The combined action of ERG11 gene overexpression and its mutations in the development of Candida albicans resistance to triazolic antifungals
- Authors: Nesvizhsky Y.V.1,2, Afanasiev S.S.2, Zverev V.V.1, Voropaev A.D.2, Afanasiev M.S.1, Voropaeva E.A.2, Budanova E.V.1, Smirnova L.M.1, Anisova S.A.1, Urban Y.N.2
-
Affiliations:
- I.M. Sechenov First Moscow State Medical University (Sechenov University)
- G.N. Gabrichevsky Moscow Research Institute for Epidemiology and Microbiology
- Issue: Vol 102, No 3 (2025)
- Pages: 325-330
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18877
- DOI: https://doi.org/10.36233/0372-9311-653
- EDN: https://elibrary.ru/QEKEIF
- ID: 18877
Cite item
Abstract
Introduction. Modern medicine is faced with the resistance of Candida spp. to antimycotics, due to changes in the expression and structure of the ERG11 gene, the molecular target of triazoles. These mechanisms often operate simultaneously, but the interaction between them remains poorly understood.
The aim of this study is to investigate the interaction between ERG11 gene overexpression and mutation in the development of triazole resistance in C. albicans.
Materials and methods. Eleven C. albicans strains from the G.N. Gabrichevsky Moscow Research Institute of Epidemiology culture collection were analyzed. Each strain was characterized by its ERG11 gene expression level, the presence of ERG11 mutations, and its susceptibility to the triazoles posaconazole, voriconazole, itraconazole and fluconazole.
Results. The C. albicans strains (n – number of tested strains) were categorized into four groups: Group 1 (n = 2, ERG11 overexpression only), Group 2 (n = 3, ERG11 mutations only), Group 3 (n = 4, both ERG11 overexpression and mutation) and Group 4 (n = 2, neither ERG11 overexpression nor mutation). The minimum inhibitory concentration (MIC) of Triazoles in Group 1 was 15.76-fold higher than in Group 2, 4.97-fold higher than in Group 3, and 2.51-fold lower than in Group 4 (p < 0.05 for all comparisons). The MIC of triazoles in Group 2 was 3.17-fold lower than in Group 3 and 40.00-fold lower than in Group 4 (p < 0.001). The MIC of triazoles in Group 3 was 12.5-fold lower than in Group 4 (p < 0.001). Population-level variation in triazoles MIC was more strongly influenced by the isolated effect of ERG11 mutations (45.94%) than by the isolated effect of ERG11 overexpression (5.27-fold less).
Conclusion. Triazole resistance in C. albicans is influenced by the combined actions of ERG11 overexpression and mutation. ERG11 overexpression appears to contribute more to the absolute level of resistance, while ERG11 mutations have a greater impact on the diversity of resistance levels within the C. albicans population.
Keywords
Full Text
Introduction
Microbial resistance to chemotherapeutic drugs is a longstanding challenge in modern medicine. While numerous mechanisms of antibiotic resistance are well-characterized, including those genetically encoded that increase antibiotic target production or alter target structure, the interplay of these mechanisms remains poorly understood [1–3]. These resistance mechanisms can operate independently or concurrently within a microbial cell, and the consequences of their combined effects require further investigation.
We investigated this issue using Candida species as a model, given their well-documented resistance to antimicrobial drugs. One resistance mechanism involves increased expression of genes encoding drug targets, notably ERG11, which encodes lanosterol 14α-demethylase. This enzyme is crucial for ergosterol biosynthesis, a key component of the fungal cell wall. ERG11 overexpression leads to increased ergosterol production, rendering Candida species insensitive to therapeutic azole concentrations [4].
However, recent studies have identified non-synonymous ERG11 mutations that modulate its effects, impacting Candida's triazole susceptibility both positively and negatively [5–9]. Our data [10] show that certain ERG11 mutations mitigated the effects of overexpression, reducing the Minimal Inhibitory Concentration (MIC) of triazole drugs in mutant Candida albicans strains by up to 100-fold. Complete reversal of resistance, however, was not observed. It is important to note that ERG11 overexpression and mutations appear to manifest relatively independently across different Candida spp. [5, 7–9, 11–15].
Both ERG11 overexpression and mutation clearly contribute to the population-level diversity in azole sensitivity observed in Candida species. However, the precise nature and outcome of the interaction between these mechanisms remain unclear. Investigating this interaction is crucial for understanding the survival strategies employed by Candida spp. under conditions of widespread drug exposure and may reveal promising avenues for combating the growing problem of antimicrobial resistance.
