Outcomes of the multicenter monitoring of the causative agent of invasive listeriosis in the metropolis
- Authors: Voronina O.L.1, Ryzhova N.N.1, Kunda M.S.1, Aksenova E.I.1, Karpova T.I.1, Melkumyan A.R.2, Klimova E.A.3, Karetkina G.N.3, Posukhovsky E.A.3, Gruzdeva O.A.4, Tartakovsky I.S.1
-
Affiliations:
- N.F. Gamaleya National Research Center for Epidemiology and Microbiology
- F.I. Inosemtsev City Clinical Hospital
- A.I. Yevdokimov Moscow State University of Medicine and Dentistry
- Russian Medical Academy of Continuous Professional Education
- Issue: Vol 100, No 3 (2023)
- Pages: 143-154
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/7321
- DOI: https://doi.org/10.36233/0372-9311-393
- EDN: https://elibrary.ru/nzfizb
- ID: 7321
Cite item
Abstract
Introduction. Invasive listeriosis is a rare disease posing a threat to high-risk groups and often leading to a fatal outcome. Its causative agent is Listeria monocytogenes, a ubiquitous saprophyte that has turned into an important foodborne pathogen with the growing industry of semi-cooked and ready-to-eat products.
The aim of the study is the characterization of L. monocytogenes isolates in the Moscow region and identification of possible causes of susceptibility to infection
Materials and methods. The multicenter monitoring of L. monocytogenes was conducted in the Moscow metropolitan area, using bacteriological and genomic methods for description of the pathogen, medical history collection and detailed analysis of patient case summaries.
Results. In the cohorts of patients with perinatal listeriosis (PL) and meningitis-septicemia (MS), invasive listeriosis had a year-round occurrence with slight upswings in March–April and July–November. During the COVID-19 pandemic, in the MS group, the minimum age of patients decreased to 31 years and the proportion of deaths increased 1.57-fold compared to 2018–2019. During the pandemic, an increase in the diversity of L. monocytogenes genotypes was observed, along with changes in the spectrum of pathogen genotypes throughout the pandemic stages.
During the monitoring, a total of 73 L. monocytogenes clinical isolates belonging to 24 genotypes were described. Seven genotypes belonged to the first phylogenetic lineage (PLI); 14 genotypes belonged to PLII. The PL cohort had the highest proportion of PLI genotypes (52%). In the MS cohort, the group of men had the widest diversity of genotypes, 6 of which were identical to genotypes of food isolates. In the analysed set of isolates, 12 new profiles of internalin genes were identified and described. The whole genome sequencing detected the presence of plasmids in 9 of 58 genomes of clinical isolates. The comparison of core genomes revealed an epidemic relationship between isolates of the same genotype for ST4, ST21, and ST425.
Conclusion. The performed study presents a detailed description of the diversity and virulence of L. monocytogenes circulating in the Moscow metropolitan area, thus providing information for timely diagnosis and treatment of invasive listeriosis.
Full Text
Introduction
Invasive listeriosis (IL) caused by a ubiquitous saprophyte Listeria monocytogenes is a rare disease associated with a high case fatality rate1. Groups at risk for IL include pregnant women, embryos, fetuses, and newborns (the perinatal listeriosis (PL) cohort) as well as elderly people and immunocompromised individuals (the meningitis and/or septicemia (MS) cohort). According to statistics, in Moscow, 18 and 21 cases of listeriosis were reported in 2020 and 2021, respectively2. However, considering the severity of the principal diagnosis in risk groups, we assume that cases of concomitant IL are underreported due to the absence of recorded results of laboratory tests. The multicenter study, which was approved for the Moscow metropolitan area at the end of 2018, was designed to analyze L. monocytogenes clinical isolates from hospitalized patients. In our project, we used the available data on the diversity of genotypes of Listeria clinical isolates in the European part of Russia; the data were obtained both during the retrospective study of isolates collected in 1971–1999 [1] and by the analysis of isolates from 2015–2017 [2]. Since the growing industry of semi-cooked and ready-to-eat products has turned L. monocytogenes into an important food-borne pathogen, the study also included isolates collected from food products at facilities supervised by the Moscow Department of Rospotrebnadzor. Although out of 6,883 food samples that were tested at laboratories in Moscow in 20203, only a small percentage (0.6%) was tested positive for Listeria, the sources of L. monocytogenes demonstrate quite a wide variety: meat, poultry, fish, dairy products [3–6]. The environment as a source of Listeria should not be overlooked, as can be seen from the studies of nature parks and water bodies in the Central Federal District [7] as well as water bodies near livestock farms in the Vologda Region [8]. The COVID-19 pandemic gave rise to a new high-risk group — patients recovered from COVID-19; they are at high risk of development of listeriosis-associated sepsis and/or meningitis/meningoencephalitis and have high chances for an adverse outcome [9]. Microbiological studies conducted during the monitoring were designed to assess changes in the pathogen, employing molecular and genetic methods used by the Centers for Disease Control and Prevention in the United States and Europe as well as by research centers in our country.
Materials and methods
During the monitoring period (from November 2018 to February 2023), we analyzed 73 L. monocytogenes clinical isolates obtained from 9 hospitals in Moscow and 44 food isolates provided by the Moscow Center for Hygiene and Epidemiology in the Central Administrative District of Moscow. The study group included hospitalized patients, whose samples were used to collect Listeria isolates for molecular and genetic studies.
The cultivation of Listeria, the analysis using molecular and genetic methods, including MLST (multilocus sequence typing), MVLST (multi-virulence-locus sequence typing) and whole genome sequencing, genome assembly and annotation were performed as described previously [10].
The analysis of MLST alleles and allelic profiles (ST, sequence type) was performed using the resources of the Bacterial Isolate Genome Sequence Database for L. monocytogenes (BIGSdb-Lm4). The analyzed isolates and new allelic profiles were deposited in the website database, ID: 49370–49375; 75929–75933; 76308–76312; 76385–76389; 77384–78379; 78656–78660; 78713–78717; 82478–82494; 98277–98278; 98280–98297; 100872–100876; 102088–102092.
