Streptococcal effective molecules as promising antiсancer agents: pros and cons
- Authors: Suvorov A.N.1,2, Tsapieva A.N.1, Chernov A.N.1,3
-
Affiliations:
- Institute of Experimental Medicine
- Saint Petersburg State University
- Saint Petersburg State Pediatric Medical University
- Issue: Vol 102, No 2 (2025)
- Pages: 223-238
- Section: SCIENCE AND PRACTICE
- URL: https://microbiol.crie.ru/jour/article/view/18830
- DOI: https://doi.org/10.36233/0372-9311-638
- EDN: https://elibrary.ru/NXCMRV
- ID: 18830
Cite item
Abstract
Oncological diseases remain the main cause of death and disability of the population worldwide. The most malignant types of cancer include of pancreas, liver and brain tumors. Modern methods of therapy (including chemoradiation, targeted and immune therapy) do not allow achieving the desired effectiveness in this group of patients. In this regard, new approaches to the treatment of oncological diseases are needed.
The aim of this review was to discuss the mechanisms of anticancer action of Strepococcus pyogenes and other streptococcal species, as well as to consider their cancer-stimulating effects and their limitations.
The review considers the current state of the problem of using bacterial oncotherapy involving streptococci A group (in particular, S. pyogenes). The involvement of S. pyogenes pathogenicity factors is discussed: M-protein, exotoxins: streptolysins S, O, superantigens, arginine deiminase, etc., as well as molecular mechanisms mediated by the host immune system cells. The authors' own data show that S. pyogenes exhibits selective M-protein-mediated cytolytic activity against C6 glioma and pancreatic adenocarcinoma (Panc) tumor cells and no activity against normal fibroblasts. The data on preclinical and clinical application of the streptococcal-based medicine OK-432 for the therapy of oncological diseases are briefly summarized. Tumor-associated properties of streptococci (induction of cytokine storm, proliferation, migration and angiogenesis of vascular epithelial cells, formation of neutrophil extracellular traps in the tumor microenvironment) caused by their interaction with immune cells of the tumor-bearing organism are also discussed.
Conclusion. The research data presented in this review convincingly demonstrate that S. pyogenes, with the participation of pathogenicity factors, can directly exert an antitumor effect on cancer cells. However, it should be noted the streptococcal vaccine should be used with caution, taking into account the individual characteristics of the patient's immune system, since S. pyogenes can also have effects of the opposite nature, requiring further research.
Full Text
Introduction
Cancer diseases are one of the main causes of disability and mortality worldwide, second only to cardiovascular pathology [1]. According to the World Health Organization and the International Agency for Research on Cancer (Globocan), 19,976,499 new cases of cancer were globally registered in 2022, of which 9,743,832 were death (48.8%)1. The World Health Organization predicts that by 2045, cancer incidence and mortality will increase by 55% to reach 31.0 million and 16.7 million cases respectively.
Recent scientific achievements in biology, medicine and oncology have been marked by the discovery of cancer stem cells, horizontal transfer of genetic information (mobile genetic elements), and the emergence of new genetic diagnostic methods: whole-genome and whole-exome sequencing. These and other advances contributed to a paradigm shift from cohort-based to individualized treatment, which led to the emergence of new scientific directions: targeting and immunotherapy, which gave hope for the cure of many types of cancer [2, 3].
On the other hand, all these successes in oncology caused new problems associated with the establishment of molecular and cellular heterogeneity of tumors, the development of multiple drug resistance of cancer cells to therapy, its high toxicity to normal tissues and cells, and the participation of tumor microenvironment cells in cancer progression [4, 5]. These obstacles required reconsideration of existing treatment methods and search for new therapeutic approaches to oncologic diseases.
The use of live bacteria that selectively colonize the tumor represents a promising prospect for cancer therapy that overcomes many problems [6]. Unlike most therapeutic agents, bacteria have multiple mechanisms targeted at inhibiting tumor growth. Microorganisms selectively colonize tumors and proliferate within them, where they initiate anticancer immune responses, which may ultimately increase survival after systemic infection in tumor models of animals [7]. For example, a Salmonella typhimurium strain VNP20009 attenuated more than 10,000-fold compared to the wild-type strain has a tumor : liver colonization ratio > 1000 : 1 and exhibits at titers of 1 × 104 – 3 × 106 CFU/mouse) a strong inhibitory effect (57-95%) on the growth of Lox, DLD-1, A549, WiDr, HTB177, MDA-MB-231 and B16F10 human tumors and the development of pulmonary metastases in mouse tumor models [8, 9]. Furthermore, such bacteria can be further programmed using simple or complex genetic and bioengineering methods for the synthesis and tumor-selective delivery of anticancer drugs [10]. For example, the use of bacteria as transport vectors can increase the poor tumor penetration and activity of preventive drugs for chemotherapy while reducing their systemic toxicity to the organism. Bacteria can be used for targeted delivery of chemotherapeutics, cytokines, immunomodulators, enzymes, prodrugs, or small interfering RNAs to tumors [6]. Furthermore, bacteria themselves can synthesize anticancer enzymes, for example, L-asparaginase and methionine-gamma-lyase [11, 12].