Therefore, the aim of this study was to investigate the interaction between ERG11 overexpression and mutation in the development of triazole resistance in C. albicans.
Materials and methods
The study was conducted on 11 C. albicans strains from the collection of the G.N. Gabrichevsky Moscow Research Institute of Epidemiology and Microbiology (Rospotrebnadzor), which were initially resistant to the effects of fluconazole and voriconazole.
Strain identification was performed using biochemical assays and real-time multiplex polymerase chain reaction (qPCR), along with ERG11 expression level analysis and mutation screening. A detailed description of the methodology is provided in another study [10].
According to the available characterization, 7 of the studied strains were carriers of 5 variants of non-synonymous mutations in the ERG11 gene (E266D, G464S, I471L, D116E, and V488I), while 6 strains showed ERG11 overexpression.
Based on these genetic characteristics, C. albicans strains were divided into 4 groups: Group 1 (n = 2) — strains with only ERG11 overexpression; Group 2 (n = 3) — strains with only ERG11 mutations; Group 3 (n = 4) — strains with simultaneous expression of both types of genetic alterations; Group 4 (n = 2) — strains without either of the genetic alterations.
The sensitivity of the studied C. albicans strains to four representatives of triazole antifungals (posaconazole, voriconazole, itraconazole, fluconazole) was investigated in accordance with the recommendations of the Interregional Association for Clinical Microbiology and Antimicrobial Chemotherapy (IACMAC) for determining the sensitivity of microorganisms to antimicrobial agents, based on CLSI M44 and M60 standards for fungi and the standards and criteria of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) for microdilution methods and bacterial cultures1.
The minimum inhibitory concentrations (MIC, mg/mL) were determined by the serial microdilution method using the Sensititre YeastOne plates (Trek diagnostic system). For this, the inoculum was prepared similarly to the disk diffusion method, after which it was introduced into a modified RPMI-1640 medium and distributed into 96-well plates for serial microdilutions with previously added triazole antifungals [11]. The results were recorded visually, comparing the growth in the well with the positive control well according to EUCAST criteria [12].
To ensure the comparability of the research results, the data for individual C. albicans strains for each triazole antifungal were weighted by the average MIC value for the given drug. Subsequently, the obtained relative values were analyzed.
Statistical analyses were conducted using Microsoft Excel, SciPy and Matplotlib. The significance of the differences was assessed using the Mann–Whitney U-test. The contribution of factors to the population variability of the trait was assessed using single-factor and two-factor ANOVA. The critical error level for testing statistical hypotheses was set at p < 0.05.
Results
The MIC of triazole antifungals with various genetic modifications in C. albicans is presented in Table 1. Statistical analysis revealed no significant differences between the individual drugs for each variant of genetic alterations, indicating a uniform directional effect across all triazoles. Due to this fact, the results of the MIC study were pooled into a single group of triazoles. The defining characteristics of each group are presented in Table 2.
Table 1. MIC of triazole antifungals in various genetic modifications in the ERG11 gene of C. albicans (X ± m)
Strain group | n | Posaconazole | Voriconazole | Itraconazole | Fluconazole |
1 | 2 | 1.361 ± 1.351 | 1.184 ± 1.045 | 1.363 ± 1.353 | 1.579 ± 1.483 |
2 | 3 | 0.008 ± 0.002 | 0.139 ± 0.000 | 0.008 ± 0.002 | 0.191 ± 0.000 |
3 | 4 | 0.028 ± 0.019 | 0.383 ± 0.244 | 0.026 ± 0.020 | 0.669 ± 0.317 |
4 | 2 | 4.068 ± 1.357 | 3.343 ± 1.115 | 4.075 ± 1.359 | 2.296 ± 0.765 |
Table 2. MIC of triazole antifungals in the studied groups
Strain group | n | X ± m | Me [Q1; Q3] |
1 | 8 | 1.371 ± 0.501 | 1.184 [0.010; 2.470] |
2 | 12 | 0.087 ± 0.024 | 0.075 [0.007; 0.139] |
3 | 16 | 0.276 ± 0.113 | 0.112 [0.006; 0.152] |
4 | 8 | 3.445 ± 0.522 | 2.889 [1.879; 3.759] |
Comparative analysis of the obtained results showed that the MIC of triazoles in Group 1 was 15.76 times higher (p < 0.05) than in Group 2, 4.97 times higher (p < 0.05) than in Group 3, and 2.51 times lower (p < 0.05) than in Group 4. In Group 2, the MIC of triazoles was 3.17 times lower than in Group 3, and 40 times lower (p < 0.001) than in Group 4. The MIC of triazoles in Group 3 was 12.5 times lower (p < 0.001) compared to Group 4.