MvLST alleles were identified using the published sequences as reference sequences. The new inlA, inlB, inlE allelic variants were deposited in GenBank (Accession Numbers: MW538938, MW965279; MW538939, MZ486423; OM240824, OQ606809). For new internalin profiles, IPs (internalin gene (inlA, inlB, inlC, inlE) profile), we used the previously established numbering system [11].
The sequenced genomes were deposited in GenBank under BioProject accession number PRJNA605697. The identified plasmids were registered under numbers MZ869810, MZ869811, OM867528–OM867530, OP752358–OP752360, OP921773, OP921774.
The core genome was analyzed following the MLST scheme for 1,748 loci [12] using an open-source bioinformatics software platform4. Virulence factors were identified using the virulence factor database5 and VF analyzer6 [13] as well as the BIGSdb-Lm database. The resistome was analyzed using the Antibiotic Resistance BIGSdb-Lm database and the data from the Comprehensive Antibiotic Resistance Database7 [14]. The MobileElementFinder tool8 was used for detection of mobile genetic elements. The comparative analysis of sequences of RepA genes encoding the replication initiator protein was performed to identify the affiliation of plasmids to RepA phylogenetic groups [15]. CLC Genomics Workbench v. 21.0.1 and the Whole Genome Alignment plugin 21.0 were used for alignment of plasmids and construction of trees, using homologous plasmids from GenBank (CP015985, MZ089999, MZ147615, MZ065170, KU513859) for reference.
Results
Characterization of identified cases of invasive listeriosis
The monitoring of the IL pathogen in the Moscow metropolitan area lasted for more than 4 years. It covered the period before the COVID-19 pandemic and the time of the pandemic. Based on the main high-risk groups, the detected IL cases were distributed into two main cohorts: PL and MS. In the subset, throughout the monitoring period, PL cases accounted for 37%, showing a slight decrease during the pandemic: from 42% to 35%. The proportion of the MS cohort increased from 58% to 65% during the pandemic due to different factors, including an increase in IL cases among individuals of a younger age than the previously identified threshold of 59 years [3].
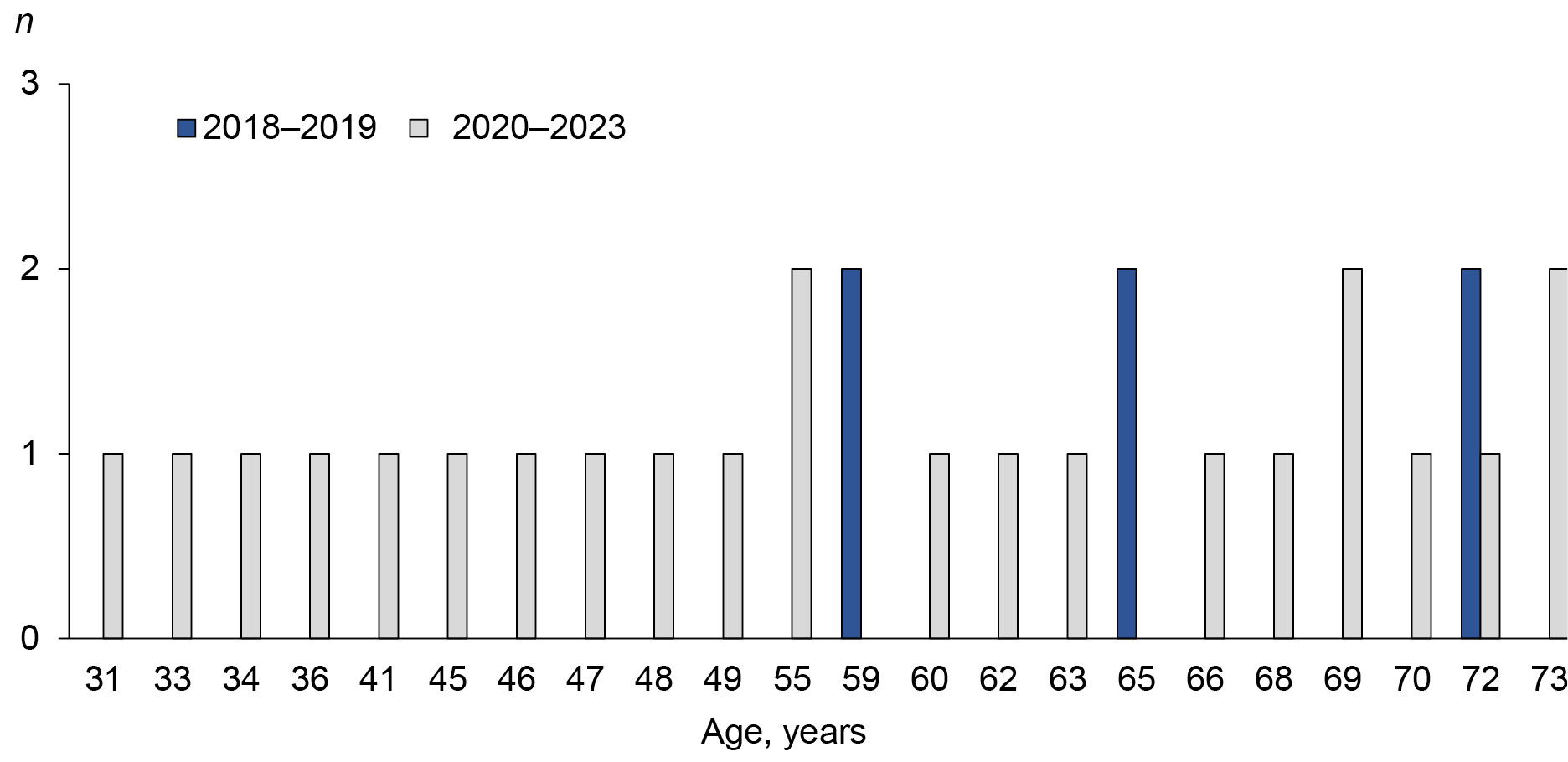
The minimum age of patients in this cohort decreased to 31 years (Fig. 1). The proportion of patients under 59 years of age was 34%. At the same time, the proportion of deaths in the study group increased 1.57-fold (from 36% before the pandemic to 57% during the COVID-19 pandemic).
