020;202(2):162–92. DOI: https://doi.org/10.1111/cei.13517
- Ожмегова Е.Н., Савочкина Т.Е., Прилипов А.Г. и др. Молекулярно-эпидемиологический анализ геновариантов SARS-CoV-2 на территории Москвы и Московской области. Вопросы вирусологии. 2022;67(6):496–505. Ozhmegova E.N., Savochkina T.E., Prilipov A.G., et al. Molecular epidemiological analysis of SARS-CoV-2 genovariants in Moscow and Moscow region. Problems of Virology. 2022;67(6):496–505. DOI: https://doi.org/10.36233/0507-4088-146 EDN: https://elibrary.ru/crgiwk
- Ghafouri F., Cohan R.A., Noorbakhsh F., et al. An in-silico approach to develop of a multi-epitope vaccine candidate against SARS-CoV-2 envelope (E) protein. Res. Sq. 2020. Preprint. DOI: https://doi.org/10.21203/rs.3.rs-30374/v1
- Ayyagari V.S., Venkateswarulu T.C., Abraham Peele K., Srirama K. Design of a multi-epitope-based vaccine targeting M-protein of SARS-CoV-2: an immunoinformatics approach. J. Biomol. Struct. Dyn. 2022;40(7):2963–77. DOI: https://doi.org/10.1080/07391102.2020.1850357
- Gupta T., Gupta S.K. Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses. Int. Immunopharmacol. 2020;86:106717. DOI: https://doi.org/10.1016/j.intimp.2020.106717
- Латышев О.Е., Зайкова О.Н., Елисеева О.В. и др. Разработка, получение и характеристика вирусоподобных частиц SARS-CoV-2 (Coronaviridae: Orthocoronavirinae: Betacoronavirus: Sarbecovirus). Вопросы вирусологии. 2024;69(2):175–86. Latyshev O.E., Zaykova O.N., Eliseeva O.V., et al. Development, production and characterization of SARS-CoV-2 virus-like particles (Coronaviridae: Orthocoronavirinae: Betacoronavirus: Sarbecovirus). Problems of Virology. 2024;69(2):175–86. DOI: https://doi.org/10.36233/0507-4088-226 EDN: https://elibrary.ru/gkxfed
- Banihashemi S.R., Es-Haghi A., Fallah Mehrabadi M.H., et al. Safety and efficacy of combined intramuscular/intranasal RAZI-COV PARS vaccine candidate against SARS-CoV-2: a preclinical study in several animal models. Front. Immunol. 2022;13:836745. DOI: https://doi.org/10.3389/fimmu.2022.836745
- Vakhrusheva A.V., Kudriavtsev A.V., Kryuchkov N.A., et al. SARS-CoV-2 subunit virus-like vaccine demonstrates high safety profile and protective efficacy: Preclinical study. Vaccines (Basel). 2022;10(8):1290. DOI: https://doi.org/10.3390/vaccines10081290
- Чернорыж Я.Ю., Кондратьева В.М., Малкова А.П. и др. Доклинические исследования безопасности интраназальной вакцины на основе вирусоподобных частиц для профилактики COVID-19. Вопросы вирусологии. 2025;70(1):35–46. Chernoryzh Ya.Y., Kondratieva V.M., Malkova A.P., et al. Pre-clinical safety studies of intranasal virus-like particles based vaccine for prevention of COVID-19. Problems of Virology. 2025;70(1):35–46. DOI: https://doi.org/10.36233/0507-4088-278 EDN: https://elibrary.ru/fzgyxe
- Yang X., Chen M., Cao L., Zhao M. Bibliometric analysis of scientific papers on adverse reactions to COVID-19 vaccines published between 2019 and 2023. Hum. Vaccin. Immunother. 2023;19(3):2270194. DOI: https://doi.org/10.1080/21645515.2023.2270194
- Kombe Kombe A.J., Li B., Zahid A., et al. Epidemiology and burden of human papillomavirus and related diseases, molecular pathogenesis, and vaccine evaluation. Front. Public Health. 2021;8:552028. DOI: https://doi.org/10.3389/fpubh.2020.552028
- Nooraei S., Bahrulolum H., Hoseini Z.S., et al. Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnology. 2021;19(1):59. DOI: https://doi.org/10.1186/s12951-021-00806-7
- Smit M.J., Sander A.F., Ariaans M.B.P.A., et al. First-in-human use of a modular capsid virus-like vaccine platform: an open-label, non-randomised, phase 1 clinical trial of the SARS-CoV-2 vaccine ABNCoV2. Lancet Microbe. 2023;4(3):e140–8. DOI: https://doi.org/10.1016/s2666-5247(22)00337-8
- Tariq H., Batool S., Asif S., et al. Virus-like particles: revolutionary platforms for developing vaccines against emerging infectious diseases. Front. Microbiol. 2022;12:790121. DOI: https://doi.org/10.3389/fmicb.2021.790121
- Okba N.M.A., Müller M.A., Li W., et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg. Infect. Dis. 2020;26(7):1478–88. DOI: https://doi.org/10.3201/eid2607.200841
- Медуницын Н.В., Олефир Ю.В., Меркулов В.А., Бондарев В.П. Персональный и коллективный иммунитет при вакцинации. БИОпрепараты. Профилактика, диагностика, лечение. 2016;16(4):195–207. Medunitsyn N.V., Olefir Yu.V., Merkulov V.A., Bondarev V.P. Vaccination contribute to the development of personal and herd immunity. Biological Products. Prevention, Diagnosis, Treatment. 2016;16(4):195–207. EDN: https://elibrary.ru/xehmax
- Кроткова Е.Н., Кузнецов О.Е., Горчакова О.В. Оценка популяционного иммунитета к вирусу SARS-CoV-2 среди населения г. Гродно. Журнал Гродненского государственного медицинского университета. 2021;19(5):489–95. Krotkova E.N., Kuznetsov O.E., Gorchakova O.V. Assessment of population immunity to the SARS-CoV-2 virus among the population of Grodno. Journal of the Grodno State Medical University. 2021;19(5):489–95. DOI: https://doi.org/10.25298/2221-8785-2021-19-5-489-495
- Lai C.C., Liu Y.H., Wang C.Y., et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J. Microbiol. Immunol. Infect. 2020;53(3):404–12. DOI: https://doi.org/10.1016/j.jmii.2020.02.012
- Liu Y., Sawalha A.H., Lu Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021;33(2):155–62. DOI: https://doi.org/10.1097/bor.0000000000000776
- Мезенцева М.В., Антошина И.Ф., Морозова О.В. Синтез цитокинов in vitro при инфекции культуры клеток человека вирусом клещевого энцефалита и в присутствии инактивированной вакцины. Инфекция и иммунитет. 2014;4(1):37–42. Mesentseva M.V., Antoshina I.F., Morozova O.V. The cytokines synthesis in vitro in the tick-borne encephalitis virus infected cells and in the presence of inactivated vaccine. Russian Journal of Infection and Immunity. 2014;4(1):37–42. EDN: https://elibrary.ru/rzjgcz
- Landete P., Quezada Loaiza C.A., Aldave-Orzaiz B., et al. Clinical features and radiological manifestations of COVID-19 disease. World. J. Radiol. 2020;12(11):247–60. DOI: https://doi.org/10.4329/wjr.v12.i11.247
- Luo X.H., Zhu Y., Mao J., Du R.C. T cell immunobiology and cytokine storm of COVID-19. Scand. J. Immunol. 2021;93(3):e12989. DOI: https://doi.org/10.1111/sji.12989
Supplementary files