Sequence analysis of the non-coding control region of John Cunningham virus isolates from patients with multiple sclerosis treated with natalizumab
- Authors: Tyumentseva M.A.1, Tyumentsev A.I.1, Zakharova M.N.2, Askarova L.S.2, Simaniv T.O.2, Piradov M.A.2, Akimkin V.G.1
-
Affiliations:
- Central Research Institute for Epidemiology
- Research Center of Neurology
- Issue: Vol 100, No 1 (2023)
- Pages: 7-25
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/4739
- DOI: https://doi.org/10.36233/0372-9311-341
- ID: 4739
Cite item
Abstract
Introduction. The John Cunningham virus (JCPyV) causes a fatal demyelinating disease of the central nervous system known as progressive multifocal leukoencephalopathy (PML). In healthy people, the JCPyV non-coding control region (NCCR) is not rearranged, while NCCRs in immunocompromised patients are characterized by frequent rearrangements and can be associated with PML development. Therefore, patients treated with natalizumab, which decreases the migration of leukocytes and monocytes through the blood-brain barrier to inflammatory foci, are at increased risk of developing PML.
The purpose of the study was to analyze NCCR sequences of JCPyV isolates from patients with multiple sclerosis (MS) treated with natalizumab.
Materials and methods. A total of 26 blood plasma samples and 8 cerebrospinal fluid samples were analyzed using nested PCR to study the JCPyV NCCR structure in Russian MS patients treated with natalizumab. The NCCRs present in the samples were cloned and sequenced by Sanger sequencing. All the JCPyV NCCR sequences were compared with the archetype sequence and mapped. The NCCR sequences were also examined for presence of putative transcription factor binding sites.
Results. A total of 48 NCCR sequences were found. The analysis showed that up to 55% of NCCRs were identified as rearranged NCCRs, while the other were archetype-like NCCRs. All the sequences can be divided into 6 types with one dominant rearrangement pattern. This rearranged NCCR was also found in a patient with the confirmed PML diagnosis and a poor prognosis. All the rearranged NCCRs were characterized by the presence of additional transcription factor binding sites.
Conclusion. The study has helped identify previously unknown NCCR patterns typical of MS patients treated with natalizumab in Russia, thus confirming the need for the further research on NCCR rearrangements in MS patients undergoing natalizumab treatment to gain better understanding of the origin of neurovirulent JCPyV variants.
Full Text
Introduction
Human polyomavirus 2, commonly referred to as the JC virus or John Cunningham virus (JCPyV), is a representative of genus Betapolyomavirus, family Polyomaviridae. JCPyV is a non-enveloped virus with double-stranded DNA genome comprised of approximately 5,100 bp. The JCPyV genome can be divided into three parts: the non-coding control region (NCCR), the early viral gene region, and the late viral gene region [1].
NCCR is a hypervariable JCPyV promoter/enhancer region approximately 400 bp long; it contains most of the elements required for initiation of early and late transcription and viral gene expression, including TATA boxes and transcription factor binding sites [2]. The early viral gene region is located leftward to the replication origin and encodes the small T-antigen, large T-antigen, and truncated version of the large T-antigen. These proteins are responsible for virus replication and late gene expression [3, 4]. The late viral gene region is located rightward to the replication origin and encodes three structural proteins: major capsid protein VP1 and minor capsid proteins VP2 and VP3. Each JCPyV virion contains a total of 72 pentameric capsomeres of VP1 with T=7 icosahedral symmetry. Inside the capsid, one molecule – VP2 or VP3 – is attached to each capsomere of VP1 [5].
JCPyV is an etiological agent causing a rare, rapidly progressing, often fatal, demyelinating disease of the central nervous system known as progressive multifocal leukoencephalopathy (PML) observed in patients with impaired humoral and cell-mediated immunity [6]. JCPyV can cross the blood-brain barrier and get into the central nervous system where it infects oligodendrocytes and astrocytes [7, 8]. Based on some data, 70-90% of people are infected with JCPyV [9, 10]. The non-rearranged archetypal form of JCPyV that can be found in healthy people is seen as harmless, while the rearranged form known as the prototype is found in the central nervous system of PML patients. The prototype nearly always has differences in NCCR compared to the JCPyV sequence found in healthy people [11, 12]. These differences in NCCR can alter JCPyV tropism by changing DNA binding sites for cellular transcription factors in cells permissive for infection [2, 13]. It is also assumed that these differences activate virus replication and gene transcription in glial cells, eventually launching the lytic cycle [14, 15].
Immunodeficiency or immunosuppression leads to JCPyV reactivation and switchover from the archetype to the prototype. There are known cases when JCPyV changed into an aggressive PML-inducing form during treatment with such agents as natalizumab, rituximab, dimethyl fumarate, fingolimod, efalizumab, brentuximab vedotin and other immunosuppressants [16–21].
Natalizumab is a humanized monoclonal antibody selectively binding to α4-integrin; it is used for treatment of relapsing forms of MS. Natalizumab is administered to patients with highly active disease. On average, PML is diagnosed in 4.19 cases per 1,000 patients treated with natalizumab (95% CI, 3.89–4.49) [22, 23].
There are three main risk factors associated with development of PML during the natalizumab therapy:
- the presence of anti-JCPyV antibodies (seropositive patients with anti-JCPyV);
- the duration of therapy, especially beyond 2 years;
- the prior treatment with immunosuppressants before the natalizumab therapy [24].
Special guidelines for management of patients with multiple sclerosis (MS) treated with natalizumab have been developed to prevent development of PML. Clinical practice incorporates risk management plans including regular monitoring of the anti-JCPyV antibody index values and potential seroconversion as well as brain MRI (for detection of any changes typical of PML) [25–29]. If PML is suspected, the PCR test is used to detect JCPyV DNA in cerebrospinal fluid (CSF) [24].
A diagnosis of probable PML is made in the presence of typical clinical and radiological findings, in the absence of positive detection of JCPyV in CSF and brain tissue by PCR; a diagnosis of laboratory confirmed PML is made when JCPyV DNA is detected in CSF of the patient. Histologically confirmed PML is diagnosed by isolation of JCPyV using PCR from the patient’s brain biopsy material. JCPyV DNA detection and NCCR sequence analysis are highly recommended to accurately identify JCPyV-infected MS patients treated with natalizumab and to identify NCCR rearrangements that can correlate with the emergence of neurovirulent variants [30].
The purpose of the study is to analyze multiple NCCR variants in plasma and CSF of natalizumab-treated patients from Russia.
Materials and methods
Samples
Plasma and CSF samples were collected from natalizumab-treated MS patients having the anti-JCPyV antibody index ≥ 1.5. Each patient was duly informed and signed their informed consent for participation in the study. The study protocol was approved by the Ethics Committee of the Research Center of Neurology (protocol No. 1-2/22, January 19, 2022).
All samples were stored frozen and thawed on ice prior to being used for viral DNA extraction.
Extraction of viral DNA
JCPyV DNA was extracted from a total of 26 plasma samples and 8 CSF samples. Blood samples were collected in VACUETTE® 4 ml LH Lithium Heparin tubes (Greiner Bio-One) and centrifuged at 3500 rpm for 10 min. DNA was extracted from 200 μl of plasma and/or CSF using the DNeasy® Blood & Tissue Kit (Qiagen Inc.); DNA was eluted using 75 μl elution buffer. JCPyV DNA concentration was measured with a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific). All the extracted DNA samples were stored frozen prior to their further use.
Quantification of JCPyV DNA
JCPyV DNA was quantified with an AmpliSens® JCV-BKV screen/monitor-FRT PCR kit (Central Research Institute of Epidemiology of Rospotrebnadzor) with the detection limit of 5 × 102 gEq/ml, in accordance with the manufacturer’s recommendations.
Amplification of JCPyV NCCR sequences
For amplification of NCCR sequences from viral DNA by nested PCR, we used two pairs of primers [31]. A pair of outer primers was used for the first round of PCR:
- 1 — forward primer 5'-GATTCCTCCCTATTCAGCACTTTG-3';
- 1 — reverse primer 5'-CACCTGTGCAAAAGTCCAGC-3'.
This pair of primers is used to amplify archetype JCPyV genome fragments (GenBank accession number AB038249 [32]) 540 bp long. A pair of inner primers was used for the second round of amplification:
- 2 — forward primer 5'-GGCCTCCTAAAAAGCC-3';
- 2 — reverse primer 5'-TCCACTCCAGGTTTTACTAA-3'.