Motility is an important property and allows bacteria to penetrate deeper into tumor tissue. In contrast to the passive distribution and limited penetration of chemopreventive agents, bacteria are complex living organisms that can obtain energy from the environment and convert it into kinetic energy of movement, which allows them to move independently deeper into the tumor [13]. Microorganisms are able to influence the tumor microenvironment and produce their own oncolytic metabolites (peptides, bacteriocins), which makes these microbes a promising approach for cancer therapy [14]. Treatment with live bacteria can be used either as monotherapy or in combination with other cancer therapies. Oncolytic bacteria include Bifdobacteria, Clostridium, Listeria monocytogenes, Salmonella typhimurium, Streptococcus bovis and S. pyogenes (Fig. 1) [14].
Fig. 1. Electron micrograph of S. pyogenes.
Source: Streptococcus pyogenes. URL: https://en.wikipedia.org/wiki/Streptococcus_pyogenes
Since S. pyogenes are a group of microorganisms that cause the development of a frequent pathogenic form of group A streptococcal infection in humans and one of the objects studied at the Department of molecular microbiology of the Institute of Experimental Medicine, these bacteria were chosen as the key object for discussion of its anticancer properties in this review.
The aim of the review was to discuss the mechanisms of anticancer action of S. pyogenes and other streptococcal species, as well as to consider their tumor-stimulating effects and the limitations that exist in this regard.
Antitumor mechanisms of Streptococcus pyogenes
Since 1891, when Dr. W. Coley first used a vaccine based on live S. pyogenes and Serratia marcescens to treat 10 patients with terminal osteosarcoma and suggested an association between the development of fever and tumor regression [15], several bacterial strains have been studied and selected for testing on patients. In order to understand the pathogenetic mechanisms of the effect of S. pyogenes on tumor cells, it is necessary to consider the associated pathogenicity factors of these microorganisms.
Pathogenicity factors
More than 40 pathogenicity factors have been found for S. pyogenes [16]. All of them can be divided into microbial cell-associated and extracellular ones.
The streptococcus cell is surrounded by a cell wall formed by the peptidoglycan polymer consisting of N-acetylglucosamine and N-acetylmuramic acid and covalently linked to them by teichoic and lipoteichoic acids [17]. Protein and polysaccharide components are connected to the cell wall (Fig. 2) [18].
Fig. 2. Schematic representation of the streptococcal cell and the main biologically active products of S. pyogenes [19].
The cell wall polysaccharides also include carboxyhydrate antigens formed by group-specific polysaccharide-A, the differences in the structure of which are the basis for the classification of streptococci [20]. Among cell wall proteins, the main pathogenicity factor is M (Emm) protein, which provides resistance of S. pyogenes to phagocytosis and multiplication in the blood [21].
The authors of the review showed that both wild-type GUR and M-protein mutant GURSA1 strains (106 CU/mL) exerted in vitro cytotoxic effect on C6 glioma cells using the xCELLigence system (Fig. 3) [22, 23].
Fig. 3. Effect of S. pyogenes strains GUR, GURSA1 on C6 cells in real time using xCELLigence system [23].
The data of Fig. 3 show that streptococcal strains GUR, GURSA1 demonstrated rapid and strong oncolytic effect against C6 glioma cells. S. pyogenes strains GUR and GURSA1 had the slowest cytotoxic effects on C6 glioma cells with the highest cell inhibition rates of 81.8 and 79.3% after 4 and 6 h, respectively. Moreover, the cytotoxic effects of S. pyogenes strains GUR and GURSA1 slightly increased over time, reaching growth inhibition rates of 85.0 and 81.5% after 8 h, respectively [22]. Similar results were obtained when S. pyogenes strains GUR and GURSA1 were tested on mouse pancreatic adenocarcinoma (Panc) cells in real time (Fig. 4) [23].
Fig. 4. Effect of S. pyogenes strains GUR, GURSA1 on Panc cells in real time using xCELLigence system [23].
The results in Figs. 3, 4 show that both strains inhibited the growth of C6 glioma and Panc cells, and the effect of GURSA1 strain was weaker than that of GUR, which confirms the presence of a cytotoxic effect due to the presence of M-protein as a pathogenicity factor. At the same time, both strains had no cytotoxic effects on normal fibroblast cells in real time (Fig. 5) [23].
Fig. 5. Effect of S. pyogenes strains GUR, GURSA1 on normal fibroblast cells in real time using xCELLigence system [23].
S. pyogenes do not synthesize a single M-protein, but there are at least up to 4 groups of M-proteins: FG I (Mrp); FG II, M-protein and H-protein; FG III (Enn); and FG IV (M-protein). The M and Enn proteins form two groups with 9 subgroups, and the Mrp proteins form 4 groups with 10 subgroups (Fig. 6) [24].
Fig. 6. Genetic network analysis of SplitsTree, including 537 genetic sequences with the allocation of clusters of M proteins [24].
a — M protein; b — Mrp (221 sequences); c — Enn proteins (262 sequences). Black dotted ellipses denote two groups of M proteins, green dotted ellipses denote chimeric M proteins, and colored ellipses denote subgroups of M proteins.
M-proteins are known to consist of α-helical fibrils with a diameter of 50-60 nm located on the bacterial cell wall. These proteins have a super-spiral structure and can form dimers with different polypeptide chain lengths (Fig. 7) [25].
Fig. 7. Three M (emm) proteins (M5, M80, and M77) [25].
