Explanatory models for tick-borne disease incidence (Astrakhan rickettsial fever and Crimean-Congo hemorrhagic fever)
- Authors: Dubyanskiy V.M.1,2, Prislegina D.A.1,2, Platonov A.E.2
-
Affiliations:
- Stavropol Plague Control Research Institute
- Central Research Institute for Epidemiology
- Issue: Vol 100, No 1 (2023)
- Pages: 34-45
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/5417
- DOI: https://doi.org/10.36233/0372-9311-344
- ID: 5417
Cite item
Abstract
Introduction. The study focuses on methods providing mathematical substantiation of discrepancies between actual incidence rates of Astrakhan rickettsial fever (ARF) and Crimean-Congo hemorrhagic fever (CCHF) and predicted rates due to the indirect impact of weather conditions during the current epidemic season.
The purpose of the study was to develop explanatory models for ARF and CCHF incidence using satellite monitoring (remote sensing) data and to present the results of their practical evaluation in the Stavropol Territory and Astrakhan Region.
Materials and methods. The materials included climate data provided by the Space Research Institute of the Russian Academy of Sciences as well as epidemiological data on CCHF and ARF incidence from 2005 to 2021. The explanatory models incorporated the Bayes theorem and Wald sequential analysis. All the calculations were completed using the Microsoft Excel 2010-based program developed by the authors.
Results. It has been found that the greatest indirect effect on development of the CCHF epidemiological situation is produced by the normalized difference vegetation index and relative air humidity in June-July in the Stavropol Territory and by the maximum, minimum and average air temperature in October as well as the minimum air temperature in July in the Astrakhan Region. ARF incidence rates depend on the indirect effect of the annual average and average annual maximum temperature, maximum temperature and the normalized difference vegetation index in April-July. The match between explanatory model-based results and prediction model-based results ranged within 46.2-100%.
Discussion. In addition to projecting incidence rates, which could be reached with the observed values of climatic factors in the current year, the explanatory models can be used for indirect verification of prediction models and for identification of factors causing differences in results.
Conclusion. The practical evaluation of explanatory models confirms the prospects and benefits of the study that should be continued, involving other regions highly endemic for tick-borne infections.
Full Text
Introduction
Forecasting of dynamics and incidence rates of tick-borne transmissible infections (TBTI) is an important component of the epidemiological surveillance over these dangerous natural-focal diseases endemic in Russia. Multiple studies address epidemiological forecasts for such TBTI diseases as tick-borne viral encephalitis, Astrakhan rickettsial fever (ARF), tick-borne borreliosis and Crimean-Congo hemorrhagic fever (CCHF) [1–10].
Prediction data for an epidemiological situation play a significant role in planning of preventive (including acaricide treatment) measures; the prediction accuracy is essential not only for justification of their scientific and economic viability, but also for application of a differentiated approach in planning. Therefore, when significant differences are observed between the predicted and reported intensity levels of the epidemic process, the factors causing them should be explored thoroughly. Such differences can be caused not only by shortcomings of the existing methods that need to be upgraded, but also by external indirect effects produced by weather conditions in the current year on the activity of arthropods transmitting TBTI, which cannot be predicted when preparing short-range (for the coming year), let alone medium and long-range forecasts. It is known that an epidemic season can start early or late, depending on the spring daytime and nighttime temperature that must be +9ºC and at least +2ºC, respectively, so that dormant species Hyalomma marginatum (the main transmitters of the CCHF pathogen) become active and start feeding on farm animals, while their parasitizing activity reaches peak levels at the monthly average temperature of +16.9ºC [11–13].
The significant indirect effect produced by climatic factors on the CCHF dynamics and incidence rates during the year is confirmed by multiple studies in other countries. For example, in Southeastern Iran, scientists, using logistic regression, found a significant relationship between the number of CCHF cases and the monthly average temperature (the direct relationship with a two-month lag and the inverse relationship with a five-month lag), monthly maximum relative humidity and accumulated precipitation with a two-month lag and a five-month lag, respectively [14]. The study based on the Poisson regression analysis and the McFadden pseudo R-squared demonstrated that in Eastern Iran the number of cases highly correlated with maximum temperature (during the previous month) and relative air humidity (during the previous month and half a year) levels [15]. In addition, using logistic regression, Iranian scientists have found that when the maximum temperature during 3 previous months increases by 1ºC and relative humidity during 2 previous months increases by 1%, the risk of disease case occurrence increases by 9% and 4%, respectively [16].