These primers were used for amplification of a 386 bp fragment of archetype JCPyV (GenBank accession number AB038249).
Phusion™ High-Fidelity DNA polymerase (Thermo Fisher Scientific) was used in the first round of amplification. The thermal profile is as follows:
- initial denaturation: 98°C for 30 sec;
- 35 cycles: 98°C for 10 sec, 55°C for 45 sec, 72°C for 30 sec;
- final elongation: 72°C for 5 min.
3 μl of the amplicon from the first round of PCR were used for the second round of PCR using Taq polymerase (the 2x PCR Blue Mix, Central Research Institute of Epidemiology of Rospotrebnadzor).
The thermal profile of amplification:
- initial denaturation: 95ºC for 3 min;
- 40 cycles: 95°C for 15 sec, 55°C for 45 sec, 72°C for 30 sec;
- final elongation: 72°C for 5 min.
The PCR products received after the second round of amplification were analyzed by 1% agarose gel electrophoresis and staining with ethidium bromide. The PCR products were used for direct Sanger sequencing and for cloning.
Cloning of JCPyV NCCR sequences
JCPyV NCCRs present in plasma and/or CSF samples were amplified using nested PCR [31] and then were cloned into the pGEM-T vector (Promega®). The plasmids obtained from cloning were transformed into chemically competent cells XL10-Gold E. coli. Transformed E. coli were grown at 37°C in Luria-Bertani agar plates with ampicillin (100 μg/ml), X-gal (80 μg/ml), and IPTG (0.5 mM). The obtained clones were screened using M13forward and M13reverse primers. After the screening, 10–20 clones obtained from each plasma and/or CSF sample were sequenced.
Sequencing JCPyV NCCR sequences
Prior to sequencing, the PCR products corresponding to JCPyV NCCRs were purified using a QIAquick PCR purification kit (Qiagen Inc.) in accordance with the manufacturer’s protocol. The DNA sequencing was performed with the Applied Biosystems 3500xL genetic analyzer.
Amplification of JCPyV VP1 sequences
To identify a JCPyV genotype of the isolates from plasma and/or CSF samples, a fragment of the viral protein 1 (VP1) gene 215 bp long was amplified using one pair of primers: JLP-15 (5'-ACAGTGTGGCCAGAATTCCACTACC-3') and JLP-16 (5'-TAAAGCCTCCCCCCCAACAGAAA-3') [33]. PCR was performed using Taq polymerase (the 2x PCR Blue Mix, Central Research Institute of Epidemiology of Rospotrebnadzor) in accordance with the following procedure:
- initial denaturation: 95°C for 3 min;
- 40 cycles: 95°C for 15 sec, 55°C for 45 sec, 72°C for 30 sec;
- final elongation: 72°C for 5 min.
The PCR products were analyzed by 1% agarose gel electrophoresis and staining with ethidium bromide, followed by direct Sanger DNA sequencing.
Sequencing DNA JCPyV NCCR and VP1 sequences
The PCR products corresponding to the NCCR and VP1 JCPyV regions were purified using a QIAquick PCR purification kit (Qiagen Inc.) in accordance with the manufacturer’s protocol. The DNA sequencing was performed with the Applied Biosystems 3500xL genetic analyzer.
All NCCR sequences were compared with the CY archetype sequence (GenBank accession number AB038249) [32]. All NCCR sequences were mapped using the SnapGene Viewer software and divided into segments A, B, C, D, E, and F composed of 25, 23, 55, 66, 18, and 69 base pairs, respectively [11].
The sequences obtained after the amplification of the VP1 region were compared with the respective sequences of JCPyV isolates of different genotypes/subtypes with accession numbers GenBank AF015526 (genotype 1A), AF281599 (genotype 1B), AF030085 (genotype 2A), AF015532 (genotype 2B), AF015534 (genotype 2C), AF015536 (genotype 2D), AF295731 (genotype 3A), U73501 (genotype 3B), AF015528 (genotype 4), AF015537 (genotype 6), AF295737 (genotype 7) and AF281623 (genotype 8). The sequence alignment and phylogenetic analysis were carried out using the MEGA 7 software [34].
Analysis of NCCR nucleotide sequences
The NCCR sequences were analyzed for the presence of putative transcription factor binding sites using the PROMO virtual laboratory resource1 [35, 36].
Accession numbers of sequences
All the NCCR sequences were deposited to the GenBank NCBI database, with assigned accession numbers OM479515–OM479562.
Statistical analysis of the data
The statistical analysis (Student’s t-test) was performed using the unpaired t-test built-in module of GraphPad Prism™ 9 software.
Results
DNA quantification and JCPyV genotypes
A total of 26 plasma samples and 8 CSF samples collected from MS patients treated with natalizumab, having anti-JCPyV antibody index ≥ 1.5, were used for quantification of JCPyV DNA (Table 1). JCPyV DNA was detected in plasma and CSF of only 1 patient (NAT-1). The patient was diagnosed with PML, and samples were collected when he was in the vegetative state. Most of the studied samples contained JCPyV DNA at the levels lower than the detection limit set for the PCR kit used for quantification of JCPyV DNA (lower than 5×102 gEq/ml). It correlates with the low number of JCPyV copies observed in most confirmed cases of PML [24].
Table 1. Characteristics of samples obtained from MS patients treated with natalizumab
No. | Patient ID | Number of natalizumab infusions, pcs | Anti-JCPyV antibody index | Quantification of JCPyV DNA, gEq/ml | JCPyV genotype |
Blood plasma | |||||
1 | NAT-1/1 | Н. д. | N. d. | Н. д. | N. d. | 6,88 × 102 | 1A |
2 | NAT-1/2 | Н. д. | N. d. | Н. д. | N. d. | ˂ 1 × 103 | 1A |
3 | NAT-2 | 36 | 2,14 | Н. о. | N. def. | Н. о. | N. def. |
4 | NAT-3 | 22 | 2,127 | Н. о. | N. def. | 1B |
5 | NAT-4/1 | 16 | 2,43 | Н. о. | N. def. | 1B |
6 | NAT-4/2 | 16 | 2,43 | Н. о. | N. def. | 1B |
7 | NAT-5 | 9 | 2,64 | Н. о. | N. def. | 1B |
8 | NAT-6 | 11 | Н. д. | N. d. | Н. о. | N. def. | 1B |
9 | NAT-7 | 32 | 2,63 | Н. о. | N. def. | Н. о. | N. def. |
10 | NAT-8/1 | 24 | 2,11 | Н. о. | N. def. | 1B |
11 | NAT-8/2 | 24 | 2,64 | Н. о. | N. def. | 1B |
12 | NAT-9 | 18 | 2,194 | Н. о. | N. def. | 1A |
13 | NAT-10/1 | 25 | 1,529 | Н. о. | N. def. | 1B |
14 | NAT-10/2 | 25 | 1,529 | Н. о. | N. def. | 1A |
15 | NAT-11 | 20 | 0,259 | Н. о. | N. def. | Н. о. | N. def. |
16 | NAT-12 | 26 | 2,582 | Н. о. | N. def. | 1B |
17 | NAT-13 | 28 | 2,38 | Н. о. | N. def. | 1B |
18 | NAT-14 | 35 | 2,179 | Н. о. | N. def. | 1A |
19 | NAT-15/1 | 27 | 2,353 | Н. о. | N. def. | 1A |
20 | NAT-16 | 12 | 2,773 | Н. о. | N. def. | Type 1 |
21 | NAT-17 | 40 | 3,4 | Н. о. | N. def. | 1B |
22 | NAT-18 | 24 | Н. д. | N. d. | Н. о. | N. def. | Н. о. | N. def. |
23 | NAT-19 | Н. д. | N. d. | Н. д. | N. d. | Н. о. | N. def. | 1B |
24 | NAT-20 | Н. д. | N. d. | Н. д. | N. d. | Н. о. | N. def. | 1B |
25 | NAT-21 | Н. д. | N. d. | Н. д. | N. d. | Н. о. | N. def. | 1B |
26 | NAT-22 | Н. д. | N. d. | 2,7 | Н. о. | N. def. | 1B |
Cerebrospinal fluid | |||||
1 | NAT-1/1 | Н. д. | N. d. | – | 1,45 × 103 | 1A |
2 | NAT-2 | 36 | – | Н. о. | N. def. | Н. о. | N. def. |
3 | NAT-3 | 22 | – | Н. о. | N. def. | 1B |
4 | NAT-4 | 16 | – | Н. о. | N. def. | 1B |
5 | NAT-5 | 9 | – | Н. о. | N. def. | 1B |
6 | NAT-10/3 | 25 | – | Н. о. | N. def. | 1B |
7 | NAT-15/2 | 27 | – | Н. о. | N. def. | 1B |
8 | NAT-18 | 24 | Н. д. | N. d. | Н. о. | N. def. | Н. о. | N. def. |
Note. N. d. — no data; N. def. — not defined.