The assessment of the impact of various genetic alterations on the degree of variation in the MIC of triazoles in the studied C. albicans population was conducted using analysis of variance (ANOVA). The single-factor model showed that the combined effect of ERG11 overexpression and mutation amounts to 58.58% (p < 0.001) of the total variance.
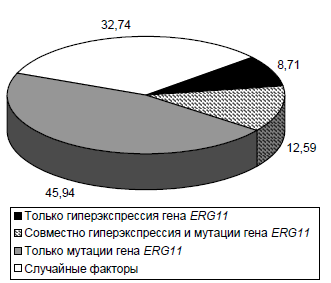
A two-factor ANOVA was performed to quantify the relative impact of these genetic alterations (Figure). The isolated effect of ERG11 mutations accounted for almost half (45.94%) of the genetic variance, which is more than 5.27 times greater than the contribution of the isolated effect of ERG11 overexpression and 3.65 times greater than the combined effect of mutations and overexpression. Taken together, the combined effect of all genetic alterations accounts for 67.26%, which is consistent with the findings of the single-factor ANOVA model.
Two-factor model of the influence of genetic alterations in the ERG11 gene on the variation of triazole MIC in the C. albicans population, %.
Discussion
This study confirms that both ERG11 overexpression and ERG11 mutations contribute to triazole resistance in C. albicans strains initially resistant to fluconazole and voriconazole. It is further demonstrated that these genetic mechanisms can act independently or synergistically in conferring resistance. While ERG11 overexpression generally exerts a more pronounced effect than ERG11 mutations alone, as confirmed in several previous studies [4-9, 16, 17], their interaction is complex.
Although an additive effect of ERG11 overexpression and mutations might be anticipated, our data reveal that certain mutations can attenuate the impact of ERG11 overexpression. This resulted in a noticeable reduction in the overall effect of ERG11 overexpression in our C. albicans strain collection. However, it is not recommended to generalize this observation to all instances of genetically mediated resistance in C. albicans; rather, this finding is interpreted as a potential characteristic specific to the strains included in this study.
The observation of high triazole resistance in C. albicans strains lacking ERG11 alterations suggests that other resistance mechanisms are also operative. For example, overexpression of efflux pump genes, such as CDR1, CDR2 and MDR1, has been reported [4, 5], although their relative contributions to resistance remain to be fully quantified.
The analysis of variance accounted for the contribution of both ERG11 overexpression and mutation to the population-level variation in triazole susceptibility among C. albicans strains. While both mechanisms contribute, the results indicate that ERG11 mutations play a dominant role in shaping this phenotypic diversity.
Evaluating the biological and medical significance of the overexpression and mutations of the ERG11 gene in C. albicans strains, it was observed that ERG11 overexpression and the associated lanosterol-14α-demethylase hyperproduction serve as a far more effective defense mechanism against the harmful effects of triazole antifungals than the synthesis of genetically modified enzyme variants. However, non-synonymous point mutations in ERG11 clearly contribute to the increased biological diversity of this yeast-like fungus, without necessarily causing a dramatic, short-term increase in its clinical threat. Therefore, from a practical perspective, identifying ERG11 overexpression may be a more appropriate initial strategy for predicting the immediate risk of triazole resistance in C. albicans isolates.
Conclusion
- Triazole resistance in albicans strains arises from the combined effects of ERG11 overexpression and mutation.
- ERG11 overexpression has a significantly greater impact on resistance levels than its non-synonymous mutations.
- Mutations within the ERG11 gene are a more significant driver of population-level triazole resistance diversity in albicans than ERG11 overexpression.
- It is recommended to test strains for ERG11 overexpression to predict the emergence of triazole resistance in albicans.
1 IACMAC Recommendations "Determination of the sensitivity of microorganisms to antimicrobial drugs (2021)». URL: https://www.antibiotic.ru/minzdrav/category/clinical-recommendations
About the authors
Yuri V. Nesvizhsky
I.M. Sechenov First Moscow State Medical University (Sechenov University); G.N. Gabrichevsky Moscow Research Institute for Epidemiology and Microbiology
Author for correspondence.