The one-way analysis of variance (ANOVA), which was performed in Bulgaria, demonstrates that the one-unit increase in the average air temperature and the normalized difference vegetation index (NDVI) results in a 5.5% increase in the intensity of the CCHF epidemic process [17]. In the meantime, no extensive studies addressing the development of methods that would identify and explain, using mathematical statistics, cause-and-effect relationships between prediction results for CCHF and ARF incidence, actual data and the impact of climatic factors in the current year have been conducted so far.
The purpose of the study was to develop explanatory models for ARF and CCHF incidence and to present the results of their practical evaluation in the Stavropol Territory and Astrakhan Region.
Materials and methods
This comprehensive study is a continuation of the previous study involving the development of prediction models. ARF and CCHF have been selected as target diseases representing the most common TBTIs in the south of Russia. The Stavropol Territory and Astrakhan Region were selected due to high intensity of epidemic processes in terms of these infections.
The study was conducted using epidemiological and statistical research methods. The retrospective epidemiological analysis was conducted using the data from databases1 for ARF and CCHF incidence, which were developed within the RSF project (No. 19-75-20088). The relative rates of CCHF and ARF incidence (per 100,000 population) for each administrative district in the selected regions were estimated using data from the Federal Statistics Service2 for each year of the studied period. Hydro-meteorological data3 (independent variables) were represented by numeric values of 13 climatic factors, which were obtained using remote sensing satellites (for each month of the studied period and annual average):
- air temperature — average, maximum and minimum (ºC);
- soil temperature at depths of 10 and 40 cm (ºC);
- soil moisture content at depths of 10 and 40 cm (%);
- snow depth (m);
- area covered by snow (%);
- pressure (Pa);
- relative air humidity (%);
- precipitation (kg/m2);
- NDVI (relative units).
Thus, a total of 169 parameters were used as initial data that were further screened and downsized to the most important (informative) parameters.
The explanatory models were developed using nonparametric statistics — Bayes theorem and Wald analysis; the informativeness coefficients for factors were calculated using the Kullback method [18–20]. A factor was seen as informative and was used for further calculations, if its informativeness value was > 0.5.
Informativeness and prediction coefficients were calculated automatically using the Microsoft Excel 2010-based program developed by the authors during their previous studies (for prediction models) [10, 21, 22].
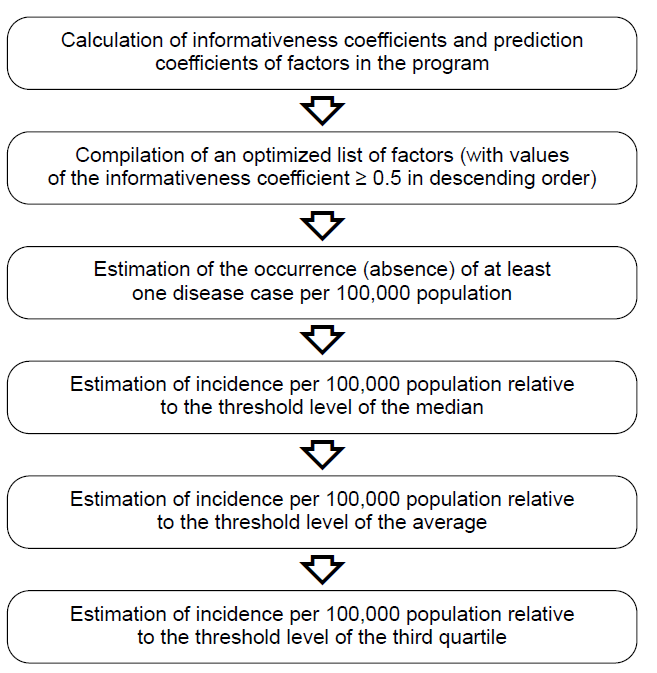
Mathematical calculations for explanatory models were performed similarly to the calculations for prediction models; the variables of climatic factors for the previous years were replaced with numeric values for the current epidemic season [21]. The step-by-step algorithm for calculating explanatory models is shown in Figure.
Algorithm for calculating explanatory models.
The threshold level of the probability of positive solution for explanatory models for CCHF incidence dynamics for the Stavropol Territory and Astrakhan Region was set at 99.0% (the error probability was 1.0%). At this stage of the study, the threshold for ARF was 90% (at the error probability of 10%), which can be explained by a low proportion of informative parameters from the list. In future, when new additional variables (such as accumulated values of climatic data) are used, the percentage of error probability can be reduced.
The calculations were made by summing up the values of prediction coefficients of the informative factors in accordance with their values for each administrative district until the numeric value of +20 or –20 was received for CCHF models and +9 or –9 for ARF models to indicate the occurrence/absence of at least 1 disease case per 100,000 population with a probability of 99% and 90%, respectively. Then, when the districts with positive results were identified, the prediction for them was made against other selected threshold levels of incidence.