The length of the M protein and the size of the repeat and non-repeat domains are shown to the scale. The A–C emm patterns are the longest M proteins with a hypervariable domain of 230 amino acids. The D and E structured M proteins have hypervariable domains consist from 150 and 100 residues, respectively. The “A” repeats are absent from the vast majority of M proteins belonging to the D and E pattern groups. The “B” repeats are present in most A–C and D M proteins, but are absent from the E–M subtypes of M proteins
M-proteins as pathogenicity factors contribute to the resistance of S. pyogenes to phagocytosis by macrophages and host antibodies [26]. M-proteins can bind blood plasma proteins: immunoglobulins G and A (Fc-fragments), fibrinogen, fibronectin, albumin, plasminogen, C4b-binding protein (C4BP), factor-H and complement proteins [19]. The interaction of M-protein with plasma factors affects the adhesive and invasive properties of S. pyogenes, and through alteration of blood coagulation capacity indirectly affects cancer cell viability. This is confirmed by the fact that the state of blood hypercoagulability as a component of tumor microenvironment promotes cancer progression [27].
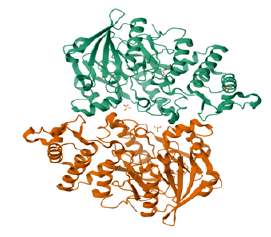
The other pathogenicity factors of S. pyogenes have been studied to a lesser extent. They include: exotoxins streptolysins S, O, superantigens, serine and cysteine proteinases, streptokinase, arginine deiminase, endo-β-N-acetylglucosaminidase, nicotinamidine dinucleotidase, etc. [19]. For example, arginine deaminase (ADI) consists of 2 domains, the first of which is formed by 5 ββαβ sites repeating around a pseudo-5-fold axis containing the active center. The second domain is a 4-domain helical chain (Fig. 8).
Fig. 8. Structure of arginine deaminase from S. pyogenes (a); crystal structure illustrating the connecting regions (b): green, blue, pink, light blue and red colors, respectively; residues of the ADI active site (c), and the ADI topological diagram (d) [28].
ADI was first isolated from the Su strain (3 μg/mL), which is capable of inhibiting the growth of transformed BALB/3T3 fibroblast cells [29]. ADI is localized in the cell wall of S. pyogenes, whereas in S. suis the same enzyme is present in the membrane fraction of cells. Its activity is associated with the ability to convert arginine to ammonia and citrulline [30]. On the other hand, arginine utilization enhances the proliferation and growth of some types of tumors such as melanoma, hepatocellular carcinoma and prostate cancer. Since arginine deficiency induces cycle arrest in G1-phase cancer cells, activation of mTOR- and GCN2-kinase genes in them, which trigger autophagy and apoptosis [31, 32], T. Fiedler et al. studied the efficacy of ADI therapy (3.5–350.0 IU/mL) and its combinations with cytostatic drugs: chloroquine (5 and 20 μM), hydroxamic acid suberoylanilide (SAHA; 0.25 and 0.5 μM) or palomide 529 (dual TORC1/TORC2 inhibitor; 7.5 μM) to inhibit the growth of 12 glioblastoma (GBM) cell lines in vitro and in vivo on male NMRI Foxn1nu mice weighing 20–25 g [33]. ADI administration inhibited GBM growth in 50% of tested lines (Fig. 9).
Fig. 9. Crystal violet staining of the GBM cells after ADI incubation during 72 h (a) and tumor growth curve (b).
Tumor volumes are given as x-fold increase compared to day 0 [33].
In cell lines for which ADI reduced cell proliferation, its combinations with chemotherapy agents were tested. Synergistic effects were observed on HROG02, HROG05 and HROG10 66% GBM cells with growth inhibition of up to 70% using the combination of ADI with Palomid 529. Similar anticancer effects (60% cell death) were established after adding chloroquine to ADI. The reason for the anticancer effects of ADI was epigenetic suppression of arginine synthesis pathway genes (argininosuccinate synthetase ASS1 and argininosuccinate lyase ASL). In an in vivo model, ADI (250 U/kg wt) and its combination with SAHA (25 mg/kg wt) inhibited 70% of HROG05 heterograft tumor size compared to control (Fig. 9, b) [32].
Fig. 10. ADI treatment induces autophagy and senescence in GBM cells [34].
Acridine orange (orange) and calcein AM (green) staining. Analysis was conducted on a laser scanning microscope (Zeiss) using a 20x objective.
C. Maletzki et al. found that ADI (35 mU/mL) induced the expression of heat shock and arginine metabolism protein genes (ASS1, ASL, ArgI, CPSI, OTC) in HROG02, HROG05, HROG52, HROG63 cells of GBM patients (Fig. 10) [34]. As in a previous study, ADI administration stimulated autophagy and senescence of tumor cells. Combinations of ADI with curcumin, resveratrol, quinacrine and sorafenib for 3 days enhanced the cytotoxicity of the enzyme by activating autophagy.
Other pathogenicity factors of S. pyogenes include: cholesterol-dependent cytolysins and streptolysin O (SLO). S.C. Feil et al. determined the three-dimensional structure of this enzyme (Fig. 11) [35].
Fig. 11. Strepolisin O molecular structure [35].