All the detected JCPyV isolates belonged to type 1 (genotypes 1A and 1B, Table 1) widely occurring in Europe [37]. The most frequently observed genotype was type 1B, being found in 75% of patients. Note that JCPyV of different genotypes (9/7/2019 – 1B, 3/10/2019 – 1A) was detected in patient NAT-10 in plasma collected during different periods of time, while JCPyV of genotype 1B was detected in the patient’s CSF collected on 21/10/2019. In patient NAT-15, JCPyV of genotype 1A was detected in the plasma collected on 1/11/2019, while JCPyV of genotype 1B was detected in CSF collected on 6/2/2020.
Types of JCPyV NCCR sequences in MS patients treated with natalizumab
JCPyV NCCRs were found in 21 MS patients treated with natalizumab: 37 independent JCPyV NCCR sequences were obtained from 26 plasma samples and 10 independent JCPyV NCCR sequences were obtained from 8 CSF samples. One case of primary immunodeficiency ID-1, where JCPyV NCCR was detected in plasma, was analyzed additionally.
Based on the NCCR classification [31], more than 50% of NCCRs obtained from plasma of MS patients treated with natalizumab belong to rearranged NCCRs of subtype II-R (52.63%; 20 of 38 JCPyV NCCR sequences). Nearly half (48.65%; 18 of 38) of JCPyV NCCRs found in plasma of MS patients treated with natalizumab belong to archetype-like NCCRs of subtype II-S. Similarly, 60% (6 of 10) of NCCRs obtained from CSF of MS patients treated with natalizumab belong to rearranged NCCRs of subtype II-R, while the other NCCRs belong to archetype-like subtype II-S (40%, 4 of 10).
Note that JCPyV NCCRs obtained from 1 patient with the confirmed diagnosis of PML and a poor prognosis (samples were collected when he was in the vegetative state) and detectable JCPyV DNA belonged to rearranged JCPyV NCCRs of subtype II-R. On the other hand, NCCRs from another MS patient treated with natalizumab, having the confirmed diagnosis of PML with the favorable outcome and undetectable JCPyV DNA, belonged to archetype-like subtype II-S.
In addition, the NCCR sequence obtained from the plasma of a patient with primary immunodeficiency (ID-1; GenBank accession number OM479515), who did not take natalizumab, is 99% homologous to the published archetype NCCR, except for 1 point mutation in segment F, and belongs to archetype NCCR subtype II-S.
All the retrieved JCPyV NCCR sequences were divided into 6 different types (without regard to point mutations): types I-IV belong to rearranged NCCRs of subtype II-R (a total of 26 NCCRs, 20 from plasma and 6 from CSF, respectively); types V and VI belong to archetype-like subtype II-S (a total of 22 NCCRs, 18 from plasma and 4 from CSF, respectively); the sequences structures are shown in Table 2. The detected types of JCPyV NCCR sequences are schematically presented in Fig. 1.
Table 2. Characterization of NCCR sequences identified in MS patients treated with natalizumab
No. | GenBank accession number | Sequence formula |
Blood plasma | ||
Type I (II-R, (BCDE)3) | ||
1 | OM479519 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
Type II (II-R, (BCDE)2) | ||
2 | OM479520 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
3 | OM479516 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
4 | OM479517 | A1-25B1-23C1-45-CCTAACCTCCTA-D3-39D46-66E1-18-F1-2-A25-B1-23C1-45 CCTAACCTCCTA-D3-39D46-66E1-18-F1-69 |
5 | OM479526 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
6 | OM479528 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
7 | OM479534 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
8 | OM479538 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
9 | OM479531 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
10 | OM479523 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
11 | OM479542 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
12 | OM479545 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
13 | OM479552 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
14 | OM479557 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
Type III (II-R, BCDEBC) | ||
15 | OM479550 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55F40-69 |
16 | OM479558 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55F40-69 |
17 | OM479561 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55F40-69 |
18 | OM479555 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-3-B1-23C1-55F40-69 |
Type IV (II-R, C1-34C5-55) | ||
19 | OM479529 | A1-25B1-23C1-34C5-55D1-39D46-66E1-18F1-69 |
20 | OM479535 | A1-25B1-23C1-34C5-55D1-39D46-66E1-18F1-69 |
Type V (II-S, ABCD30 and other truncated NCCR) | ||
21 | OM479522 | A1-25B1-23C1-55D1-30 |
22 | OM479530 | A1-25B1-23C1-55D1-30 |
23 | OM479541 | A1-25B1-23C1-55D1-30 |
24 | OM479544 | A1-25B1-23C1-55D1-30 |
25 | OM479559 | A1-25B1-23C1-55D1-30 |
26 | OM479562 | A1-25B1-23C1-55D1-30 |
27 | OM479533 | A1-25B1-23C1-55D1-39D46-66 |
Type VI (II-S, archetype-like with Box D typical deletion) | ||
28 | OM479518 | A1-25B1-23C1-55D1-39D46-66E1-18F1-69 |
29 | OM479532 | A1-25B1-23C1-55D1-39D46-66E1-18F1-69 |
30 | OM479539 | A1-25B1-23C1-55D1-39D46-66E1-18F1-69 |
31 | OM479536 | A1-25B1-23C1-55D1-39D46-66E1-18F1-69 |
32 | OM479540 | A1-25B1-23C1-55D1-39D46-66E1-18F1-69 |
33 | OM479543 | A1-25B1-23C1-55D1-39D46-66E1-18F1-69 |
34 | OM479560 | A1-24B1-23C1-55D1-39D46-66E1-18F1-69 |
35 | OM479549 | A1-25B1-23C1-55D1-39D46-66E1-18F1-69 |
36 | OM479551 | A1-25B1-23C1-55D1-39D46-66E1-18F1-69 |
37 | OM479556 | A1-25B1-23C1-55D1-39D46-66E1-18F1-69 |
Archetype | ||
38 | OM479515 | A1-25B1-23C1-55D1-66E1-18F1-69 |
Cerebrospinal fluid | ||