The median, average and third quartile values for models for CCHF incidence dynamics for the Stavropol Territory were 0.9, 3.5 and 4.7; 0.5, 1 and 2 for the Astrakhan Region; and for the model for ARF incidence dynamics in the Astrakhan Region — 25, 39.5 and 62.4, respectively.
The calculations for explanatory models for CCHF incidence dynamics for the Stavropol Territory were based on retrospective data for 2005–2019, for CCHF and ARF in the Astrakhan Region - for 2013-2019.
The pilot testing of the performance of the models was conducted using retrospective data for 2018-2020; the evaluation was performed using data for 2021.
Results and discussion
The explanatory models were developed, addressing the following tasks:
1) assessment of the indirect relationship between the weather conditions during the current epidemic season and the intensity of the CCHF and ARF epidemic process, using informativeness coefficients;
2) estimation of the incidence rate that could be reached at the observed values of climatic factors during the current year (exclusive of the impact of the hydro-meteorological data of the previous year) and its subsequent comparison with the actual rates;
3) indirect verification of prediction models for CCHF and ARF incidence to identify the factors causing erroneous results or, on the opposite, to confirm the accuracy of the performance of the above models.
During the assessment stage it was found that the climatic factors that were critical for development of explanatory models for different regions differed significantly. Based on the calculated coefficients of informativeness, the most informative factors for the Stavropol Territory were NDVI and relative air humidity in June–July, demonstrating complete consistency with the published data [23–27]. For the CCHF model in the Astrakhan Region, the highest values of informativeness coefficients were received for the maximum, minimum and average air temperature in October as well as for the minimum air temperature in July, thus also showing no discrepancy with the published data on the impact of weather conditions on tick activity [25–28]. Meanwhile, such differences are expected and can be explained by significant differences in landscape, climatic, hydrological and other conditions in the Stavropol Territory and Astrakhan Region, which have a significant effect on the epizootological and epidemiological CCHF situation [29–31]. The most informative parameters for the explanatory model for ARF incidence dynamics in the Astrakhan Region were the annual average and average annual maximum temperature, maximum air temperature and NDVI in April–July, which is also consistent with the published data [25, 28].
The accuracy of explanatory models was verified by comparison of the calculated data with the reported incidence rates for each administrative district.
As with prediction models, the model-based erroneous predictions were divided into 4 categories [21]:
- false-positive — the result of the explanatory model for the first stage is positive; however, no disease cases have been reported;
- false-negative — the result for the first stage is negative; however, disease cases (at least 1) have been reported;
- overestimated — the actual incidence rate is lower than the estimated rate at the set threshold level;
- underestimated — the actual incidence rate is higher than the estimated rate at the set threshold level.
The results of testing and evaluation of explanatory models are presented in Tables 1–3.
Table 1. Testing results for the explanatory model for CCHF incidence dynamics for 2018–2020 (retrospectively) and evaluation results in 2021 (the Stavropol Territory)
Result | Years | |||||||
2018 | 2019 | 2020 | 2021 | |||||
number of districts | % | number of districts | % | number of districts | % | number of districts | % | |
Correct | 10 | 38.5 | 13 | 50.0 | 8 | 30.8 | 16 | 61.5 |
False positive | 8 | 30.8 | 7 | 26.9 | 13 | 50.0 | 5 | 19.2 |
False negative | – | – | 2 | 7.7 | 1 | 3.8 | 2 | 7.7 |
Overestimated | 8 | 30.8 | 4 | 15.4 | 4 | 15.4 | 3 | 11.5 |
Table 2. Testing results for the explanatory model for CCHF incidence dynamics for 2018–2020 (retrospectively) and evaluation results in 2021 (the Astrakhan Region)
Result | Years | |||||||
2018 | 2019 | 2020 | 2021 | |||||
number of districts | % | number of districts | % | number of districts | % | number of districts | % | |
Correct | 5 | 41.7 | 7 | 58.3 | 2 | 16.7 | 12 | 100.0 |
False positive | 5 | 41.7 | 4 | 33.3 | 10 | 83.3 | – | – |
False negative | – | – | – | – | – | – | – | – |
Overestimated | 2 | 16.7 | 1 | 8.3 | – | – | – | – |
Table 3. Testing results for the explanatory model for ARF incidence dynamics for 2018–2020 (retrospectively) and evaluation results in 2021 (the Astrakhan Region)
Result | Years | |||||||
2018 | 2019 | 2020 | 2021 | |||||
number of districts | % | number of districts | % | number of districts | % | number of districts | % | |
Correct | 9 | 75.0 | 8 | 66.7 | 3 | 25.0 | 6 | 50.0 |
False positive | – | – | – | – | 4 | 33.3 | – | – |
False negative | 1 | 8.3 | 1 | 8.3 | – | – | – | – |
Overestimated | 2 | 16.7 | 3 | 25.0 | 5 | 41.7 | 6 | 50.0 |
Thus, the accuracy of the obtained results compared to the actual data for explanatory models for CCHF incidence dynamics in the Stavropol Territory ranged from 30.8% (8 districts) in 2020 to 61.5% (16 districts) in 2021; in the Astrakhan Region — from 16.7% (2 districts) in 2020 to 100% (12 districts) in 2021. The verification of ARF models showed that the accuracy of results ranged from 25% in 2020 (3 districts) to 75% (9 districts) in 2018. However, if like with the earlier developed prediction models (and considering the below-given explanations about a significant effect produced by factors of the previous year on the formation of tick populations during the current epidemic season), the focus of error assessment is shifted to false-negative results that are seen as truly erroneous, the average accuracy of CCHF models in the studied period for the Stavropol Territory and Astrakhan Region will be 95.2% and 100%, respectively, and 95.9% for the ARF model.