Fig. 1. Age of patients with IL before and during the COVID-19 pandemic in the MS cohort.
Based on the number of IL cases accumulated throughout the monitoring period, the patients were distributed by months of the year, and the date of diagnosis was taken into consideration (Fig. 2). In both cohorts, IL occurred throughout the year, demonstrating slight upswings in March–April and July–November.
Fig. 2. Distribution of detected IL cases by months. MS — meningitis-septicemia; PL — perinatal listeriosis.
Molecular and genetic analysis of L. monocytogenes isolates
Diversity of genotypes during the monitoring period. 73 L. monocytogenes clinical isolates collected during the monitoring belonged to 24 genotypes. More virulent PLI included 7 genotypes (ST1, ST4, ST5, ST6, ST194, ST204, ST219); PLII included 14 genotypes (ST8, ST7, ST14, ST17, ST20, ST21, ST29, ST37, ST101, ST121, ST155, ST241, ST391, ST425, ST451, ST1365, ST2096).
The COVID-19 pandemic split the time of IL pathogen monitoring into two periods: 2018–2019 and 2020–2023. The latter, in its turn, can be divided into three stages based on genetic characteristics of circulating SARS-CoV-2 variants:
- Stage I (March 2020 – May 2021) — circulation of various SARS-CoV-2 Pango lineages [16];
- Stage II (June 2021 – January 2022) — dominance of the delta variant [17];
- Stage III (February 2022 – present) — dominance of the omicron variant [18].
During the pandemic stages, we observed changes in the spectrum of genotypes of L. monocytogenes clinical isolates (Fig. 3). Autochthonous ST7 (PLII) and imported ST6 (PLI) that prevailed before the pandemic [3, 10] were detected very rarely during stage I. The detected ST4, ST219 (PLI) and ST391 (PLII) were genotypes new for Russia. L. monocytogenes ST21, ST37, and ST425 that were isolated during pandemic stage I had not been previously detected in human IL cases. Another 2 new genotypes of clinical isolates were detected during stage II: ST8 (PLII) and ST194 (PLI). During stage III, 4 L. monocytogenes genotypes, which had never been reported in Russia, were detected: ST17, ST29, ST204, and ST1365 — all representing PLII, and ST121 (PLII), which had not been previously detected in clinical isolates.
Fig. 3. Diversity of genotypes of L. monocytogenes clinical isolates before and during the COVID-19 pandemic. I–III — periods of the COVID-19 pandemic; isolates of phylogenetic lineage I are marked with red dots; new genotypes for L. monocytogenes clinical isolates are framed in purple. The presence of a plasmid in the genome is marked by pl shown in an oval.
Diversity of genotypes in study groups. We compared the diversity of genotypes of L. monocytogenes isolates in the analyzed cohorts during the monitoring, having divided the MS cohort into 2 groups based on the gender of patients (MS_M — males; MS_F — females). As can be seen in Fig. 4, all groups had ST4, ST6, ST7 and ST8 isolates. The isolates from the MS_F group had 1 common genotype with isolates from PL (ST21) and MS_M (ST425) group, while the isolates from PL and MS_M groups had 3 common genotypes (ST1, ST194 and ST451). The isolates from the MS_M group had the largest number of genotypes (17); the Shannon index was 3.7. Isolates ST37 had the highest occurrence (21%) in the MS_M group, but they were not detected in other groups. The isolates of this genotype ranked second (20.5%) by the occurrence frequency in the group of food isolates (Fig. 5, a) and were found in all varieties of food products (Fig. 5, b).
Fig. 4. Diversity of genotypes of L. monocytogenes clinical isolates in study groups. MS_F — a group of women in the meningitis-septicemia cohort; MS_M — group of men in the meningitis-septicemia cohort; PL — perinatal listeriosis. The pink frame — genotypes common for all groups; the blue frame — genotypes common for MS_M and PL; the purple frame — genotypes common for MS_F and PL; the green frame — genotypes common for MS_M and MS_F.
Fig. 5. L. monocytogenes isolates from food product. а — the frequency of occurrence of different L. monocytogenes genotypes in products; b — distribution of the most represented L. monocytogenes genotypes by product categories. The red frame shows the most represented L. monocytogenes genotypes.
Generally, the number of genotypes matching those in the group of food isolates was higher in the MS_M group: there were 6 matching genotypes (ST7, ST8, ST37, ST121, ST155, ST451), while PL and MS_F groups had only 2–3 genotypes matching genotypes of food isolates.
The food and clinical isolates with matching genotypes required close attention. First of all, their internalin profile (IP) was analyzed. Note that in food isolates of the most frequent genotypes (ST121, ST37, ST7), IPs were identical to profiles of clinical isolates. However, new profiles of IP genes were detected in isolates of other genotypes.