Type I (II-R, (BCDE)3) | ||
1 | OM479554 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
Type II (II-R, (BCDE)2) | ||
2 | OM479525 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
3 | OM479527 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
4 | OM479553 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55D1-39D46-66E1-18F1-69 |
Type III (II-R, BCDEBC) | ||
5 | OM479521 | A1-25B1-23C1-55D1-39D46-66E1-18-F1-2-A25-B1-23C1-55F40-69 |
6 | OM479546 | A1-24B1-23C1-55D1-39D46-66E1-18-GGGAAT-B4-20-ATG-C1-52-CCCCA-F42-67 |
Type V (II-S, ABCD30 and other truncated NCCR) | ||
7 | OM479547 | A1-25B1-23C1-55D1-30 |
Type VI (II-S, archetype-like with Box D typical deletion) | ||
8 | OM479524 | A1-25B1-23C1-55D1-39D46-66E1-18F1-69 |
9 | OM479537 | A1-25B1-23C1-55D1-39D46-66E1-18F1-69 |
10 | OM479548 | A1-25B1-23C1-55D1-39D46-66E1-18F1-69 |
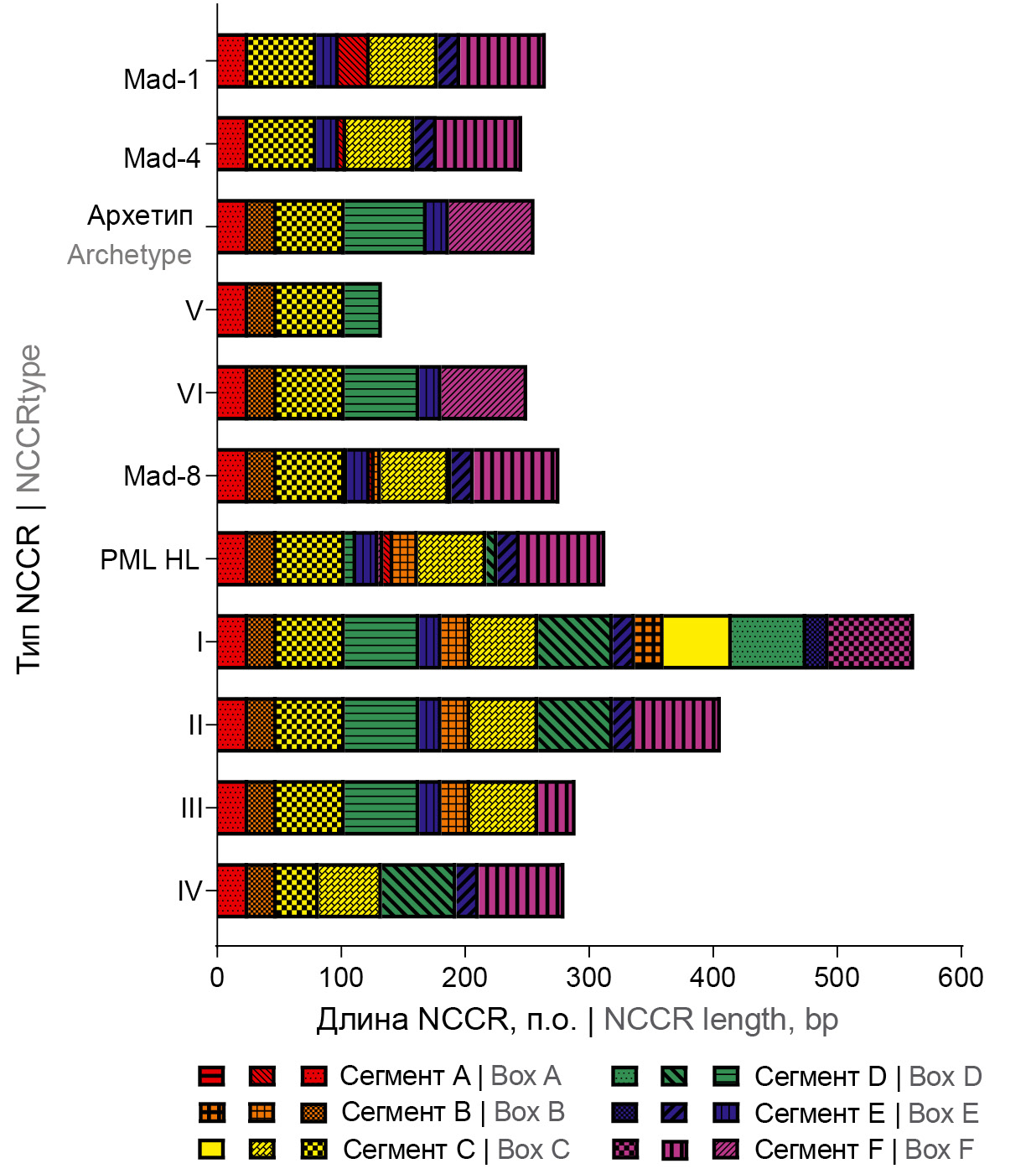
Fig. 1. Schematic representation of the structure of JCPyV NCCR sequences identified in MS patients treated with natalizumab.
Type I is represented by NCCR of type II-R, in which the fragment composed of segments B, C, D, and E (BCDE), tandemly repeated for 3 times. NCCR of type I was detected in a plasma sample from patient NAT-3 (5%; 1 of 20 rearranged NCCRs) and in a CSF sample from patient NAT-18 (16.7%, 1 of 6 rearranged NCCRs). Like NCCR of type I, type II is represented by NCCR of type II-R, but BCDE-fragment is tandemly repeated twice. NCCR of type II, most frequently found in NCCR samples, was detected in 13 plasma samples (65%; 13 of 20 rearranged NCCRs) and 3 CSF (50%; 3 of 6 rearranged NCCRs).
NCCR of type I is a new, unique, rearranged NCCR variant that has no homology with the NCCR sequences published previously in NCBI GenBank (confirmed by the nucleotide BLAST analysis). NCCR of type II is also a rare rearranged NCCR variant that has homology with the NIID11-68 NCCR sequence (GenBank accession number LC164353), which was deposited to the NCBI GenBank. The NCCR variant of type II was identified for the first time in plasma and CSF of a patient with the confirmed diagnosis of PML, a poor prognosis and detectable JCPyV DNA.
NCCR of type III belongs to type II-R that has an additional copy of the BC-fragment, which is integrated between segments E and F. NCCR of type III was found in 4 plasma samples (20%; 4 of 20 rearranged NCCRs) and in 2 CSF samples (33.3%; 2 of 6 rearranged NCCRs).
Type IV is represented by II-R NCCR, where segment C is repeated tandemly. This NCCR variant was found only in plasma from 2 MS patients treated with natalizumab, NAT-8 and NAT-10 (10%; 2 of 20 rearranged NCCRs).
NCCRs of types V and VI are archetype-like NCCRs of subtype II-S. Type V is a shortened NCCR variant, which lacks segments E and F (8 of 9 non-rearranged NCCRs) or segments A, B, and C (1 of 9 non-rearranged NCCRs). NCCRs of type V were most frequently detected in plasma (8 of 9 non-rearranged NCCRs), and 1 time in the CSF sample from patient NAT-15.
Type VI is represented by NCCR of type II-S having more than 98% homology with the published archetype CY NCCR (GenBank accession number AB038249), except for the 6-bp nucleotide deletion in segment D. More than half (55.5%) of NCCRs of type VI were detected in plasma samples (10 of 18 II-S NCCRs) and 75% (3 of 4 II-S NCCRs) – in CSF samples. It should be noted that NCCR of type VI was detected in plasma and CSF of the patient with the PML diagnosis, favorable outcome, and unquantifiable JCPyV DNA.
Comparative analysis of occurrence of putative transcription factor binding sites in JCPyV NCCR in MS patients treated with natalizumab
The comparative analysis of the occurrence of putative transcription factor binding sites included sequences of archetype NCCR (as a reference sequence) and sequences of NCCR prototypes: Mad-1 [12, 38], Mad-4 [39, 40], Mad-8 [40], and PML HL [41]. The analysis showed that all types of NCCRs contain the number of binding sites of transcription factors comparable with archetypal NCCR, including Pentamut, L3 (Spi-B), L6 (Spi-B), L18 (Spi-B), L8 (Spi-B), Pseudo-NF-1 site, p53, AP-1-like site, AP-4, PEA3, GABP, GABP-alpha, TATA box, TFIID, and TBP. At the same time, the number of transcription factor binding sites such as GA box (SP-1 site), c-Jun, NF-1 motif, NF-1, c-Fos, HMG I(Y), MEF-2A, C/EBPalpha, TEF-1, and GF1 in rearranged NCCRs of types I-IV was larger compared to archetype-like NCCRs (archetype, type V, and type VI) (Table 3).
Table 3. Putative binding sites of transcription factors in JCPyV NCCR in MS patients treated with natalizumab
Note. White indicates putative transcription factor binding sites that have not changed in number compared to the archetype sequence; light grey indicates putative transcription factor binding sites that have decreased in number compared to the archetype sequence; dark grey indicates putative transcription factor binding sites that have increased in number compared to the archetype sequence.
It has also been found that in NCCR sequences, which belong to type II-R (type I, II, III, IV, Mad-8, and PML HL), putative transcription factor binding sites NF-1, GF1, and AP-1 occur significantly more frequently (p ˂ 0.0001) than in NCCR sequences, which belong to type II-S (the archetype of types V and VI) (Fig. 2).
Fig. 2. Comparative analysis of the occurrence of putative binding sites for transcription factors NF-1, GF1, and AP-1
in JCPyV NCCR in MS patients treated with natalizumab. 22 II-S archetype-like NCCR samples and 26 II-R rearranged NCCR samples were analyzed. ****p ˂ 0.0001.