The obtained results demonstrate satisfactory performance of the developed models and confirm that the right method has been selected for achievement of the study objectives.
Special attention should be given to the possibility to compare the results of explanatory models with those obtained by using the prediction models and the actual data; this comparison provides answers to several important questions. Firstly, climatic factors, which most likely were the reason for the prediction failure, can be identified in each case of the wrongly predicted and the correct explanatory result. For example, when the weather conditions were favorable for formation of high numbers of tick populations during the previous year, the climatic factors, which were unfavorable for the activity of transmitters during the spring-summer period of the current year (the low temperature in May, continuous rainy days in June-July), contributed indirectly to lower incidence rates among the population, which matched the reported rates. On the contrary, the air temperature that was higher compared to the mean temperature in April–May could bring along the earlier beginning of the epidemic season; its length can be also increased due to warm, calm weather in September. Considering such unpredictable weather changes, our prediction models incorporate a calculated error probability of 1% and 10%, respectively. Secondly, the above comparison can be used for indirect verification of the performance of prediction models. For example, false-positive or overestimated results obtained concurrently in both models can be seen as a proof of accuracy of the prediction model and can imply that not all cases have been detected due to under-diagnosis of mild cases or due to other external factors that are not directly related to the TBTI epidemic process, as we are going to discuss further in the article. Thirdly, the concurrent false-negative results can suggest the existence of an imported case (when a patient got infected (was bitten by a tick) during their stay in another administrative district or outside the region) or can suggest the existence of factors, which cannot be taken into consideration (for example, a person can get infected through the close contact with tick-infested cattle driven from another district highly endemic for CCHF).
The retrospective data for 2018-2020 show that the match between the results of explanatory models for CCHF incidence dynamics and the similar estimated data for prediction models for the Stavropol Territory ranged from 46.2% in 2018 (12 districts) to 100% in 2019-2020 (26 districts), and for the Astrakhan region, it ranged from zero due to the absence of results in 2020 to 100% in 2019 (12 districts). For the ARF models, the proportion of matching results ranged from 66.7% in 2020 (8 districts) to 83.3% (10 districts) in 2019.
The comparison results for 2021 are presented in Tables 4–6.