The SLO molecule consists of 571 amino acids, which form 4 domains having a β-folded structure. The first N-terminal domain (70 amino acids) is cleaved off by proteolysis by streptococcal proteases after secretion. In addition to the domains, SLO contains 2 transmembrane regions, TMH1 (residues 259–288) and TMH2 (residues 359-386), which have an α-helical structure. SLO includes the 11-amino acid sequence ECTGLAWEWWR, rich in tryptophan residues, which promotes the incorporation of the molecule into cell membranes [35]. As a result of oligo- and polymerization, SLOs form large pores in cell membranes. These mechanisms lead to structural damage, severe ATP depletion and cell death by necrosis. Consequently, this mechanism of action of SLO can be used for cytolysis of cancer cells. In this regard, C. Gruber et al. using RNA trans-splicing developed 3’-pre-trans-splicing molecules (PTM) containing an embedded SLO gene in the target gene of matrix metalloproteinase-9 (MMP9) in highly aggressive squamous cell carcinoma cells [36]. This technique allowed the replacement of a tumor-specific transcript with a peptide/toxin encoding gene, resulting in cell death. A fluorescent tag was used to visualize the transcript (Fig. 12).
Fig. 12. Schematic representation of the fluorescent trans-splicing assay system [36].
PTM is the engineered mRNA linked via an antisense BD domain to MMP9 intron 1. BP is the branch point; PPT is the polypyrimidine sequence.
Trans-splicing between the 50th and 30th nucleotides of MMP9 and PTM genes resulted in the formation of mRNA encoding a full-length GFP-protein containing a DsRed tag. Transfection of HEK293 human squamous cell carcinoma and embryonic kidney HEK293 cancer cells with SLO-PTM induced death in them due to SLO expression (Fig. 13).
Fig. 13. Fluorescence microscopy of transfected HEK293 cells [36].
Control was transfected with a construct containing DsRed dye and full-length cDNA of GFP. Cells expressing GFP indicate trans-splicing in the target gene and the PTM carrying one of the three BD domains (BD1, BD2, and BD4). Scale bar is 20 mm.
Thus, the use of SLO is a new approach in bacterial therapy of human malignant tumors [37]. In other Streptococcus species, such as S. bovis those belonging to group D, bacteriocin bovicin HC5 synthesized in ribosomes has been found [37]. This cationic peptide has a cytolytic effect against MCF-7 breast adenocarcinoma and HepG2 hepatocellular carcinoma cells [38].
Mediated mechanisms of S. pyogenes' anticancer action
In addition to direct cytolytic action on tumor cells, S. pyogenes in the body can also exert cytostatic anticancer effects mediated by immune cells and factors. Once in the body, streptococci are perceived by the immune system as foreign and trigger an inflammatory response. During this reaction, a cytokine and chemokine storm develops due to increased levels of tumor necrosis factor-α (TNF-α), interferon-γ, interleukins (IL-1β, IL-6, IL-10), monocyte chemoattractant protein-1 [38]. In addition to these changes, Y.H. Liu et al. found that during infection with the A20 strain of S. pyogenes, the patient’s brain cells expressed glial fibrillary acidic protein, inducible nitric oxide synthase, components of nicotinamideadenine dinucleotide phosphate oxidase, all at a high level, which stimulated the production of reactive oxygen species. Myeloperoxidase secreted by activated macrophages and neutrophils was also expressed in the cortex and hippocampus of infected mice (Fig. 14) [39].
Fig. 14. Changes in GFAP (a), iNOS (b), MPO (c) and MMP-9 (d) levels in the brain during infection with S. pyogenes strain A20 in a patient [39].
Increased levels of IL-10 and IL-12 in blood activate CD18+-monocytes and CD11c-CD123+-plasmacytoid and CD11c+CD123–-myeloid dendritic cells (DCs) through the Toll-like receptor (TLR) signaling pathway [40]. T.G. Loof et al. showed that when KTL3 was stimulated by S. pyogenes strain through TLR4 activation in MyD88–DCs, the expression of costimulatory molecules CD40, CD80, CD86 and the production of inflammatory cytokines were decreased in them [41, 42]. Activation of receptors and CD54, CD70, CD83, CCR7 molecules triggers p38MAPK, ERK, JNK, PI3K/Akt/NF-κB signaling cascades in DCs that enhance their proliferation and differentiation [43, 44]. Recently, X. Chen et al. found that TLR4 also stimulates the cGAS-STING/NF-κB signaling pathway, which mediates DC maturation and IL-6 secretion [45]. These results suggest that S. pyogenes through stimulation of monocytes, macrophages, may exhibit their anticancer effects.
Furthermore, S. pyogenes stimulate a subpopulation of interferon-producing DCs and natural killers. Activation of these cells enhances processing, tumor antigen presentation, TNF-α-induced TRAIL- and Fas-ligand-dependent apoptosis [46]. In turn, plasmacytoid myeloid DCs activate tumor-specific cytotoxic CD8+-, CD4+-T-lymphocytes and their secretion of cytokines [40, 47]. According to the study of W. Li et al. factors involved in DC activation in hepatocellular carcinoma are presented in Fig. 15.
Fig. 15. Mechanisms of streptococcal activation of DCs targeted at inhibiting of hepatocellular carcinoma growth [47].
The key pathogenicity factor, M-protein or acidic streptococcal glycoprotein (SAGP) binds to an IAP-specific coupled receptor, which activates proteintyrosine phosphatase dephosphorylates epidermal growth factor and blocks the p42/44MAPK-signaling cascade, thereby inhibiting proliferation of A431 epidermoid carcinoma cells [48]. S. pyogenes secret exotoxins SpeB, SpeA, which have cysteine protease activity and inactivate inflammatory factors IL-8, C5a, cathelicidin LL-37, and inhibit neutrophil chemotaxis [26, 47, 49, 50]. DNAases SpnA and SdaI of S. pyogenes suppress the formation of neutrophil extracellular traps, SpeB suppresses the activity of antimicrobial chemokines (Fig. 16). Furthermore, SpeB promote the formation of gazdermin D, which forms pores, induces pyroptosis, inhibits IL-1β and H-kininogen, which exert potent proinflammatory effects [47]. Consequently, secretion of streptococcal exotoxins can inhibit tumor growth and development.