New IP profiles. 12 new profiles of IP genes (56, 58–62, 66–71) were reported in this study in 2020–2023 (Table). Only one new IP was detected in PLI isolate for ST219 that was new for Russia, the other were detected in PLII isolates belonging to new genotypes (26, 391, 1365, 204, 17, 29) and to the genotypes that had been previously detected in isolates from other sources. For example, the ST21 isolate obtained from a goat with listeriosis in the North Caucasian Federal District in 1975 (BIGSdb-Lm ID 41525) [1] had a different IP compared to ST21 clinical isolates obtained in 2020, demonstrating differences in the allele at the inlC locus. The ST425 clinical isolates also differed in IP from the isolate obtained in 2009 from the environment (from feces of deer Cervus elaphus, BIGSdb-Lm ID 5909 [19]): differences in inlB alleles. The ST155 isolates demonstrated various IPs. Before the COVID-19 pandemic, in the multicenter study, ST155 isolates were obtained twice: from food products (fish) and from a patient. IPs of the isolates differed in alleles at the inlE locus. The isolate obtained from fish in 2021 matched the clinical isolate by IP; however, the isolate that was also obtained from fish in 2022 demonstrated new IP having differences at the inlE locus. Having compared the ST155 isolates from our subset with the isolates collected from rodents in the Far Eastern Federal District in 2006 [20], we found a difference at another locus — inlB. The ST451 and ST403 isolates had been previously obtained in Russia [2]; however, the types of their IPs were not identified; therefore, IPs of isolates of these genotypes were recorded for the first time.
New Internalin Profile
Isolate | BIGSdb-Lm ID | CC | ST | inlA | inlB | inlC | inlE | IP | PL | Source |
GIMC2056:LmcEH-1 | 49374 | CC21 | 21 | 12 | 14 | 7 | 8 | 56 | II | Clinical |
GIMC2060:LmcH24-1 | 75931 | CC11 | 451 | 22 | 23 | 6 | 6 | 58 | II | Clinical |
GIMC2062:Lmc5157 | 75933 | CC26 | 26 | 7 | 14 | 6 | 6 | 59 | II | Food |
GIMC2073:LmcUH25 | 77384 | CC4 | 219 | 23 | 8 | 4 | 3 | 60 | I | Clinical |
GIMC2077:LmcBH-1 | 78714 | CC89 | 391 | 15 | 14 | 17 | 6 | 61 | II | Clinical |
GIMC2079:LmcUH26 | 78377 | CC90 | 425 | 13 | 24 | 13 | 8 | 62 | II | Clinical |
GIMC2097:Lmc929 | 82487 | CC403 | 403 | 9 | 23 | 21 | 17 | 66 | II | Food |
GIMC2117:Lmc22984 | 98289 | CC155 | 155 | 10 | 14 | 19 | 18 | 67 | II | Food |
GIMC2120:LmcH51-1 | 98292 | ST1365 | 1365 | 22 | 20 | 6 | 9 | 68 | II | Clinical |
GIMC2124:LmcUH35 | 98296 | CC204 | 204 | 7 | 13 | 18 | 6 | 69 | II | Clinical |
GIMC2126:LmcH51-3 | 100872 | CC8 | 17 | 12 | 14 | 6 | 17 | 70 | II | Clinical, Food |
GIMC2133:LmcZhH_1 | 102090 | CC29 | 29 | 9 | 14 | 19 | 6 | 71 | II | Clinical |
Note. IP 57 (7; 13, 16, 6) was identified by S. Ermolaeva and E. Psareva (the data have not been published); IP 63–65 were published in [4]; CC — clonal complex; ST — sequence type; IP — internalin gene (inlA, inlB, inlC, inlE) profile; PL — phylogenetic lineage.
Analysis of genomes of L. monocytogenes isolates
Comparison of core genomes (cgMLST). The comparison of core genomes (1,748 loci) gives the most complete understanding of the epidemic relationship of isolates belonging to the same genotype. As can be seen in Fig. 6, ST4, ST425, and ST21 isolates obtained from different patients had 0–5 differences, thus providing a proof of an epidemic relationship of isolates belonging the same genotype. The core genomes of ST8, ST37 and ST451 isolates differed by 22–74 loci, demonstrating that Listeria of these genotypes are associated with multiple sources of infection.
Fig. 6. Genomic characteristics of L. monocytogenes isolates. a — the number of loci differentiating core genomes of the same genotype; b — plasmidome of the isolate subset. The orange line indicates the permissible number of differences (7–10), based on which isolates can be assigned to the same epidemic outbreak. The Listeria species indicated on the phylogenetic tree: L.m — L. monocytogenes, L.w — L. welshimeri. Countries of origin of isolates: IT — Italy; PL — Poland; RU — Russia. Sources of isolates: F — food; E — environment; FPE — food production environment; CL — clinical isolates. Colored circles: red — clinical isolates; blue — food isolates; green — isolates from the environment.
Plasmids in L. monocytogenes genomes. Plasmids in Listeria genomes, especially in the genomes of clinical isolates, have rare occurrence [21]. Out of 72 Listeria spp. genomes sequenced in our projects (PRJNA658237, PRJNA326717, PRJNA326713, PRJNA605697), only 11 (15%) contained plasmids: 1 — in the genome of L. welshimeri isolate, ST2331, obtained at a meat processing factory [11]; 9 — in the genomes of L. monocytogenes clinical isolates, PLII (ST8, ST20, ST121, ST425); 1 — in the genome of the L. monocytogenes food isolate, ST121. The plasmids differed in size and replication group. The G1 RepA group included plasmids of L. welshimeri isolates of ST2331 (57530 bp) and L. monocytogenes isolates of ST20, ST425 (72763 bp); the G2 RepA group included plasmids of ST121 (62207 bp) and ST8 (86632 bp) isolates. Cadmium resistance genes were present in all plasmids. The plasmid of ST20 and ST425 isolates contained genes resistant to other heavy metals, oxidative stress, changes in pH and osmosis; the plasmid of ST121 contained genes of the type IV secretion system, which are responsible for conjugation transfer. The largest plasmid of ST8 isolates included all the above genes.