Table 4. Comparison of results obtained by using prediction and explanatory models for CCHF incidence dynamics for the Stavropol Territory (for 2021)
Administrative district | Prediction result | Interpretation of prediction | Interpretation of explanation | Explanation result | Actual incidence per 100,000 population) |
Alexandrovsky | ≤ 0,000009 | Correct | ≤ 0,000009 | Correct | 0 |
Andropovsky | ≤ 0.000009 | Correct | ≤ 0.000009 | Correct | 0 |
Apanasenkovsky | > 4.7 | Correct | > 4.7 | Correct | 10.1 |
Arzgirsky | > 4.7 | Correct | > 4.7 | Correct | 8.3 |
Blagodarnensky | > 4.7 | Correct | > 4.7 | Correct | 5.2 |
Budennovsky | > 4.7 | False positive | > 4.7 | False positive | 0 |
Georgievsky | > 0.9 | False positive | ≤ 0.000009 | Correct | 0 |
Grachevsky | ≤ 0.000009 | False negative | ≤ 0.000009 | False negative | 2.7 |
Izobilnensky | > 4.7 | False positive | ≤ 0.000009 | Correct | 0 |
Ipatovsky | > 4.7 | Correct | > 4.7 | Correct | 5.4 |
Kirovsky | ≤ 0.000009 | Correct | ≤ 0.000009 | Correct | 0 |
Kochubeevsky | ≤ 0.000009 | Correct | ≤ 0.000009 | Correct | 0 |
Krasnogvardeisky | > 4.7 | Correct | > 4.7 | Correct | 5.4 |
Kursky | > 4.7 | False positive | > 4.7 | False positive | 0 |
Levokumsky | > 4.7 | False positive | > 4.7 | False positive | 0 |
Mineralovodsky | ≤ 0.000009 | Correct | ≤ 0.000009 | Correct | 0 |
Neftekumsky | > 4.7 | Overestimated | > 4.7 | Overestimated | 1.6 |
Novoaleksandrovsky | > 4.7 | False positive | ≤ 0.000009 | Correct | 0 |
Novoselytsky | > 4.7 | False positive | ≤ 0.000009 | Correct | 0 |
Petrovsky | > 4.7 | Overestimated | > 4.7 | Overestimated | 1.4 |
Predgornyy | ≤ 0.000009 | Correct | ≤ 0.000009 | Correct | 0 |
Soviet | > 4.7 | False positive | ≤ 0.000009 | Correct | 0 |
Stepnovsky | > 4.7 | False positive | > 4.7 | False positive | 0 |
Trunovsky | > 4.7 | Overestimated | > 4.7 | Overestimated | 3.4 |
Turkmensky | > 4.7 | False positive | > 4.7 | False positive | 0 |
Shpakovsky | ≤ 0.000009 | False negative | ≤ 0.000009 | False negative | 1.3 |
Table 5. Comparison of results obtained by using prediction and explanatory models for CCHF incidence dynamics for the Astrakhan Region (for 2021)
Administrative district | Prediction result | Interpretation of prediction | Interpretation of explanation | Explanation result | Actual incidence per 100,000 population) |
Astrakhan | > 2 | False positive | ≤ 0,000009 | Correct | 0 |
Akhtubinsky | ≤ 0.000009 | Correct | ≤ 0.000009 | Correct | 0 |
Volodarsky | > 2 | False positive | ≤ 0.000009 | Correct | 0 |
Enotaevsky | > 2 | False positive | ≤ 0.000009 | Correct | 0 |
Ikryaninsky | > 2 | False positive | ≤ 0.000009 | Correct | 0 |
Kamyzyaksky | > 2 | False positive | ≤ 0.000009 | Correct | 0 |
Krasnoyarsky | > 2 | False positive | ≤ 0.000009 | Correct | 0 |
Limansky | > 2 | False positive | ≤ 0.000009 | Correct | 0 |
Narimanovsky | > 2 | False positive | ≤ 0.000009 | Correct | 0 |
Privolzhskiy | > 2 | False positive | ≤ 0.000009 | Correct | 0 |
Kharabalinsky | > 2 | False positive | ≤ 0.000009 | Correct | 0 |
Chernoyarsky | ≤ 0.000009 | Correct | ≤ 0.000009 | Correct | 0 |
Table 6. Comparison of results obtained by using prediction and explanatory models for ARF incidence dynamics for the Astrakhan Region (for 2021)
Administrative district | Prediction result | Interpretation of prediction | Interpretation of explanation | Explanation result | Actual incidence per 100,000 population) |
Astrakhan | ≤ 62.4 | Overestimated | ≤ 39.5 | Overestimated | 3.24 |
Akhtubinsky | > 62.4 | False positive | ≤ 0.000009 | Correct | 0.00 |
Volodarsky | ≤39.5 | Overestimated | ≤ 25 | Correct | 2.18 |
Enotaevsky | > 62.4 | Overestimated | ≤ 25 | Correct | 4.06 |
Ikryaninsky | ≤ 62.4 | Overestimated | ≤ 39.5 | Overestimated | 15.24 |
Kamyzyaksky | ≤ 39.5 | Overestimated | ≤ 25 | Correct | 6.54 |
Krasnoyarsky | > 62.4 | Overestimated | > 62.4 | Overestimated | 32.82 |
Limansky | > 62.4 | Overestimated | ≤ 39.5 | Overestimated | 6.93 |
Narimanovsky | > 62.4 | Overestimated | ≤ 39.5 | Overestimated | 17.04 |
Privolzhskiy | ≤ 62.4 | Overestimated | ≤ 39.5 | Correct | 25.95 |
Kharabalinsky | > 62.4 | Overestimated | > 62.4 | Overestimated | 46.09 |
Chernoyarsky | ≤ 25 | False positive | ≤ 0.000009 | Correct | 0.00 |
The above tables also demonstrate that the offered models provide clear explanation of differences between the actual and predicted rates due to the effects of factors during the current year. For example, for 5 administrative districts in the Stavropol Territory (Georgiyevsky, Izobilnensky, Novoalexandrovsky, Novoselitsky and Sovetsky) having false-positive predicted results, the explanatory model provided the results matching the actual data (absence of CCHF cases) mostly due to the adverse weather conditions during the spring-summer period. For the models used in the Astrakhan Region, the similar situation for CCHF was observed in 2 cases — in Astrakhan and the Kharabalinsky District; for ARF — for 2 districts having false-positive predicted results (Akhtubinsky and Chernoyarsky Districts) and 4 districts with overestimated predicted results (Volodarsky, Yenotayevsky, Kamyzyaksky and Privolzhsky Districts). The evaluation results show that explanatory models can and should be used to achieve the set objectives.