Heterogeneity of JCPyV NCCR populations in MS patients treated with natalizumab
The performed analysis showed that 12 MS patients treated with natalizumab had several circulating variants of the virus (Table 4). Two NCCR variants were detected in 6 patients, 3 variants were found in 4 patients, 2 patients had 4 variants (Table 4). NCCRs of type I and type IV (2 of 12) were detected most rarely in combinations of virus variants; NCCR of type III was detected 5 times in the found combinations; NCCRs of types II and V were detected 7 times. Archetype-like NCCR of type VI was the most frequently occurring NCCR in combinations of virus variants (9 of 12).
Table 4. Multiple NCCR variants found in MS patients treated with natalizumab
No. | Patient ID | Blood plasma, NCCR type | Cerebrospinal fluid, NCCR type |
1 | NAT-3 | I, II | III |
2 | NAT-4 | II, V | II, VI |
3 | NAT-8 | II, IV, V | Н. д. | N. d. |
4 | NAT-9 | V, VI | Н. д. | N. d. |
5 | NAT-10 | II, IV, VI | VI |
6 | NAT-13 | V, VI | Н. д. | N. d. |
7 | NAT-14 | II, VI | Н. д. | N. d. |
8 | NAT-15 | II, V | III, V, VI |
9 | NAT-18 | II, III, VI | I, II |
10 | NAT-19 | III, VI | Н. д. | N. d. |
11 | NAT-21 | V, VI | Н. д. | N. d. |
12 | NAT-22 | III, V | Н. д. | N. d. |
Note. N. d. — no data.
Note that different variants of the virus were found in different body compartments (plasma and CSF). However, no significant differences in the frequency of occurrence between rearranged and archetype-like NCCR variants in different body compartments were found.
The analysis of NCCR variants detected in paired plasma and CSF samples (8 patients) also showed that one NCCR variant was in 3 cases in plasma and CSF (type II in 2 cases, type VI – in 1 case), while in the other cases, there were different (multiple) variants of the virus (Table 5).
Table 5. NCCR variants detected in paired plasma and cerebrospinal fluid samples from MS patients treated with natalizumab
No. | Patient ID | Blood plasma, NCCR type | Cerebrospinal fluid, NCCR type |
1 | NAT-1 | II | II |
2 | NAT-2 | VI | VI |
3 | NAT-3 | I, II | III |
4 | NAT-4 | II, V | II, VI |
5 | NAT-5 | II | II |
6 | NAT-10 | II, IV, VI | VI |
7 | NAT-15 | II, V | III, V, VI |
8 | NAT-18 | II, III, VI | I, II |
Discussion
Development of PML in MS patients treated with immunosuppressive agents such as natalizumab requires search for biological factors (predictors) contributing to the risk of development of this serious brain infection [42]. Studying of the structure of the JCPyV NCCR sequence is one of the promising approaches. During this study, for the first time in Russia, JCPyV NCCR sequences were analyzed and the unique pattern of duplications of NCCR segments was identified, having no homology with NCCR sequences, which were described earlier.
NCCR is a hypervariable regulatory JCPyV region approximately 400 bp long, containing most of the elements required for initiation of early and late transcription and viral gene expression, including TATA boxes and transcription factor binding sites [2]. The archetype NCCR JCPyV is divided into 6 segments – A, B, C, D, E, and F composed of 25, 23, 55, 66, 18, and 69 bp, respectively [11]. Neurovirulent JCPyV NCCRs are rearranged. For example, the prototype JCPyV NCCR sequence, known as Mad-1, which was first isolated from a PML patient [12, 38], has deletions in segment D and consists only of segments A, C, and E represented by 98-bp tandem repeats followed by segment F [43–45]. There are multiple rearranged JCPyV isolates [46], including other Mad-isolates, which were obtained from tissues of PML patients [47], for example, Mad-8, which is identical to Mad-1 in its segmental composition, but contains part of segment B as well as single insertions. At the same time, the Mad-8 variant is more common among NCCRs found in PML patients than Mad-1 [43, 47, 48].
As is known, JCPyV NCCRs are classified into four groups [49]: I-S, I-R (like Mad-1 and Mad-4), II-S and II-R (like Mad-7 and Mad-8). NCCRs of type I do not contain insertions, while NCCRs of type II contain an insertion of at least a portion of the sequence from one of the sections B or D. Subtypes S (for singular) do not contain segments, while subtypes R (for repeat) have them. NCCRs of type II-S are referred to as archetypes or archetype-like if they contain deletions. NCCRs of type II-R are generally referred to as rearranged NCCRs [49].
Our study has revealed that up to 55% of JCPyV NCCRs in MS patients treated with natalizumab belong to rearranged NCCRs of subtype II-R, while the other represent archetype-like subtypes II-S. Our findings slightly differ from the previously published data, where up to 80–100% of NCCRs identified in CSF samples and/or plasma/serum samples from patients having PML or treated with natalizumab belong to rearranged NCCRs of subtype II-R [31, 50, 51]. In addition, the analysis of NCCR sequences in MS patients treated with natalizumab revealed only 4 NCCR rearrangement patterns (types I–IV) and 2 archetype-like patterns (types V and IV; Table 2). These findings differ from those reported earlier by Reid et al., showing that each PML patient had a unique set of deletions and duplications within NCCR [50]. Types I and III are unique rearranged NCCRs, which were detected for the first time. NCCR variants of type IV were described earlier for patients with primary immunodeficiency syndrome, PML and MS patients treated with natalizumab [50–53]. Shortened NCCRs were also described earlier in MS patients treated with natalizumab [50].
NCCR of type II is most common among samples of rearranged NCCRs, having been detected in more than 30% of cases (16 of 48); it is considered rare and has been found only in 1.7% (17 of 989) of the studied cases worldwide [46]. Previously, such NCCRs were found only in CSF [46], while in our study, more than 80% of such NCCRs were found in plasma from MS patients treated with natalizumab.
Mutations in rearranged NCCRs are complex and, most likely, owe their existence to homologous recombination, which leads to large deletions and tandem duplications of segments in NCCR [43], like it was observed in NCCRs detected during our study.
The comparative analysis has shown that flanking segments A and F have never been affected during the rearrangement, except for NCCR of type III, where part of segment F was removed. These findings correlate with NCCR rearrangements found in PML patients treated with natalizumab [50].
Duplicated segment B is often detected as a portion of relatively long tandem or single repeats (BCDE or BC in our study) in rearranged JCPyV NCCRs in MS patients treated with natalizumab. The similar duplications of segment B (partial or full-length) were detected in Mad-8 JCPyV NCCR sequences found in brain tissues from PML patients [47], in Mad-8 JCPyV NCCR sequences from tonsil tissues of children [40] and in some NCCRs in PML patients treated with natalizumab [50, 51, 55]. Only NCCR of type IV contained the only copy of segment B.
Note that segment C was always duplicated in rearranged JCPyV NCCRs of the studied cohorts treated with natalizumab, as a portion of relatively long repeats (BCDE or BC) in NCCR of type I-type III or as a single duplication in NCCR of type IV. Duplications of segment C are typical of many NCCRs found in patients with primary immunodeficiency syndrome, PML and MS patients treated with natalizumab [50–53].
Note that segment D was present in all NCCRs from MS patients treated with natalizumab. This observation differs from the previous reports describing its complete or partial absence in NCCRs from plasma and/or CSF of PML patients treated with natalizumab [46, 50]. Most of the NCCRs detected during the study had an identical unique deletion 6 bp long (AAACCA) in segment D. This deletion was detected in 41 of 48 NCCR sequences obtained from plasma and CSF samples in all types of NCCRs (rearranged and archetype-like), except for shortened NCCRs (7 of 48), which belonged to type V. It should be noted that when this deletion was present in the repeated fragment BCDE, it was common for all copies, thus leading to the assumption that the deletion occurred prior to the duplication, as it was assumed previously [50, 55]. Apparently, this unique deletion is caused by natalizumab in the studied cohort (the result of the selective pressure caused by natalizumab treatment), as it was found in most of the analyzed NCCR sequences; however, it was absent in NCCRs from plasma and CSF from the patient with primary immunodeficiency.
Segment E was present in all NCCRs in MS patients treated with natalizumab. In NCCRs of types I and II, it was duplicated as part of relatively long repeat BCDE. This observation correlates with the previously described NCCR structures in PML patients [47, 50, 51, 54]. NCCRs of type III had the only copy of segment E.