Email: nesviz@mail.ru
ORCID iD: 0000-0003-0386-3883
Dr. Sci. (Med.), Professor, Department of microbiology, virology and immunology, I.M. Sechenov First Moscow State Medical University (Sechenov University); chief researcher, Laboratory of clinical microbiology and biotechnology, G.N. Gabrichevsky Research Institute for Epidemiology and Microbiology
Russian Federation, Moscow; MoscowStanislav S. Afanasiev
G.N. Gabrichevsky Moscow Research Institute for Epidemiology and Microbiology
Email: afanasievss409.4@bk.ru
ORCID iD: 0000-0001-6497-1795
Dr. Sci. (Med.), Professor, chief researcher, Laboratory of clinical microbiology and biotechnology
Russian Federation, MoscowVitaly V. Zverev
I.M. Sechenov First Moscow State Medical University (Sechenov University)
Email: vitalyzverev@outlook.com
ORCID iD: 0000-0001-5808-2246
Dr. Sci. (Biol.), Professor, RAS Full Member, Head, Department of microbiology, virology and immunology
Russian Federation, MoscowAlexander D. Voropaev
G.N. Gabrichevsky Moscow Research Institute for Epidemiology and Microbiology
Email: advoropaev@gmail.com
ORCID iD: 0000-0002-6431-811X
Cand. Sci. (Med.), junior researcher, Laboratory of clinical microbiology and biotechnology
Russian Federation, MoscowMaxim S. Afanasiev
I.M. Sechenov First Moscow State Medical University (Sechenov University)
Email: maxim.afanasyev78@gmail.com
ORCID iD: 0000-0002-5860-4152
Dr. Sci. (Med.), Prof., Department of clinical allergology and immunology
Russian Federation, MoscowElena A. Voropaeva
G.N. Gabrichevsky Moscow Research Institute for Epidemiology and Microbiology
Email: voropaevaea2011@gmail.com
ORCID iD: 0000-0002-0463-0136
Dr. Sci. (Biol.), Prof., chief researcher, Laboratory of clinical microbiology and biotechnology
Russian Federation, MoscowElena V. Budanova
I.M. Sechenov First Moscow State Medical University (Sechenov University)
Email: e.v.budanova@mail.ru
ORCID iD: 0000-0003-1864-5635
Cand. Sci. (Med.), Assoc. Prof., Department of microbiology, virology and immunology
Russian Federation, MoscowLudmila M. Smirnova
I.M. Sechenov First Moscow State Medical University (Sechenov University)
Email: lmsmirnova1306@gmail.com
ORCID iD: 0000-0002-6581-4529
Cand. Sci. (Med.), Assoc. Prof., Department of skin and venereal diseases
Russian Federation, MoscowSofia A. Anisova
I.M. Sechenov First Moscow State Medical University (Sechenov University)
Email: sofaanisova@ya.ru
ORCID iD: 0009-0002-1099-4451
student, Filatov Clinical Institute of Child Health
Russian Federation, MoscowYulia N. Urban
G.N. Gabrichevsky Moscow Research Institute for Epidemiology and Microbiology
Email: urbanek@mail.ru
ORCID iD: 0000-0003-0189-3608
Cand. Sci. (Biol.), senior researcher, Laboratory for clinical microbiology and biotechnology
Russian Federation, MoscowReferences
- Xiong L., Wang X., Wang Y., et al. Molecular mechanisms underlying bacterial resistance to ceftazidime/avibactam. WIREs Mech. Dis. 2022;14(6):e1571. DOI: https://doi.org/10.1002/wsbm.1571
- Azargun R., Gholizadeh P., Sadeghi V., et al. Molecular mechanisms associated with quinolone resistance in Enterobacteriaceae: review and update. Trans. R. Soc. Trop. Med. Hyg. 2020; 114(10):770–81. DOI: https://doi.org/10.1093/trstmh/traa041
- Gogry F.A., Siddiqui M.T., Sultan I., Haq Q.M.R. Current update on intrinsic and acquired colistin resistance mechanisms in bacteria. Front. Med. (Lausanne). 2021;8:677720. DOI: https://doi.org/10.3389/fmed.2021.677720
- Biswas C., Chen S.C., Halliday C., et al. Identification of genetic markers of resistance to echinocandins, azoles and 5-fluorocytosine in Candida glabrata by next-generation sequencing: a feasibility study. Clin. Microbiol. Infect. 2017;23(9):676.e7–10. DOI: https://doi.org/10.1016/j.cmi.2017.03.014