When analyzing the performance of the models, it should be remembered that 2 years of testing and evaluation (2020 and partially 2021) coincided with the COVID-19 pandemic period. The decrease in the incidence of almost all TBTI forms, which was observed during that period due to the implementation of restrictive measures, the reduced number of specific laboratory tests for diagnosis verification, the probability of under-diagnosis of mild CCHF and ARF cases because of high overload and repurposing of treatment and preventive care facilities, may have affected the accuracy of results obtained by using explanatory models for 2020. The external impact on the performance of the models is also demonstrated by a high percentage of concurrently obtained false-positive (overestimated) results both for prediction and explanation for the same districts – for CCHF in the Stavropol Territory (17 districts) and for ARF in the Astrakhan Region (7 districts). Therefore, the accurate assessment of the effectiveness and accuracy of the models can be made only after stabilization of the COVID-19 epidemiological situation.
Conclusion
The authors have made an attempt to solve the problem addressing the interrelated and divergent effects produced by the factors of the previous and current years on the intensity of TBTI epidemic processes in the south of Russia and to receive a mathematically grounded answer to the common question "Why did the epidemiological forecast fail to come true?". During the study conducted using data for the Stavropol Territory and Astrakhan Region, we developed explanatory models for incidence dynamics of ARF and CCHF, which are the most common TBTIs in the Southern and North Caucasian Federal Districts. The results obtained after testing and evaluation of the models are quite satisfactory and prove that they can be used independently for assessment of the impact of weather conditions during the current epidemic season on the epidemiological situation for TBTI as well as for verification of the earlier developed prediction models and identification of the factors causing differences between the predicted and the reported incidence rates.
The performance of the models needs further verification, especially during the stabilization of the epidemiological situation for COVID-19, and further improvement to increase accuracy of the obtained results (the search for additional informative climatic and other factors such as accumulated temperature, precipitation, etc.). The study will be continued, focusing on development of similar models for other regions in the south of Russia, which are highly endemic for TBTI (Rostov and Volgograd Regions).
About the authors
Vladimir M. Dubyanskiy
Stavropol Plague Control Research Institute; Central Research Institute for Epidemiology
Email: daria775@rambler.ru
ORCID iD: 0000-0003-3817-2513
D. Sci. (Biol.), Head, Department of epizootological monitorning and prognostication, member of the temporary research team for the implementation of the grant of the Russian Science Foundation Central Research
Russian Federation, Stavropol; MoscowDaria A. Prislegina
Stavropol Plague Control Research Institute; Central Research Institute for Epidemiology
Email: daria775@rambler.ru
ORCID iD: 0000-0002-9522-129X
Cand. Sci. (Med.), senior researcher, Laboratory of epidemiology, member of the temporary research team for the implementation of the grant of the Russian Science Foundation
Russian Federation, Stavropol; MoscowAlexander E. Platonov
Central Research Institute for Epidemiology
Author for correspondence.