High frequency of repeats in NCCR sequences results in the increasing number of enhancer elements, which presumably are of critical importance for viral pathogenesis [46]. Each BCDE-fragment contains several enhancer elements, including binding sites of transcription factors NF1, CRE-TAR, GA-domain (the binding site of the SP-1 transcription factor), GF-1, AP-1, etc. (Table 3). It is known that these enhancer elements modulate transcription, expression, and replication of JCPyV as well as its tropism [13, 44, 56–61].
Our analysis has shown that segment D contains several enhancer elements, including 4 binding sites, TFIID, TBP, TEF-1, and c-Fos, which were predicted by the PROMO tool [35, 36]. The unique 6-bp deletion (AAACCA) detected in segment D caused the loss of the predicted c-Fos binding site (the prediction was made for the human factor and viral sites) compared to the archetype sequence. c-Fos is a nuclear phosphoprotein, which forms a tight, but not covalently linked complex with the JUN/AP-1 transcription factor [62, 63]. c-Fos is expressed with low tissue specificity and is found almost in all body compartments2. It is known that c-Fos activates JCPyV early and late promoters [64]; therefore, the loss of this putative binding site in segment D may not result in switchover to the neurovirulent variant; however, this circumstance requires further research.
It should be noted that multiple NCCR variants were found in 12 MS patients treated with natalizumab; the variants detected in plasma and CSF from one patient were different. Earlier, it was found that in a human body, JCPyV existed as a quasispecies (i.e. the population of closely related species), which was common for CSF and plasma from one patient [53]. In the meantime, in most of the paired samples, NCCR variants detected in plasma and CSF were different. It is known that bone marrow cells and respective B lymphocytes are seen as potential sites for neurotropic transformation of JCPyV due to inherent capability of DNA rearranging, which can contribute to emergence of rearranged JCPyV variants [65–67]. It was also assumed that both lymphocytes infected with the virus and the cell-free virus participated in the spread of the virus by crossing the blood-brain barrier [67–70]. Most likely, rearrangements in JCPyV NCCRs in the infected lymphocytes that crossed the blood-brain barrier continue, leading to the emergence of NCCR variants in CSF, which differ (completely or partially) from those that are detected in plasma.
The changeover of JCPyV genotypes, which we observed in paired samples from two patients (NAT-10 and NAT-15), demonstrates that in immunosuppressive conditions (associated with natalizumab treatment), JCPyV is highly variable not only in the NCCR region, but also in the region encoding VP1. The existence of multiple JCPyV variants, their different compartmentalization in the body of the patient as well as the changeover of JCPyV genotypes over time and depending on the analyzed body compartment (plasma or CSF) are associated with the processes, during which the virus is continuously adapting to its cellular environment, as it was also described by other researchers [53].
Conclusion
The study has for the first time identified NCCR patterns typical of MS patients treated with natalizumab in Russia and detected a new rearranged NCCR variant; it has also highlighted the pressing need for further research on JCPyV NCCR rearrangements in MS patients treated with natalizumab to gain a new insight into the origin of neurovirulent JCPyV variants. In future, identification of JCPyV NCCR rearrangements can improve PML risk stratification, expedite diagnostic tests, and optimize the healthcare resource utilization, contributing to the reduction of direct and indirect costs associated with the MS disease.
1 URL: http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB = TF_8.3
2 URL: https://www.proteinatlas.org/ENSG00000170345-FOS/tissue
About the authors
Marina A. Tyumentseva
Central Research Institute for Epidemiology
Email: tyumentseva@cmd.su
ORCID iD: 0000-0002-3145-3702
Cand. Sci. (Biol.), Head, Laboratory of genome editing, Department of molecular diagnostics and epidemiology
Russian Federation, MoscowAleksandr I. Tyumentsev
Central Research Institute for Epidemiology
Email: tyumentseva@cmd.su
ORCID iD: 0000-0003-0537-2586
Cand. Sci. (Biol.), Head, Laboratory of experimental pharmacology, Department of molecular diagnostics and epidemiology
Russian Federation, MoscowMaria N. Zakharova
Research Center of Neurology
Email: tyumentseva@cmd.su
ORCID iD: 0000-0002-1072-9968
D. Sci. (Med.), Professor, chief researcher, Head, 6th Department of neurology, Institute of Clinical and Preventive Neurology
Russian Federation, MoscowLola S. Askarova
Research Center of Neurology
Email: tyumentseva@cmd.su
ORCID iD: 0000-0003-1606-7245
Cand. Sci. (Med.), senior researcher, 6th Department of neurology, Institute of Clinical and Preventive Neurology
Russian Federation, MoscowTaras O. Simaniv
Research Center of Neurology
Email: tyumentseva@cmd.su
ORCID iD: 0000-0001-7256-2668
Cand. Sci. (Med.), senior researcher, 6th Department of neurology, Institute of Clinical and Preventive Neurology
Russian Federation, MoscowMikhail A. Piradov
Research Center of Neurology
Email: tyumentseva@cmd.su
ORCID iD: 0000-0002-6338-0392
D. Sci. (Med.), Professor, Full Member of the Russian Academy of Sciences, Professor, Director
Russian Federation, MoscowVasily G. Akimkin
Central Research Institute for Epidemiology
Author for correspondence.
Email: tyumentseva@cmd.su
ORCID iD: 0000-0003-4228-9044
D. Sci. (Med.), Professor, Full Member of the Russian Academy of Sciences, Director
Russian Federation, MoscowReferences
- Wharton K.A. Jr., Quigley C., Themeles M., Dunstan R.W., Doyle K., Cahir-McFarland E., et al. JC Polyomavirus abundance and distribution in progressive multifocal leukoencephalopathy (PML) brain tissue implicates myelin sheath in intracerebral dissemination of infection. PLoS One. 2016; 11(5): e0155897. https://doi.org/10.1371/journal.pone.0155897
- Marshall L.J., Major E.O. Molecular regulation of JC virus tropism: insights into potential therapeutic targets for progressive multifocal leukoencephalopathy. J. Neuroimmune. Pharmacol. 2010; 5(3): 404–17. https://doi.org/10.1007/s11481-010-9203-1
- Frisque R.J. Structure and function of JC virus T’ proteins. J. Neurovirol. 2001; 7(4): 293–7. https://doi.org/10.1080/13550280152537120
- Prins C., Frisque R.J. JC virus T’ proteins encoded by alternatively spliced early mRNAs enhance T antigen-mediated viral DNA replication in human cells. J. Neurovirol. 2001; 7(3): 250–64. https://doi.org/10.1080/13550280152403290
- Gasparovic M.L., Gee G.V., Atwood W.J. JC virus minor capsid proteins Vp2 and Vp3 are essential for virus propagation. J. Virol. 2006; 80(21): 10858–61. https://doi.org/10.1128/JVI.01298-06
- Del Valle L., Piña-Oviedo S. HIV disorders of the brain: pathology and pathogenesis. Front. Biosci. 2006; 11(1): 718–32. https://doi.org/10.2741/1830
- Major E.O. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu. Rev. Med. 2010; 61(1): 35–47. https://doi.org/10.1146/annurev.med.080708.082655
- White M.K., Khalili K. Pathogenesis of progressive multifocal leukoencephalopathy – revisited. J. Infect. Dis. 2011; 203(5): 578–86. https://doi.org/10.1093/infdis/jiq097
- Delbue S., Ferraresso M., Ghio L., Carloni C., Carluccio S., Belingheri M., et al. A review on JC virus infection in kidney transplant recipients. Clin. Dev. Immunol. 2013; 2013: 926391. https://doi.org/10.1155/2013/926391