Fig. 16. Molecular and cellular mechanisms of S. pyogenes pathogenesis [26].
Not only live streptococci have cytotoxic activity, but also the preparation OK-432 (picibanil, NSC-B116209; Fig. 16) obtained on their basis, the administration of which (0.001–1.0 μg/mL) caused CD56+NK activation. This resulted in growth inhibition by 7–14 days of MADB106 mammary carcinoma in an F344 rats` intraperitoneal model [51]. OK-432 administration promoted the activation of T-helper lymphocytes and leukocytes by increasing the number of neutrophils [26]. In the review by Y. Ryoma et al. summarize the mechanisms of anticancer activity of OK-432, showing that picibacil induces direct anticancer action by inhibiting RNA synthesis and cell cycle arrest. Also OK-432 stimulates the synthesis of TNF-α, perforin and interferon-γ, which activate the expression of intercellular adhesion molecules (ICAM-1), HLA-DR and eventually apoptosis of cancer cells [52]. OK-432 stimulates the production of IL-8, macrophage factors (G-CSF, GM-CSF) that enhance proliferation and maturation of granulocytes, monocytes, lymphocytes and platelets [52]. Recent results convincingly demonstrate that collagen-like protein-1 (Scl1) of S. pyogenes inhibits the formation of neutrophil extracellular traps in a rat model with Panc02 pancreatic ductal adenocarcinoma [53].
In this regard, it is interesting to note that streptococci can infect the tumor, forming biofilms on it. Streptococcal infection activates the body’s immune system, which increases the number of neutrophils and lymphocytes in the focus of infection [14, 54]. Thus, bacterial components can enhance the interaction between the tumor and the immune system, acting as adjuvants, promoting the presentation of tumor antigens and activation of the immune system [55].
Preclinical and clinical studies
The results of in vitro and in vivo experiments with S. pyogenes, which established their anticancer effects and mechanisms, stimulated further preclinical and clinical trials with these microorganisms. For example, T. Iwai et al. recently conducted a preclinical study to evaluate the efficacy of intratumoral administration of OK-432 or its combination with radiofrequency ablation on 4–5 weeks old male Sprague-Dawley rats (n = 145; 80–90 g) with metastatic osteosarcoma [55]. The animals were divided into 4 groups to evaluate overall survival and tumor size: control (no treatment), radiofrequency ablation, OK-432, and RFA + OK-432. The median survival of rats in the control group was 28.4 days (11–51 days) and 38.4 days (10–51 days) in OK-432 groups, 40.0 days (12–51 days; p = 0.14) in RFA and 47.3 days (15–51 days; p = 0.084) in RFA + OK-432 groups (p = 0.046) [56].
Japanese scientists M.S. Oba et al. conducted a meta-analysis including 14 studies involving 796 patients with stage III or IV gastric cancer after surgical resection to evaluate the efficacy of OK-432 immunochemotherapy compared to standard chemotherapy. The primary endpoint was overall survival [57]. The control and OK-432 groups included 726 and 796 patients, respectively. The median overall survival was 42.6 months for the OK-432 group and 32.3 months for the control group. The overall hazard ratio was 0.88 (95% confidence interval 0.77-1.00; p = 0.050; Fig. 17) [57]. The authors conclude that immunochemotherapy with OK-432 may be effective for patients with gastric cancer in stages III or IV after surgery [57].
Fig. 17. Overall survival of gastric cancer patients who received OK-432 immunotherapy [57].
In an earlier study, the same authors, summarizing 6 randomized trials involving 1522 patients with gastric cancer, showed that systemic administration of OK-432 had a significant effect on increasing life expectancy (OR = 0.81; 95% CI = 0.65–0.99; p = 0.044) and 3-year survival. This rate was 67.5 and 62.6% in the immunochemotherapy and chemotherapy groups, respectively [58].
It should be noted that mild adverse reactions such as: fatigue, anorexia, local inflammation, hyperemia, chest and abdominal pain were observed during clinical use of OK-432. The most frequent symptom was fever controlled with antipyretic therapy. Intracranial pressure was rarely reported. Only a few patients experienced fatal outcomes due to the development of embolism, acute nephritis, hemorrhage, and improperly dosed injections of the drug [59]. Serious side effects require identification of their causes and relationship to the current therapy, perhaps they were due to incorrect dosage or development of individual intolerance to the drug.
Mechanisms of cancer-stimulating effects of S. pyogenes
In the process of studying the anticancer effect of S. pyogenes, data of the opposite nature were gradually accumulated. For example, D. Kong et al. studied the effects of OK-432 (sapilin) in patients with breast cancer and found increased secretion of cytokines IL-1a, IL-6, fibroblast growth factor-β, vascular endothelial growth factor and transforming growth factor-β1 in drainage fluids. Furthermore, its administration enhanced proliferation, migration and angiogenesis of HUVEC, HFL1 epithelial cells, fibroblasts and collagen deposition [60]. These results indicate that S. pyogenes through activation of proliferation, migration and angiogenesis of epithelial cells and fibroblasts can stimulate the development of breast cancer. Exotoxins SpnA, SpeB and serine protease SpyCEP of S. pyogenes take part in the degradation of IL-8, IL-1β, complement factor C5a, which inhibit the migration (chemotaxis) of neutrophils (Fig. 18). In turn, SpeB induces IgG proteolysis, degradation of antimicrobial peptides, chemokines, which promotes the formation of neutrophil extracellular traps and cancer development [61].