Analysis of virulence factors in genomes of isolates of the detected genotypes. Virulence factors (VFs) in L. monocytogenes genomes can be searched using the virulence factor database including 45 factors or the BIGSdb-Lm database with an extended list of 76 factors, including genes contained within pathogenicity islands LIPI-3 and LIPI-4. Most of the VFs presented by the databases were detected in 21 genotypes of sequenced genomes belonging to both phylogenetic lineages: PLI — 7 ST; PLII — 14 ST. With reference to the complete list of VFs, all representatives of PLII lacked the aut_IVb gene (LMOF2365_RS00075) encoding the invasion factor as well as the LIPI-3 and LIPI-4 genes. No aut (lmo1076, invasion), tagB (lmo1088, teichoic acid biosynthesis) and inlL (LMON_RS10535, internalin) genes were detected in L. monocytogenes genomes of PLI. Among the representatives of genotypes belonging to PLII, the inlL gene was detected only in genomes of 6 of 14 STs; vip (lmo0320, invasion) was also found in 6 of 14 ST; inlG (lmo0262, internalin) was detected in 11 of 14 STs. In genomes of PLI, the inlG gene was detected only in L. monocytogenes ST6. LIPI-3 and LIPI-4 were not represented in all genomes of PLI. LIPI-3 (synthesis of listeriolysin S) was found in genomes of ST1, ST6, ST4, ST219 isolates, but in ST194, ST2, ST145 isolates, it was absent. LIPI-4 (the sugar-transporting phosphotransferase system, PTS) was detected only in genomes of ST4, ST219 (CC4) isolates and ST194 (CC315) isolates.
Antibiotic resistance genes were grouped in a separate Antibiotic Resistance database by the developers of the BIGSdb-Lm database. The database includes 25 loci [12] represented by the chromosome genes and the genes that can be introduced by plasmids and transposons. All the analyzed genomes had 5 genes: fosX (lmo1702), lin (lmo0919), mprF (lmo1695), sul (lmo0224) and norB (lmo2818) providing resistance to fosfomycin, lincosamide, cationic peptides, sulfonamide and quinolones, according to the developers. The detected genes had genotype-specific alleles. The analysis of sul (lmo0224) gene sequences showed that they encoded dihydropteroate synthase that participated in the folate biosynthesis and served as the target for sulfonamide. Only amino acid substitutions at positions 58–71 (based on the E. coli numbering system), which form a connecting loop between β-strand 5 and α-helix E, help reduce the affinity to sulfonamide and provide resistance [22]. These substitutions were not detected in the sequenced genomes. Therefore, it is more accurate to refer to lmo0224 as folP and, if there are no substitutions, classify it as a gene of the core genome.
In addition to the MFS transporter (norB), which is involved in the efflux of antibiotics, the resistance to quinolones can be induced by modifications of enzymes — targets for this class of antibacterial drugs. These enzymes include gyrase (GyrA, lmo0007) and topoisomerase (ParC, lmo1287). Mutations at positions 83 and 87 (the E. coli GyrA numbering system) in one of the two enzymes are most frequently associated with resistance [23]. In all the analyzed genomes, we detected Ser83 and Glu87 in the ParC sequence, which shows the consistency with characteristics of quinolone-resistant topoisomerases.
Additional resistance genes were detected only in one culture sample isolated from secretions of the cervical canal of a puerperal. The whole genome sequencing showed that the culture contained a small amount of admixture of ST41 Enterococcus faecalis. The ermB and cat genes responsible for resistance to macrolides, lincosamide, streptogramin B and fenicols were detected in the plasmid, while the tetM, dfrD genes responsible for resistance to tetracycline and trimethoprim were detected in the mobile element of the E. faecalis chromosome.
Discussion
The prolonged monitoring of IL cases in the Moscow metropolitan area expanded the collection of described L. monocytogenes clinical isolates obtained in the European part of Russia. A total of 73 isolates from 2018–2023 were added to 26 isolates from 1971–2017 in the BIGSdb-Lm database. The monitoring revealed the increased diversity of genotypes of L. monocytogenes clinical isolates both in the first and in the second phylogenetic lineages. In the clinical isolates, the proportion of genotypes belonging to PLI increased from 15% in 1971–2017 to 37% in 2018–2023. The PL group demonstrated the most substantial increase in the proportion of genotypes of PLI (52% over the entire period) — from 38% in 2018–2019 to 58% in 2020–2023. The comparison with the data from the largest European collection of clinical isolates, which were obtained from the French National Reference Center for Listeria (2,584 PLI and PLII isolates collected in 2005–2013), shows that in the PL group, the proportion of PLI has almost reached the levels of France (PLI — 66%) [24]; for our subset of 2018–2023, this proportion is almost 2 times as low. The diversity of hypervirulent genotypes among the isolates obtained in the PL group — ST1, ST4, ST6, ST219, ST194 — is alarming. In their tests with animal models, Maury et al., who coined the term "hypervirulent", provided the evidence for a stronger association of CC4 (ST4, ST219) with perinatal infection and for the role of LIPI-4 (the sugar-transporting phosphotransferase system, PTS) in the ability of bacteria to cross the placental barrier [24]. LIPI-4 was also detected in the genomes of ST194 (CC315) isolates. While CC4 isolates had not been isolated from food products in Russia, the ST194 (CC315) isolates were detected by Psareva et al. in the collection of isolates obtained from dairy products in 2005 [4]. Out of isolates of PLII genotypes, strictly associated with food products in France (ST9, ST121) [24], the ST121 isolate was detected among the clinical isolates during the monitoring. In our list of PLII genotypes, 14 of 17 STs were identical to those listed by France in reference to intermediate isolates that are associated both with clinical manifestations and with food products. Among the recent large-scale studies, the study of isolates from cattle abortions (191 isolates collected in 2013–2018) in Latvia that borders Russia deserves special attention [25]. In that study, the proportion of PLI isolates was 2%. More than half of the isolates belonged to ST29, ST37, ST451 and ST7 [25], which we also detected during our monitoring. In total, 11 of 17 STs were the same both in the monitoring-based list of PLII genotypes and the list of genotypes from Latvia.