- Kmieciak D., Debicki S., Trzeciak W.H. Occurrence rate and genotype distribution of the JC virus (JCV) in a sample from the Polish population. J. Med. Virol. 2008; 80(6): 1079–83. https://doi.org/10.1002/jmv.21153
- Yogo Y., Kitamura T., Sugimoto C., Ueki T., Aso Y., Hara K., et al. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J. Virol. 1990; 64(6): 3139–43. https://doi.org/10.1128/JVI.64.6.3139-3143.1990
- Frisque R.J., Bream G.L., Cannella M.T. Human polyomavirus JC virus genome. J. Virol. 1984; 51(2): 458–69. https://doi.org/10.1128/JVI.51.2.458-469.1984
- Marshall L.J., Dunham L., Major E.O. Transcription factor Spi-B binds unique sequences present in the tandem repeat promoter/enhancer of JC virus and supports viral activity. J. Gen. Virol. 2010; 91(Pt. 12): 3042–52. https://doi.org/10.1099/vir.0.023184-0
- Gosert R., Kardas P., Major E.O., Hirsch H.H. Rearranged JC virus noncoding control regions found in progressive multifocal leukoencephalopathy patient samples increase virus early gene expression and replication rate. J. Virol. 2010; 84(20): 10448–56. https://doi.org/10.1128/JVI.00614-10
- Daniel A.M., Swenson J.J., Mayreddy R.P., Khalili K., Frisque R.J. Sequences within the early and late promoters of archetype JC virus restrict viral DNA replication and infectivity. Virology. 1996; 216(1): 90–101. https://doi.org/10.1006/viro.1996.0037
- McGuigan C., Craner M., Guadagno J., Kapoor R., Mazibrada G., Molyneux P., et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J. Neurol. Neurosurg. Psychiatry. 2016; 87(2): 117–25. https://doi.org/10.1136/jnnp-2015-311100
- Carson K.R., Evens A.M., Richey E.A., Habermann T.M., Focosi D., Seymour J.F., et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009; 113(20): 4834–40. https://doi.org/10.1182/blood-2008-10-186999
- Diebold M., Altersberger V., Décard B.F., Kappos L., Derfuss T., Lorscheider J. A case of progressive multifocal leukoencephalopathy under dimethyl fumarate treatment without severe lymphopenia or immunosenescence. Mult. Scler. 2019; 25(12): 1682–5. https://doi.org/10.1177/1352458519852100
- Berger J.R., Cree B.A., Greenberg B., Hemmer B., Ward B.J., Dong V.M., et al. Progressive multifocal leukoencephalopathy after fingolimod treatment. Neurology. 2018; 90(20): e1815–21. https://doi.org/10.1212/wnl.0000000000005529
- Schwab N., Ulzheimer J.C., Fox R.J., Schneider-Hohendorf T., Kieseier B.C., Monoranu C.M., et al. Fatal PML associated with efalizumab therapy: insights into integrin αLβ2 in JC virus control. Neurology. 2012; 78(7): 458–67; discussion 465. https://doi.org/10.1212/WNL.0b013e3182478d4b
- Carson K.R., Newsome S.D., Kim E.J., Wagner-Johnston N.D., von Geldern G., Moskowitz C.H., et al. Progressive multifocal leukoencephalopathy associated with brentuximab vedotin therapy: A report of 5 cases from the Southern Network on Adverse Reactions (SONAR) project: Brentuximab-Associated PML. Cancer. 2014; 120(16): 2464–71. https://doi.org/10.1002/cncr.28712
- Vukusic S., Rollot F., Casey R., Pique J., Marignier R., Mathey G., et al. Progressive multifocal leukoencephalopathy incidence and risk stratification among natalizumab users in France. JAMA Neurol. 2020; 77(1): 94–102. https://doi.org/10.1001/jamaneurol.2019.2670
- Campagnolo D., Dong Q., Lee L., Ho P.R., Amarante D., Koendgen H. Statistical analysis of PML incidences of natalizumab-treated patients from 2009 to 2016: outcomes after introduction of the Stratify JCV® DxSelectTM antibody assay. J. Neurovirol. 2016; 22(6): 880–1. https://doi.org/10.1007/s13365-016-0482-z
- Datapharm. Physician information and management guidelines for patients with multiple sclerosis receiving TYSABRI therapy. Available at: https://www.medicines.org.uk/emc/rmm/2196/Document
- Gorelik L., Lerner M., Bixler S., Crossman M., Schlain B., Simon K., et al. Anti-JC virus antibodies: implications for PML risk stratification: Anti-JCV Antibodies. Ann. Neurol. 2010; 68(3): 295–303. https://doi.org/10.1002/ana.22128
- Viscidi R.P., Khanna N., Tan C.S., Li X., Jacobson L., Clifford D.B., et al. JC virus antibody and viremia as predictors of progressive multifocal leukoencephalopathy in human immunodeficiency virus-1-infected individuals. Clin. Infect. Dis. 2011; 53(7): 711–5. https://doi.org/10.1093/cid/cir507
- Bloomgren G., Richman S., Hotermans C., Subramanyan M., Goelz S., Natarajam A., et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. Surv. Anesthesiol. 2012; 56(6): 329–30. https://doi.org/10.1097/01.sa.0000422027.26626.8d
- Sindic C.J., Trebst C., Van Antwerpen M.P., Frye S., Enzensberger W., Hunsmann G., et al. Detection of CSF-specific oligoclonal antibodies to recombinant JC virus VP1 in patients with progressive multifocal leukoencephalopathy. J. Neuroimmunol. 1997; 76(1-2): 100–4. https://doi.org/10.1016/s0165-5728(97)00037-4
- Khanna N., Wolbers M., Mueller N.J., Garzoni C., Du Pasquier R.A., Fux C.A., et al. JC virus-specific immune responses in human immunodeficiency virus type 1 patients with progressive multifocal leukoencephalopathy. J. Virol. 2009; 83(9): 4404–11. https://doi.org/10.1128/JVI.02657-08
- Prezioso C., Zingaropoli M.A., Iannetta M., Rodio D.M., Altieri M., Conte A., et al. Which is the best PML risk stratification strategy in natalizumab-treated patients affected by multiple sclerosis? Mult. Scler. Relat. Disord. 2020; 41(102008): 102008. https://doi.org/10.1016/j.msard.2020.102008
- L’Honneur A.S., Leh H., Laurent-Tchenio F., Hazan U., Rozenberg F., Bury-Moné S. Exploring the role of NCCR variation on JC polyomavirus expression from dual reporter minicircles. PLoS One. 2018; 13(6): e0199171. https://doi.org/10.1371/journal.pone.0199171
- Kato A., Sugimoto C., Zheng H.Y., Kitamura T., Yogo Y. Lack of disease-specific amino acid changes in the viral proteins of JC virus isolates from the brain with progressive multifocal leukoencephalopathy. Arch. Virol. 2000; 145(10): 2173–82. https://doi.org/10.1007/s007050070047
- Pagani E., Delbue S., Mancuso R., Borghi E., Tarantini L., Ferrante P. Molecular analysis of JC virus genotypes circulating among the Italian healthy population. J. Neurovirol. 2003; 9(5): 559–66. https://doi.org/10.1080/13550280390241269
- Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016; 33(7): 1870–4. https://doi.org/10.1093/molbev/msw054
- Messeguer X., Escudero R., Farré D., Núñez O., Martínez J., Albà M.M. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002; 18(2): 333–4. https://doi.org/10.1093/bioinformatics/18.2.333
- Farré D., Roset R., Huerta M., Adsuara J.E., Roselló L., Albà M.M., et al. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic. Acids. Res. 2003; 31(13): 3651–3. https://doi.org/10.1093/nar/gkg605
- Hirsch H.H., Kardas P., Kranz D., Leboeuf C. The human JC polyomavirus (JCPyV): virological background and clinical implications. APMIS. 2013; 121(8): 685–727. https://doi.org/10.1111/apm.12128
- Padgett B., Zurhein G., Walker D., Eckroade R., Dessel B. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971; 297(7712): 1257–60. https://doi.org/10.1016/s0140-6736(71)91777-6
- Marshall L.J., Moore L.D., Mirsky M.M., Major E.O. JC virus promoter/enhancers contain TATA box-associated Spi-B-binding sites that support early viral gene expression in primary astrocytes. J. Gen. Virol. 2012; 93(Pt. 3): 651–61. https://doi.org/10.1099/vir.0.035832-0
- Monaco M.C., Jensen P.N., Hou J., Durham L.C., Major E.O. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J. Virol. 1998; 72(12): 9918–23. https://doi.org/10.1128/JVI.72.12.9918-9923.1998