Email: daria775@rambler.ru
ORCID iD: 0000-0001-7450-0081
D. Sci. (Biol.), Prof., chief researcher, Laboratory of zoonoses
Russian Federation, MoscowReferences
- Ugleva S.V., Akimkin V.G., Ponezheva Zh.B., Akhmerova R.R., Spirenkova A.E., Chernikova Yu.Yu., et al. Epidemiological community and differences of transmissive infections in Astrakhan region. Epidemiologiya i vaktsinoprofilaktika. 2021; 20(6): 63–71. https://doi.org/10.31631/2073304620212066371 (in Russian)
- Il’in V.P., Andaev E.I., Balakhonov S.V., Pakskina N.D. Morbidity rate forecasting for 2014 as regards tick-borne viral encephalitis in the territory of the Russian Federation based on multi-factor regression models. Problemy osobo opasnykh infektsiy. 2014; (2): 48–52. https://doi.org/10.21055/0370-1069-2014-2-48-52 (in Russian)
- Berikov V.B., Lbov G.S., Polyakova G.L., Bakhvalova V.N., Panov V.V., Shchuchinova L.D., et al. Analysis of factors influencing the incidence of tick-borne encephalitis, using logical-and-probabilistic and correlation-regression models. Epidemiologiya i vaktsinoprofilaktika. 2011; (6): 25–34. (in Russian)
- Shirokostup S.V., Shevchenko V.V., Luk’yanenko N.V. Results of evaluation of risk factors influence on disease incidence of tick-borne viral encephalitis and ixodic tick-borne borreliosis in combined foci of these infections in altai territory. Meditsinskiy al’manakh. 2014; (4): 67–70. (in Russain)
- Haemig P.D., Sjöstedt de Luna S., Grafström A., Lithner S., Lundkvist Å., Waldenström J., et al. Forecasting risk of tick-borne encephalitis (TBE): using data from wildlife and climate to predict next year’s number of human victims. Scand. J. Infect. Dis. 2011; (5): 366–72. https://doi.org/10.3109/00365548.2011.552072
- Mills J.N., Gage K.L., Khan A.S. Potential influence of climate change on vector-borne and zoonotic diseases: a review and proposed research plan. Environ. Health Perspect. 2010; (11): 1507–14. https://doi.org/10.1289/ehp.0901389
- Estrada-Peña A., Zatansever Z., Gargili A., Aktas M., Uzun R., Ergonul O., et al. Modeling the spatial distribution of Crimean-Congo hemorrhagic fever outbreaks in Turkey. Vector. Borne. Zoonotic. Dis. 2007; 7(4): 667–78. https://doi.org/10.1089/vbz.2007.0134
- Dubyanskiy V.M., Prislegina D.A., Kulichenko A.N. Risk-oriented model for predicting epidemiological situation with crimean-congo hemorrhagic fever (on the example of Stavropol region). Analiz riska zdorov’yu. 2018; (1): 13–21. https://doi.org/10.21668/health.risk/2018.1.02 (in Russian)
- Prislegina D.A., Dubyanskiy V.M., Maletskaya O.V., Kulichenko A.N., Vasilenko N.F., Manin E.A., et al. Crimean-congo hemorrhagic fever in the Stavropol region: contemporaryclinical and epidemio-logical aspects and new approach to forecasting of morbidity. Infektsionnye bolezni: novosti, mneniya, obuchenie. 2018; 7(3): 49–56. https://doi.org/10.24411/2305-3496-2018-13007 (in Russian)
- Prislegina D.A., Maletskaya O.V., Dubyanskiy V.M., Platonov A.E. Crimean-congo hemorrhagic fever in the north caucasian federal district: overview of the epidemiological situation and improvement of morbidity forecasting method. Infektsiya i immunitet. 2022; 12(2): 357–65. https://doi.org/10.15789/2220-7619-CHF-1827 (in Russian)
- Tokhov Yu.M., Degtyarev D.Yu., Dubyanskiy V.M. Ixodid Ticks (Morphology, Medical Significance, Population Regulation) [Iksodovye kleshchi (morfologiya, meditsinskoe znachenie, regulyatsiya chislennosti)]. Stavropol’; 2015. (in Russian)
- Trukhachev V.I., Tokhov Yu.M., Lutsuk S.N., Dylev A.A., Tolokonnikov V.P., D’yachenko Yu.V. Distribution and ecological characteristics of Hyalomma ixodid ticks in the ecosystems of the Stavropol region. Yug Rossii: ekologiya, razvitie. 2016; 11(2): 59–69. https://doi.org/10.18470/1992-1098-2016-2-59-69 (in Russian)
- Tokhov Yu.M., Chumakova I.V., Lutsuk S.N., D’yachenko Yu.V., Kotenev E.S., Zaytsev A.A. Tick as the reservoir of contagious diseases in the Stavropol territory. Vestnik veterinarii. 2013; (2): 19–21. (in Russian)
- Ansari H., Shahbaz B., Izadi S., Zeinali M., Tabatabaee S.M., Mahmoodi M., et al. Crimean-Congo hemorrhagic fever and its relationship with climate factors in southeast Iran: a 13-year experience. J. Infect. Dev. Ctries. 2014; 8(6):749–57. https://doi.org/10.3855/jidc.4020
- Mostafavi E., Chinikar S., Bokaei S., Haghdoost A. Temporal modeling of Crimean-Congo hemorrhagic fever in eastern Iran. Int. J. Infect. Dis. 2013; 17(7): 524–28. https://doi.org/10.1016/j.ijid.2013.01.010
- Mostafavi E., Haghdoost A., Irani A.D., Bokaei S., Chinikar S. Temporal modeling of Crimean-Congo hemorrhagic fever in Iran. J. Med. Microbiol. Infec. Dis. 2014; 1(2): 1–7.