Fig. 18. Total scheme of the S. pyogenes effects to immune cells [61].
NADase S5nA inhibits phagocytic activity of macrophages against tumor cells, inhibits IgG proteolysis and antibody-mediated killing of cancer cells by neutrophils (opsonophagocytosis). The peptidases EndoS/S2, IdeS/Mac-1 and Mac-2, SpeB are involved in the hydrolysis of glycans and ubiquitin-binding proteins that inhibit apoptosis, and M-protein (SAGP) suppresses T-lymphocyte proliferation [61]. All these events indirectly stimulate the cell cycle, proliferation, migration of tumor cells observed predominantly in chronic streptococcal infection in cancer patients.
Conclusions
Contrary to established views, streptococcal infection or fever may not always be undesirable in cancer patients and does not always lead to a decrease in immune system reserves in the cancer patient. The research evidence presented in that review strongly suggests that S. pyogenes can, with the involvement of pathogenicity factors, directly exert anticancer effects on cancer cells. Furthermore, these bacteria stimulate interleukins, chemokines of the host immune system cells (neutrophils, T-, B-lymphocytes, macrophages, dendritic cells), which, in turn, participate in anticancer immune responses.
Modern oncological therapeutic methods (chemotherapy, radiation therapy) have a number of disadvantages associated with non-selectivity of action on cancer cells, as well as, due to cellular and molecular-genetic heterogeneity of tumors, the development of therapeutic resistance, which leads to the inability to monitor the effectiveness of treatment in dynamics. All these problems cause high oncological morbidity and mortality, progression of oncological diseases with the emergence of more resistant forms of tumors with a high rate of metastasis.
Thus, it is necessary to improve cancer therapy regimens using new approaches that include selective anticancer activity combined with minimal toxicity to normal tissues of the body, as well as the ability to overcome the constant dynamic molecular and genetic changes in neoplastic cells. The use of S. pyogenes as a therapeutic strategy in cancer patients meets all of these criteria. However, it should be noted that streptococcal vaccine should be used with caution, taking into account the individual characteristics of the patient’s immune system, because S. pyogenes may have opposite effects that require further research.
1 International Agency for Research of Cancer (Globocan). URL: https://globocan.iarc.fr
About the authors
Alexander N. Suvorov
Institute of Experimental Medicine; Saint Petersburg State University
Email: alexander_suvorov1@hotmail.com
ORCID iD: 0000-0003-2312-5589
Dr. Sci. (Med.), Professor, Corresponding Member of the RАS, Head, A.A. Totolyan microbiology department, Head, Department of fundamental problems of medicine and medical technologies
Russian Federation, Saint Petersburg; Saint PetersburgAnna N. Tsapieva
Institute of Experimental Medicine
Email: anna.tsapieva@gmail.com
ORCID iD: 0000-0001-7878-6339
Cand. Sci. (Biol.), senior researcher, Laboratory of biomedical microecology, A.A. Totolyan microbiology department
Russian Federation, Saint PetersburgAlexander N. Chernov
Institute of Experimental Medicine; Saint Petersburg State Pediatric Medical University
Author for correspondence.
Email: al.chernov@mail.ru
ORCID iD: 0000-0003-2464-7370
Cand. Sci. (Biol.), senior researcher, Department of general pathology and pathological physiology, A.A. Totolyan microbiology department, Assistant, Department of Biological Chemistry
Russian Federation, Saint Petersburg; Saint PetersburgReferences
- Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015 A Systematic analysis for the Global burden of disease study. Global Burden of Disease Cancer Collaboration. JAMA Oncol. 2017;3(4):524–48. DOI: https://doi.org/10.1001/jamaoncol.2016.5688
- Lee Y.T., Tan Y.J., Oon C.E. Molecular targeted therapy: treating cancer with specificity. Eur. J. Pharmacol. 2018;834:188–96. DOI: https://doi.org/10.1016/j.ejphar.2018.07.034
- Kennedy L.B., Salama A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020;70(2):86–104. DOI: https://doi.org/10.3322/caac.21596
- Zhang S., Xiao X., Yi Y., et al. Tumor initiation and early tumorigenesis: molecular mechanisms and interventional targets. Sig. Transduct. Target Ther. 2024;9(1):149. DOI: https://doi.org/10.1038/s41392-024-01848-7
- Wang Q., Shao X., Zhang Y., et al. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023;12(10):11149–65. DOI: https://doi.org/10.1002/cam4.5698
- Duong M.TQ., Qin Y., You SH., et al. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp. Mol. Med. 2019; 51(12):1–15. DOI: https://doi.org/10.1038/s12276-019-0297-0