- Naess H., Glad S., Storstein A., Rinaldo C.H., Mørk S.J., Myhr K.M., et al. Progressive multifocal leucoencephalopathy in an immunocompetent patient with favourable outcome. A case report. BMC Neurol. 2010; 10: 32. https://doi.org/10.1186/1471-2377-10-32
- Pavlovic D., Patera A.C., Nyberg F., Gerber M., Liu M. Progressive Multifocal Leukeoncephalopathy Consortium. Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther. Adv. Neurol. Disord. 2015; 8(6): 255–73. https://doi.org/10.1177/1756285615602832
- Cortese I., Reich D.S., Nath A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat. Rev. Neurol. 2021; 17(1): 37–51. https://doi.org/10.1038/s41582-020-00427-y
- Ferenczy M.W., Marshall L.J., Nelson C.D.S., Atwood W.J., Nath A., Khalili K., et al. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin. Microbiol. Rev. 2012; 25(3): 471–506. https://doi.org/10.1128/CMR.05031-11
- Ciardi M.R., Zingaropoli M.A., Iannetta M., Prezioso C., Perri V., Pasculli P., et al. JCPyV NCCR analysis in PML patients with different risk factors: exploring common rearrangements as essential changes for neuropathogenesis. Virol. J. 2020; 17(1): 23. https://doi.org/10.1186/s12985-020-1295-5
- Wilczek M.P., Pike A.M.C., Craig S.E., Maginnis M.S., King B.L. Rearrangement in the hypervariable region of JC Polyomavirus genomes isolated from patient samples and impact on transcription factor-binding sites and disease outcomes. Int. J. Mol. Sci. 2022; 23(10): 5699. https://doi.org/10.3390/ijms23105699
- Martin J.D., King D.M., Slauch J.M., Frisque R.J. Differences in regulatory sequences of naturally occurring JC virus variants. J. Virol. 1985; 53(1): 306–11. https://doi.org/10.1128/JVI.53.1.306-311.1985
- Martin J.D., Foster G.C. Multiple JC virus genomes from one patient. J. Gen. Virol. 1984; 65(Pt. 8): 1405–11. https://doi.org/10.1099/0022-1317-65-8-1405
- Jensen P.N., Major E.O. A classification scheme for human polyomavirus JCV variants based on the nucleotide sequence of the noncoding regulatory region. J. Neurovirol. 2001; 7(4): 280–7. https://doi.org/10.1080/13550280152537102
- Reid C.E., Li H., Sur G., Carmillo P., Bushnell S., Tizard R., et al. Sequencing and analysis of JC virus DNA from natalizumab-treated PML patients. J. Infect. Dis. 2011; 204(2): 237–44. https://doi.org/10.1093/infdis/jir256
- Marshall L.J., Ferenczy M.W., Daley E.L., Jensen P.N., Ryschkewitsch C.F., Major E.O. Lymphocyte gene expression and JC virus noncoding control region sequences are linked with the risk of progressive multifocal leukoencephalopathy. J. Virol. 2014; 88(9): 5177–83. https://doi.org/10.1128/JVI.03221-13
- Nakamichi K., Tajima S., Lim C.K., Saijo M. High-resolution melting analysis for mutation scanning in the non-coding control region of JC polyomavirus from patients with progressive multifocal leukoencephalopathy. Arch. Virol. 2014; 159(7): 1687–96. https://doi.org/10.1007/s00705-014-1988-4
- Van Loy T., Thys K., Ryschkewitsch C., Lagatie O., Monaco M.C., Major E.O., et al. JC virus quasispecies analysis reveals a complex viral population underlying progressive multifocal leukoencephalopathy and supports viral dissemination via the hematogenous route. J. Virol. 2015; 89(2): 1340–7. https://doi.org/10.1128/jvi.02565-14
- Muiño E., Rubio M.A., Navalpotro I., Munteis E. Progressive multifocal leukoencephalopathy in an immunocompetent patient. Neurologia. 2017; 32(5): 337–9. https://doi.org/10.1016/j.nrleng.2015.08.011
- Johnson E.M., Wortman M.J., Dagdanova A.V., Lundberg P.S., Daniel D.C. Polyomavirus JC in the context of immunosuppression: a series of adaptive, DNA replication-driven recombination events in the development of progressive multifocal leukoencephalopathy. Clin. Dev. Immunol. 2013; 2013: 197807. https://doi.org/10.1155/2013/197807
- Kumar K.U., Devireddy L.R., Tang S.C., Pater A., Pater M.M. Human JC virus nuclear factor 1 binding motifs and large tumor antigen region required for transactivation of late promoter. J. Neurochem. 2002; 67(2): 473–81. https://doi.org/10.1046/j.1471-4159.1996.67020473.x
- Mason S., Piper M., Gronostajski R.M., Richards L.J. Nuclear factor one transcription factors in CNS development. Mol. Neurobiol. 2009; 39(1): 10–23. https://doi.org/10.1007/s12035-008-8048-6
- Rodio D.M., Anzivino E., Mischitelli M., Bellizzi A., Scrivo R., Scribano D., et al. Increased prevalence of human Polyomavirus JC viruria in chronic inflammatory rheumatic diseases patients in treatment with anti-TNF α: A 18 month follow-up study. Front. Microbiol. 2016; 7: 672. https://doi.org/10.3389/fmicb.2016.00672
- Sumner C., Shinohara T., Durham L., Traub R., Major E.O., Amemiya K. Expression of multiple classes of the nuclear factor-1 family in the developing human brain: differential expression of two classes of NF-1 genes. J. Neurovirol. 1996; 2(2): 87–100. https://doi.org/10.3109/13550289609146542
- Ravichandran V., Sabath B.F., Jensen P.N., Houff S.A., Major E.O. Interactions between c-Jun, nuclear factor 1, and JC virus promoter sequences: Implications for viral tropism. J. Virol. 2006; 80(21): 10506–13. https://doi.org/10.1128/jvi.01355-06
- Chen N.N., Kerr D., Chang C.F., Honjo T., Khalili K. Evidence for regulation of transcription and replication of the human neurotropic virus JCV genome by the human S(mu)bp-2 protein in glial cells. Gene. 1997; 185(1): 55–62. https://doi.org/10.1016/s0378-1119(96)00630-0
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. Rev. Cancer. 1991; 1072(2-3): 129–57. https://doi.org/10.1016/0304-419x(91)90011-9
- Wisdom R. AP-1: one switch for many signals. Exp. Cell. Res. 1999; 253(1): 180–5. https://doi.org/10.1006/excr.1999.4685
- Sadowska B., Barrucco R., Khalili K., Safak M. Regulation of human Polyomavirus JC virus gene transcription by AP-1 in glial cells. J. Virol. 2003; 77(1): 665–72. https://doi.org/10.1128/jvi.77.1.665-672.2003
- Houff S.A., Major E.O., Katz D.A., Kufta C.V., Sever J.L., Pittaluga S., et al. Involvement of JC virus-infected mononuclear cells from the bone marrow and spleen in the pathogenesis of progressive multifocal leukoencephalopathy. N. Engl. J. Med. 1988; 318(5): 301–5. https://doi.org/10.1056/NEJM198802043180507
- Marzocchetti A., Wuthrich C., Tan C.S., Tompkins T., Bernal-Cano F., Bhargava P., et al. Rearrangement of the JC virus regulatory region sequence in the bone marrow of a patient with rheumatoid arthritis and progressive multifocal leukoencephalopathy. J. Neurovirol. 2008; 14(5): 455–8. https://doi.org/10.1080/13550280802356837
- Monaco M.C., Atwood W.J., Gravell M., Tornatore C.S., Major E.O. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J. Virol. 1996; 70(10): 7004–12. https://doi.org/10.1128/JVI.70.10.7004-7012.1996
- Chapagain M.L., Verma S., Mercier F., Yanagihara R., Nerurkar V.R. Polyomavirus JC infects human brain microvascular endothelial cells independent of serotonin receptor 2A. Virology. 2007; 364(1): 55–63. https://doi.org/10.1016/j.virol.2007.02.018
- Chapagain M.L., Nerurkar V.R. Human polyomavirus JC (JCV) infection of human B lymphocytes: a possible mechanism for JCV transmigration across the blood-brain barrier. J. Infect. Dis. 2010; 202(2): 184–91. https://doi.org/10.1086/653823
- Tornatore C., Berger J.R., Houff S.A., Curfman B., Meyers K., Winfield D., et al. Detection of JC virus DNA in peripheral lymphocytes from patients with and without progressive multifocal leukoencephalopathy. Ann. Neurol. 1992; 31(4): 454–62. https://doi.org/10.1002/ana.410310426
Supplementary files