- Cernicka J., Subik J. Resistance mechanisms in fluconazole-resistant Candida albicans isolates from vaginal candidiasis. Int. J. Antimicrob. Agents. 2006;27(5):403–8. DOI: https://doi.org/10.1016/j.ijantimicag.2005.12.005
- Lim H.J., Shin J.H., Kim M.N., et al. Evaluation of two commercial broth microdilution methods using different interpretive criteria for the detection of molecular mechanisms of acquired azole and echinocandin resistance in four common Candida species. Antimicrob. Agents Chemother. 2020;64(11):e00740-20. DOI: https://doi.org/10.1128/AAC.00740-20
- Lopes W., Vainstein M.H., Schrank A. Revealing colonial characteristics of Candida tropicalis by high-resolution scanning electron microscopy. Clin. Microbiol. Infect. 2019;25 (2):188–9. DOI: https://doi.org/10.1016/j.cmi.2018.06.032
- Pappas P.G., Kauffman C.A., Andes D.R., et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 2016;62(4):e1–50. DOI: https://doi.org/10.1093/cid/civ933
- Castanheira M., Deshpande L.M., Messer S.A., et al. Analysis of global antifungal surveillance results reveals predominance of Erg11 Y132F alteration among azole-resistant Candida parapsilosis and Candida tropicalis and country-specific isolate dissemination. Int. J. Antimicrob. Agents. 2020;55(1):105799. DOI: https://doi.org/10.1016/j.ijantimicag.2019.09.003
- Несвижский Ю.В., Афанасьев С.С., Воропаев А.Д. и др. Спектр и функциональные свойства мутаций гена ERG11 флуконазол-резистентныйх грибов Candida albicans, выделенных от ВИЧ-инфицированных пациентов. ЖУРНАЛ МИКРОБИОЛОГИИ, ЭПИДЕМИОЛОГИИ И ИММУНОБИОЛОГИИ. 2023;100(4):285–92. Nesvizhsky Yu.V., Afanasiev S.S., Voropaev A.D., et al. Spectrum and functional properties of ERG11 gene mutations in fluconazole-resistant Candida albicans strains isolated from HIV-infected patients. Journal of Microbiology, Epidemiology and Immunobiology. 2023;100(4):285–92. DOI: https://doi.org/10.36233/0372-9311-407 EDN: https://elibrary.ru/pxrovi
- Godinho C.P., Sá-Correia I. Physiological genomics of multistress resistance in the yeast cell model and factory: аocus on MDR/MXR transporters. In: Sá-Correia I., eds. Yeasts in Biotechnology and Human Health. Progress in Molecular and Subcellular Biology, Volume 58. Cham;2019:1–35. DOI: https://doi.org/10.1007/978-3-030-13035-0_1
- Xu Y., Chen L., Li C. Susceptibility of clinical isolates of Candida species to fluconazole and detection of Candida albicans ERG11 mutations. J. Antimicrob. Chemother. 2008;61(4):798-804. DOI: https://doi.org/10.1093/jac/dkn015
- Kakeya H., Miyazaki Y., Miyazaki H., et al. Genetic analysis of azole resistance in the Darlington strain of Candida albicans. Antimicrob. Agents Chemother. 2000;44(11):2985–90. DOI: https://doi.org/10.1128/AAC.44.11.2985-2990.2000
- Finkina E.I., Bogdanov I.V., Ignatova A.A., et al. Antifungal activity, structural stability, and immunomodulatory effects on human immune cells of defensin from the lentil Lens culinaris. Membranes (Basel). 2022;12(9):855. DOI: https://doi.org/10.3390/membranes12090855
- Lee Y., Puumala E., Robbins N., Cowen L.E. Antifungal drug resistance: molecular mechanisms in Candida albicans and beyond. Chem. Rev. 2021;121(6):3390–411. DOI: https://doi.org/10.1021/acs.chemrev.0c00199
- Katsipoulaki M., Stappers M.H.T., Malavia-Jones D., et al. Candida albicans and Candida glabrata: global priority pathogens. Microbiol. Mol. Biol. Rev. 2024;88(2):e0002123. DOI: https://doi.org/10.1128/mmbr.00021-23
- Mahdizade A.H., Hoseinnejad A., Ghazanfari M., et al. The TAC1 gene in Candida albicans: structure, function, and role in azole resistance: a mini-review. Microb. Drug Resist. 2024;30(7): 288–96. DOI: https://doi.org/10.1089/mdr.2023.0334
Supplementary files