The decrease in the minimum age of patients with IL and the significantly increased proportion of deaths in the MS cohort during the COVID-19 pandemic are causing concern. Most of the patients of a younger age had laboratory-confirmed COVID-19 prior to IL. Assumedly, it was COVID-19-associated hospitalization that contributed to the development of the epidemic relationship among L. monocytogenes isolates of the same genotype representing ST4, ST425, ST21. For example, during the COVID-19 pandemic (in September–October 2020), Italy reported an outbreak of hospital-acquired listeriosis caused by L. monocytogenes ST451. The contamination was found in the meat on the hospital kitchen. A total of 4 patients (1 immunocompromised, 3 with cancer) were infected [26]. In Germany, 13 of 39 listeriosis cases caused by L. monocytogenes ST8 were detected at health-care facilities where patients had ready-to-eat meat products from the same manufacturer. The core genomes of isolates had differences in 0–10 loci, thus being indicative of the epidemic relationship [27].
The analysis of antibiotic resistance genes showed that all sequenced genomes had genotype-specific alleles fosX (lmo1702), lin (lmo0919), mprF (lmo1695), norB (lmo2818), parC (lmo1287), which were responsible for resistance to fosfomycin, lincosamide, cationic peptides and quinolones. The fact that additional resistance genes (ermB, cat, tetM, dfrD) were detected in the culture with E. faecalis admixture highlights the importance of inspection of the culture for purity before the test, especially when the isolation involves such biotope as the cervical canal, in which E. faecalis is within the normal range in 18% of pregnant women and is detected 2 times as often during inflammatory processes [28].
Additional genes associated with resistance to disinfectants and environmental stress factors were detected in genomes of plasmid-containing isolates: ST8, ST20, ST121, ST425. When such isolates are detected, disinfection of hospital and industrial premises requires new combinations of disinfectants.
Conclusion
The multicenter monitoring of IL in the Moscow metropolitan area revealed that not only L. monocytogenes hypervirulent genotypes belonging to the first phylogenetic lineage (PLI), but also and primarily hypovirulent genotypes of PLII are responsible for the infection in patients of high-risk groups. The new high-risk group including patients recovered from COVID-19, the risk of hospital-acquired infection, the increased diversity of L. monocytogenes genotypes, the emergence of strains with plasmids carrying genes resistant to disinfectants and environmental stress factors — these are new realities that should be given attention in IL diagnosis and treatment.
Ethics approval. The study was conducted with the informed consent of the patients. The research protocol was approved by the Biomedical Ethics Committee of the N.F. Gamaleya National Research Center for Epidemiology and Microbiology (protocol No. 14, July 4, 2018).
Acknowledgement. We thank the Institut Pasteur for the curation and maintenance of BIGSdb-Pasteur databases at https://bigsdb.pasteur.fr/.
Funding source. The study was carried out with the financial support of the State Assignment of the N.F. Gamaleya.
Conflict of interest. The authors declare no apparent or potential conflicts of interest related to the publication of this article.
1 WHO. Listeriosis. URL: https://www.who.int/news-room/fact-sheets/detail/listeriosis (дата обращения 10.06.2023).
2 Moscow Department of the Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing. Dynamics of infectious and parasitic morbidity in the population of Moscow in January-December 2021 compared to the same period last year. URL: https://77.rospotrebnadzor.ru (accessed on 10 May 2023)
3 Moscow Department of the Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing. Information on the activities of laboratories of the sanitary-hygienic, microbiological and parasitological profile of federal budgetary healthcare institutions - centers of hygiene and epidemiology for 2020. URL: https://77.rospotrebnadzor.ru (accessed on 10 May 2023)
4 Bacterial Isolate Genome Sequence Database for L. monocytogenes. URL: https://bigsdb.pasteur.fr/listeria/
5 Virulence Factors Database. URL: http://www.mgc.ac.cn/VFs/
6 URL: http://www.mgc.ac.cn/cgi-bin/VFs/v5/main.cgi?func=VFanalyzer/
7 Comprehensive Antibiotic Resistance Database.
URL: https://card.mcmaster.ca/
8 URL: https://cge.food.dtu.dk/services/
About the authors
Olga L. Voronina
N.F. Gamaleya National Research Center for Epidemiology and Microbiology
Email: olv550@gmail.com
ORCID iD: 0000-0001-7206-3594
Cand. Sci. (Biol.), Assistant Professor, Head, Laboratory of genome analysis
Russian Federation, MoscowNatalia N. Ryzhova
N.F. Gamaleya National Research Center for Epidemiology and Microbiology
Email: rynatalia@yandex.ru
ORCID iD: 0000-0001-5361-870X
Cand. Sci. (Biol.), senior researcher, Laboratory of genome analysis
Russian Federation, MoscowMarina S. Kunda
N.F. Gamaleya National Research Center for Epidemiology and Microbiology
Email: markunda99@gmail.com
ORCID iD: 0000-0003-1945-0397
Cand. Sci. (Biol.), senior researcher, Laboratory of genome analysis
Russian Federation, MoscowEkaterina I. Aksenova
N.F. Gamaleya National Research Center for Epidemiology and Microbiology
Author for correspondence.