- Fan J.Y., Huang Y, Li Y., et al. Bacteria in cancer therapy: a new generation of weapons. Cancer Med. 2022;11(23):4457–68. DOI: https://doi.org/10.1002/cam4.4799
- Clairmont C., Lee K.C., Pike J., et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J. Infect. Dis. 2000;181(6):1996–2002. DOI: https://doi.org/10.1086/315497
- Luo X., Li Z., Lin S., et al. Antitumor effect of VNP20009, an attenuated Salmonella, in murine tumor models. Oncol. Res. 2001;12(11-12):501–8. DOI: https://doi.org/10.3727/096504001108747512
- Allemailem K.S. Innovative approaches of engineering tumor-targeting bacteria with different therapeutic payloads to fight cancer: a smart strategy of disease management. Int. J. Nanomedicine. 2021;16:8159–84. DOI: https://doi.org/10.2147/IJN.S338272
- Alrumman S.A., Mostafa Y.S., Al-Izran K.A., et al. Production and anticancer activity of an L-asparaginase from Bacillus licheniformis isolated from the Red Sea, Saudi Arabia. Sci. Rep. 2019; 9(1):3756. DOI: https://doi.org/10.1038/s41598-019-40512-x
- Pokrovsky V.S., Anisimova N.Yu., Davydov D.Zh., et al. Methionine gamma lyase from Clostridium sporogenes increases the anticancer effect of doxorubicin in A549 cells and human cancer xenografts. Invest. New Drugs. 2019;37(2):201–9. DOI: https://doi.org/10.1007/s10637-018-0619-4
- Toley B.J., Forbes N.S. Motility is critical for effective distribution and accumulation of bacteria in tumor tissue. Integr. Biol. (Camb.). 2011;4(2):165–76. DOI: https://doi.org/10.1039/c2ib00091a
- Yarahmadi A., Zare M., Aghayari M., et al. Therapeutic bacteria and viruses to combat cancer: double-edged sword in cancer therapy: new insights for future. Cell Commun. Signal. 2024;22(1):239. DOI: https://doi.org/10.1186/s12964-024-01622-w
- Coley W. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am. J. Med. Sci. 1893;105(6):487–511.
- Ferretti J.J., McScan W.M., Ajdic D., et al. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA. 2001;98(8):4658–63. DOI: https://doi.org/10.1073/pnas.071559398
- Nakata M., Kreikemeyer B. Genetics, structure, and function of group A streptococcal pili. Front. Microbiol. 2021;12:616508. DOI: https://doi.org/10.3389/fmicb.2021.616508
- Ward I.B. Teichoic and teichuronic acids: biosynthesis, assembly and location. Microb. Rev. 1981;45(2):211–43. DOI: https://doi.org/10.1128/mr.45.2.211-243.1981
- Бурова Л.А., Тотолян А.А. Основные факторы патогенности Streptococcus pyogenes. Инфекция и иммунитет. 2022;12(1):33–50. Burova L.A., Totolian A.A. Major pathogenicity factors of Streptococcus pyogenes. Russian Journal of Infection and Immunity. 2022;12(1):33–50. DOI: https://doi.org/10.15789/2220-7619-MPF-1723, EDN: https://elibrary.ru/lwwogs
- Lancefield R.C. A serological differentiation of human and other groups of hemolytic streptococci. J. Exp. Med. 1933;57(4):571–95. DOI: https://doi.org/10.1084/jem.57.4.571
- Ferretti J.J., Stevens D.L. Fischetti V.A., eds. M protein and other surface proteins on Streptococci. In: Streptococcus pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City;2016.
- Chernov A.N., Tsapieva A.N., Alaverdian D.A., et al. In vitro evaluation of the cytotoxic effect of Streptococcus pyogenes strains, protegrin PG-1, cathelicidin LL-37, nerve growth factor and chemotherapy on the C6 glioma cell line. Molecules. 2022;27(2):569. DOI: https://doi.org/10.3390/molecules27020569
- Tsapieva A.N., Chernov A.N., Duplik N.V., et al. Studying the oncolytic activity of Streptococcus pyogenes strains against hepatoma, glioma, and pancreatic cancer in vitro and in vivo. Microorganisms. 2025;13(1):76. DOI: https://doi.org/10.3390/microorganisms13010076
- Frost H.R., Guglielmini J., Duchêne S., et al. Promiscuous evolution of Group A Streptococcal M and M-like proteins. Microbiology (Reading). 2023;169(1):001280. DOI: https://doi.org/10.1099/mic.0.001280
- McMillan D.J., Drèze P.A., Vu T., et al. Updated model of Group A Streptococcus M proteins based on a comprehensive worldwide study. Clin. Microbiol. Infect. 2013;19(5):E222–9. DOI: https://doi.org/10.1111/1469-0691.12134
- Brouwer S., Rivera-Hernandez T., Curren B.F., et al. Pathogenesis, epidemiology and control of Group A Streptococcus infection. Nat. Rev. Microbiol. 2023;21(7):431–47. DOI: https://doi.org/10.1038/s41579-023-00865-7
- Zhang Y., Zeng J., Bao S., et al. Cancer progression and tumor hypercoagulability: a platelet perspective. J. Thromb. Thrombolysis. 2024;57(6):959–72. DOI: https://doi.org/10.1007/s11239-024-02993-0
- Henningham A., Ericsson D.J., Langer K., et al. Structure-informed design of an enzymatically inactive vaccine component for Group A Streptococcus. mBio. 2013;4(4):e00509-13. DOI: https://doi.org/10.1128/mBio.00509-13