- Vescio F.M., Busani L., Mughini-Gras L., Khoury C., Avellis L., Taseva E., et al. Environmental correlates of Crimean-Congo haemorrhagic fever incidence in Bulgaria. BMC Public Health. 2012; 12: 1116. https://doi.org/10.1186/1471-2458-12-1116
- Gubler E.V. Computational Methods for Analysis and Identification of Pathological Processes [Vychislitel’nye metody analiza i raspoznavaniya patologicheskikh protsessov]. Leningrad: Meditsina; 1978. (in Russian)
- Gubler E.V., Genkin A.A. Application of Statistical Non-Parametric Criteria in Medico-Biological Investigations [Primenenie neparametricheskikh kriteriev statistiki v mediko-biologicheskikh issledovaniyakh]. Leningrad: Meditsina; 1973. (in Russian)
- Dubyanskiy M.A., Kenzhebaev A., Stepanov V.M., Asenov G.A., Dubyanskaya L.D. Prognostication of Plague Epizootic Activity in Sub-Aral and Kyzylkum Areas [Prognozirovanie epizooticheskoy aktivnosti chumy v Priaral’e i Kyzylkumakh]. Nukus: Karakalpakstan; 1992. (in Russian)
- Dubyanskiy V.M., Prislegina D.A., Platonov A.E. Predicting incidence of crimean-congo hemorrhagic fever using satellite monitoring (remote sensing) data in the Stavropol territory. Zhurnal mikrobiologii, epidemiologii i immunobiologii. 2022; 99(3): 322–35. https://doi.org/10.36233/0372-9311-213 (in Russian)
- Prislegina D.A., Dubyanskiy V.M., Kulichenko A.N. Particular dangerous arbovirus fevers in the south of Russia: improvement of monitoring with modern information technology. Meditsina truda i ekologiya cheloveka. 2019; (4): 50–8. https://doi.org/10.24411/2411-3794-2019-10047 (in Russian)
- Kotti B.K., Zaikina I.N., Zhil’tsova M.V. Distribution of the ixodid tick hyalomma marginatum koch (acari: ixodidae) in the Stavropol region. Nauka. Innovatsii. Tekhnologii. 2017; (3): 161–74. (in Russian)
- Tokhov Yu.M. Ixodid Ticks of the Stavropol Territory and Their Epidemiological Significance [Iksodovye kleshchi Stavropol’skogo kraya i ikh epidemiologicheskoe znachenie]. Stavropol’; 2008. (in Russian)
- Balashov Yu.S. Ixodid Ticks are Parasites and Vectors of Infectious Diseases [Iksodovye kleshchi — parazity i perenoschiki infektsiy]. St. Petersburg: Nauka; 1998. (in Russian)
- Koshkina N.A., Goryachaya E.V. The description of tick morphobiology Hyalomma marginatum marginatum and its control. Rossiyskiy parazitologicheskiy zhurnal. 2013; (2): 10–4. (in Russian)
- Nili S., Khanjani N., Jahani Y., Bakhtiari B. The effect of climate variables on the incidence of Crimean Congo Hemorrhagic Fever (CCHF) in Zahedan, Iran. BMC Public Health. 2020; 20(1): 1893. https://doi.org/10.1186/s12889-020-09989-4
- Zimina Yu.V., Kulikova L.N., Sal’ko V.N., Kovtunov A.I. Ixodid ticks of the Astrakhan region, their role in the formation of natural foci and the transmission of arboviruses to humans. In: Questions of Rickettsiology and Virology [Voprosy rikketsiologii i virusologii]. Astrakhan’–Moscow; 1996: 58–62. (in Russian)
- Shuvaev N.S., Barmin A.N., Kolchin E.A., Barmina E.A., Kolchina L.V. Conflicts in nature management of the Astrakhan region as a source of threat and risk of violation of region’s sustainable development. Geograficheskiy vestnik. 2012; (4): 21–8. (in Russian)
- Federal Service of Geodesy and Cartography of Russia. Atlas of the Astrakhan Region [Atlas Astrakhanskoy oblasti]. Moscow; 1997. (in Russian)
- Shal’nev V.A. Landscapes of the Stavropol Territory [Landshafty Stavropol’skogo kraya]. Stavropol ‘; 1995. (in Russian)