Email: aksenova16@yandex.ru
ORCID iD: 0000-0003-2704-6730
Cand. Sci. (Biol.), senior researcher, Laboratory of genome analysis
Russian Federation, MoscowTatiana I. Karpova
N.F. Gamaleya National Research Center for Epidemiology and Microbiology
Email: dragovtceva@yandex.ru
ORCID iD: 0000-0002-9633-7876
D. Sci. (Biol.), leading researcher, Laboratory of legionellosis
Russian Federation, MoscowAlina R. Melkumyan
F.I. Inosemtsev City Clinical Hospital
Email: alinamelkumyan@yandex.ru
ORCID iD: 0000-0002-5494-415X
Cand. Sci. (Med.), Нead, Laboratory diagnostics center
Russian Federation, MoscowElena A. Klimova
A.I. Yevdokimov Moscow State University of Medicine and Dentistry
Email: elena_klimova_@mail.ru
ORCID iD: 0000-0003-4319-8144
D. Sci. (Med.), Professor, Department of infectious diseases and epidemiology
Russian Federation, MoscowGalina N. Karetkina
A.I. Yevdokimov Moscow State University of Medicine and Dentistry
Email: karetkinagn@mail.ru
ORCID iD: 0000-0001-7850-2826
Cand. Sci. (Med.), Assistant Professor, Department of infectious diseases and epidemiology
Russian Federation, MoscowEvgeniy A. Posukhovsky
A.I. Yevdokimov Moscow State University of Medicine and Dentistry
Email: kaempfer@inbox.ru
ORCID iD: 0000-0002-7763-0313
resident doctor, Department of infectious diseases and epidemiology
Russian Federation, MoscowOlga A. Gruzdeva
Russian Medical Academy of Continuous Professional Education
Email: gruzdeva_oa@mail.ru
ORCID iD: 0000-0002-1244-1925
D. Sci. (Med.), Professor, Department of epidemiology
Russian Federation, MoscowIgor S. Tartakovsky
N.F. Gamaleya National Research Center for Epidemiology and Microbiology
Email: itartak@list.ru
ORCID iD: 0000-0003-4825-8951
D. Sci. (Biol.), Professor, Head, Laboratory of legionellosis
Russian Federation, MoscowReferences
- Psareva E.K., Egorova I.Y., Liskova E.A., et al. Retrospective study of Listeria monocytogenes isolated in the territory of inner Eurasia from 1947 to 1999. Pathogens. 2019;8(4):184. DOI: https://doi.org/10.3390/pathogens8040184
- Асташкин Е.И., Алексеева Е.А., Борзенков В.Н. и др. Молекулярно-генетическая характеристика полирезистентных штаммов Listeria monocytogenes и идентификация новых сиквенс-типов. Молекулярная генетика, микробиология и вирусология. 2021;39(4):3–13. DOI: https://doi.org/10.17116/molgen2021390413 EDN: https://www.elibrary.ru/eixxrf. Astashkin E.I., Alekseeva E.A., Borzenkov V.N., et al. Molecular-genetic characteristics of polyresistant Listeria monocytogenes strains and identification of new sequence types. Molecular Genetics, Microbiology and Virology. 2021; 36(4): 159–69. DOI: https://doi.org/10.3103/S0891416821040029 EDN: https://www.elibrary.ru/xverak
- Воронина О.Л., Кунда М.С., Рыжова Н.Н. и др. Листериоз: генотипирование как ключ к выявлению возможного источника заражения. Клиническая микробиология и антимикробная химиотерапия. 2019;21(4):261–73. Voronina O.L., Kunda M.S., Ryzhova N.N., et al. Listeriosis: genotyping as a key for identification a possible source of infection. Clinical Microbiology and Antimicrobial Chemotherapy. 2019;21(4):261–73. DOI: https://doi.org/10.36488/cmac.2019.4.261-273 EDN: https://www.elibrary.ru/chgmuc
- Psareva E.K., Liskova E.A., Razheva I.V., et al. Diversity of Listeria monocytogenes strains isolated from food products in the Central European part of Russia in 2000-2005 and 2019-2020. Foods. 2021;10(11):2790. DOI: https://doi.org/10.3390/foods10112790
- Yushina Yu.K., Kuznetsova O.A., Tutelyan A.V., et al. Prevalence of Listeria monocytogenes in meat products during 2017–2019 depending on technological factors and seasons. Theory and Practice of Meat Processing. 2022; 7(4): 238–46. DOI: https://doi.org/10.21323/2414-438X-2022-7-4-238-246
- Белова Л.В., Репникова Н.Е., Сухова Е.А. Результаты микробиологических исследований рыбы и рыбопродуктов и требования по профилактике заболеваний листериозом. Проблемы медицинской микологии. 2022;24(2):35–7. Belova L.V., Repnikova N.E., Sukhova E.A. Results of microbiological studies of fish and fish products and requirements for the prevention of listeriosis diseases. Problems in Medical Mycology. 2022;24(2):35–7. DOI: https://doi.org/10.24412/1999-6780-2022-2-34-37 EDN: https://www.elibrary.ru/ctfvvk
- Voronina O.L., Ryzhova N.N., Kunda M.S., et al. Diversity and pathogenic potential of Listeria monocytogenes isolated from environmental sources in the Russian Federation. IJMER. 2015;5(3):5–15.
- Алексеева E.A., Полосенко О.В., Фурсова Н.К. и др. Первый случай выявления Listeria monocytogenes сиквенс-типов ST7, ST20, ST425 в сточных водах при обследовании водных объектов Вологодской области. Журнал микробиологии, эпидемиологии и иммунобиологии. 2022;99(4):453–64. Alekseeva E.A., Polosenko O.V., Fursova N.K., et al. The first case of detection of Listeria monocytogenes sequence types ST7, ST20, ST425 in wastewater during an investigation of water bodies in the Vologda region. Journal of Microbiology Epidemiology and Immunobiology. 2022;99(4):453–64. DOI: https://doi.org/10.36233/0372-9311-266 EDN: https://www.elibrary.ru/gashhr
- Климова Е.А., Воронина О.Л., Кареткина Г.Н. и др. Листериоз и пандемия COVID-19. Инфекционные болезни: новости, мнения, обучение. 2022;11(1):102–12. Klimova E.A., Voronina O.L., Karetkina G.N., et al. Listeriosis and the COVID-19 pandemic. Infectious Diseases: News, Opinions, Training. 2022;11(1):102–12. DOI: https://doi.org/10.33029/2305-3496-2022-11-1-102-112 EDN: https://www.elibrary.ru/cgttxv
- Воронина О.Л., Тартаковский И.С., Ющук Н.Д. и др. Анализ спорадических случаев инвазивного листериоза в мегаполисе. Журнал микробиологии, эпидемиологии и иммунобиологии. 2020;97(6):547–55. Voronina O.L., Tartakovskii I.S., Yushchuk N.D., et al. Analysis of sporadic cases of invasive listeriosis in a metropolis. Journal of Microbiology Epidemiology and Immunobiology. 2020;97(6):547–55. DOI: https://doi.org/10.36233/0372-9311-2020-97-6-3 EDN: https://www.elibrary.ru/dziqzj
- Воронина О.Л., Рыжова Н.Н., Аксёнова Е.И. и др. Генетическое разнообразие листерий, выявленных в производственной среде переработки мяса. Молекулярная генетика, микробиология и вирусология. 2023;41(1):24–31. Voronina O.L., Ryzhova N.N., Aksenova E.I., et al. Genetic diversity of listeria found in the meat processing environment. Molecular Genetics, Microbiology and Virology. 2023;41(1):24–31. DOI: https://doi.org/10.17116/molgen20234101124 EDN: https://www.elibrary.ru/wtlkqz
- Moura A., Criscuolo A., Pouseele H., et al. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat. Microbiol. 2016;2:16185. DOI: https://doi.org/10.1038/nmicrobiol.2016.185.
- Chen L., Yang J., Yu J., et al. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res. 2005;33(Issue suppl. 1):D325–8. DOI: https://doi.org/10.1093/nar/gki008
- Alcock B.P., Huynh W., Chalil R., et al. CARD 2023: expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023;51(D1):D690–9. DOI: https://doi.org/10.1093/nar/gkac920
- Chmielowska C., Korsak D., Chapkauskaitse E., et al. Plasmidome of Listeria spp. — the repA-family business. Int. J. Mol. Sci. 2021;22(19):10320. DOI: https://doi.org/10.3390/ijms221910320
- Gushchin V.A., Dolzhikova I.V., Shchetinin A.M., et al. Neutralizing activity of sera from Sputnik V-vaccinated people against variants of concern (VOC: B.1.1.7, B.1.351, P.1, B.1.617.2, B.1.617.3) and Moscow endemic SARS-CoV-2 variants. Vaccines. 2021;9(7):779. DOI: https://doi.org/10.3390/vaccines9070779
- Klink G.V., Safina K.R., Nabieva E., et al. The rise and spread of the SARS-CoV-2 AY.122 lineage in Russia. Virus Evol. 2022;8(1):veac017. DOI: https://doi.org/10.1093/ve/veac017
- Gangavarapu K., Latif A.A., Mullen J.L., et al. Outbreak.info genomic reports: scalable and dynamic surveillance of SARS-CoV-2 variants and mutations. Nat. Methods. 2023; 20(4): 512–22. DOI: https://doi.org/10.1038/s41592-023-01769-3
- Воронина О.Л., Кунда М.С., Рыжова Н.Н. и др. Закономерности селекции полигостальных убиквитарных микроорганизмов на примере представителей трех таксонов. Молекулярная биология. 2015;49(3):430–41. DOI: https://doi.org/10.7868/S0026898415030179 EDN: https://www.elibrary.ru/tqqvfh. Voronina O.L., Kunda M.S., Ryzhova N.N., et al. Regularities of the ubiquitous polyhostal microorganisms selection by the example of three taxa. Molecular Biology. 2015;49(3):380–90. DOI: https://doi.org/10.1134/S0026893315030176 EDN: https://www.elibrary.ru/ueytrz
- Adgamov R., Zaytseva E., Thiberge J.M., et al. Genetically related Listeria monocytogenes strains isolated from lethal human cases and wild animals. In: Caliskan M., ed. Genetic Diversity in Microorganisms. Chapter 9. IntechOpen; 2012. DOI: https://doi.org/10.5772/32913
- Lebrun M., Loulergue J., Chaslus-Dancla E., Audurier A. Plasmids in Listeria monocytogenes in relation to cadmium resistance. Appl. Environ. Microbiol. 1992;58(9):3183–6. DOI: https://doi.org/10.1128/aem.58.9.3183-3186.1992
- Vedantam G., Guay G.G., Austria N.E., et al. Characterization of mutations contributing to sulfathiazole resistance in Escherichia coli. Antimicrob. Agents Chemother. 1998;42(1):88–93. DOI: https://doi.org/10.1128/AAC.42.1.88
- Aldred K.J., Kerns R.J., Osheroff N. Mechanism of quinolone action and resistance. Biochemistry. 2014;53(10):1565–74. DOI: https://doi.org/10.1021/bi5000564
- Maury M.M., Tsai Y.H., Charlier C., et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2016;48(3):308–13. DOI: https://doi.org/10.1038/ng.3501
- Šteingolde Ž., Meistere I., Avsejenko J., et al. Characterization and genetic diversity of Listeria monocytogenes isolated from cattle abortions in Latvia, 2013–2018. Vet. Sci. 2021;8(9):195. DOI: https://doi.org/10.3390/vetsci8090195
- Russini V., Spaziante M., Zottola T., et al. A nosocomial outbreak of invasive listeriosis in an Italian hospital: epidemiological and genomic features. Pathogens. 2021;10(5):591. DOI: https://doi.org/10.3390/pathogens10050591
- Lachmann R., Halbedel S., Adler M., et al. Nationwide outbreak of invasive listeriosis associated with consumption of meat products in health care facilities, Germany, 2014–2019. Clin. Microbiol. Infect. 2021;27(7):1035.e1–e5. DOI: https://doi.org/10.1016/j.cmi.2020.09.020
- Каганова М.А., Спиридонова Н.В., Казакова А.В. и др. Особенности микробиоты цервикального канала при дородовом излитии околоплодных вод и доношенной беременности. Акушерство и гинекология. 2019;(5):77–84. Kaganova M.A., Spiridonova N.V., Kazakova A.V., et al. Features of the cervical canal microbiota in prenatal amniorrhea and full-term pregnancy. Obstetrics and Gynecology. 2019;(5):77–84.DOI: https://doi.org/10.18565/aig.2019.5.77-84 EDN: https://www.elibrary.ru/hscrpw
Supplementary files