- Yoshida J., Takamura S., Suzuki S. Cell growth inhibitory action of SAGP, an antitumor glycoprotein from Streptococcus pyogenes (Su strain). Jpn. J. Pharmacol. 1987;5(2):143–7. DOI: https://doi.org/10.1254/jjp.45.143
- Старикова Э.А., Соколов А.В., Бурова Л.А. и др. Роль аргининдеиминазы пиогенного стрептококка в подавлении синтеза монооксида азота (NO) макрофагами. Инфекция и иммунитет. 2018;8(2):211–8. Starikova E.A., Sokolov A.V., Burova L.A., et al. The role of arginine deiminase from Streptococcus pyogenes in inhibition macrophages nitrogen monoxide (NO) synthesis. Russian Journal of Infection and Immunity. 2018;8(2):211–8. EDN: https://elibrary.ru/rufnrr, DOI: https://doi.org/10.15789/2220-7619-2018-2-211-218
- Albaugh V.L., Pinzon-Guzman C., Barbul A. Arginine metabolism and cancer. J. Surg. Oncol. 2016;115(3):273–80. DOI: https://doi.org/10.1002/jso.24490
- Jewell J.L., Guan K.L. Nutrient signaling to mTOR and cell growth. Trends Biochem. Sci. 2013;38(5):233–42. DOI: https://doi.org/10.1016/j.tibs.2013.01.004
- Fiedler T., Strauss M., Hering S., et al. Arginine deprivation by arginine deiminase of Streptococcus pyogenes controls primary glioblastoma growth in vitro and in vivo. Cancer Biol. Ther. 2015;16(7):1047–55. DOI: https://doi.org/10.1080/15384047.2015.1026478
- Maletzki C., Rosche Y., Riess C., et al. Deciphering molecular mechanisms of arginine deiminase-based therapy — comparative response analysis in paired human primary and recurrent glioblastomas. Chem. Biol. Interact. 2017;278:179–88. DOI: https://doi.org/10.1016/j.cbi.2017.10.007
- Feil S.C., Ascher D.B., Kuiper M.J., et al. Structural studies of Streptococcus pyogenes streptolysin O provide insights into the early steps of membrane penetration. J. Mol. Biol. 2013;426(4):785–92. DOI: https://doi.org/10.1016/j.jmb.2013.11.020
- Gruber C., Gratz I.K., Murauer E.M., et al. Spliceosome-mediated RNA trans-splicing facilitates targeted delivery of suicide genes to cancer cells. Mol. Cancer Ther. 2011;10(2):233–41. DOI: https://doi.org/10.1158/1535-7163.MCT-10-0669
- Paiva A.D., Irving N., Breukink E., Mantovani H.C. Interaction with lipid II induces conformational changes in bovicin HC5 structure. Antimicrob. Agents Chemother. 2012;56(9):4586–93. DOI: https://doi.org/10.1128/AAC.00295-12
- Rodrigues G., Silva G.G.O., Buccini D.F., et al. Bacterial proteinaceous compounds with multiple activities toward cancers and microbial infection. Front. Microbiol. 2019;10:1690. DOI: https://doi.org/10.3389/fmicb.2019.01690
- Liu Y.H., Wu P.H., Kang C.C., et al. Group A Streptococcus subcutaneous infection-induced central nervous system inflammation is attenuated by blocking peripheral TNF. Front. Microbiol. 2019;10:265. DOI: https://doi.org/10.3389/fmicb.2019.00265
- Veckman V., Julkunen I. Streptococcus pyogenes activates human plasmacytoid and myeloid dendritic cells. J. Leukoc. Biol. 2008;83(2):296–304. DOI: https://doi.org/10.1189/jlb.0707457
- Loof T.G., Goldmann O., Medina E. Immune recognition of Streptococcus pyogenes by dendritic cells. Infect. Immun. 2008; 76(6):2785–92. DOI: https://doi.org/10.1128/IAI.01680-07.
- Apetoh L., Ghiringhelli F., Tesniere A., et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13(9):1050–9. DOI: https://doi.org/10.1038/nm1622
- Chen L., Zhou S., Qin J., et al. Combination of SLC administration and Tregs depletion is an attractive strategy for targeting hepatocellular carcinoma. Mol. Cancer. 2013;12(1):153. DOI: https://doi.org/10.1186/1476-4598-12-153
- Yang M., Zhang Z., Chen J., et al. Soluble fibrinogen-like protein 2 promotes the growth of hepatocellular carcinoma via attenuating dendritic cell-mediated cytotoxic T cell activity. J. Exp. Clin. Cancer Res. 2019;38(1):351. DOI: https://doi.org/10.1186/s13046-019-1326-5
- Chen X., Tang Q., Wang J., et al. A DNA/DMXAA/metal-organic framework activator of innate immunity for boosting anticancer immunity. Adv. Mater. 2023;35(15):e2210440. DOI: https://doi.org/10.1002/adma.202210440
- Koya T., Yanagisawa R., Higuchi Y., et al. Interferon-α-inducible dendritic cells matured with OK-432 exhibit TRAIL and Fas ligand pathway-mediated killer activity. Sci. Reports. 2017;7:42145. DOI: https://doi.org/10.1038/srep42145
- Li W., Chen G., Peng H., et al. Research progress on dendritic cells in hepatocellular carcinoma immune microenvironments. Biomolecules. 2024;14(9):1161. DOI: https://doi.org/10.3390/biom14091161
- Yoshida J., Ishibashi T., Nishio M. Growth-inhibitory effect of a streptococcal antitumor glycoprotein on human epidermoid carcinoma A431 cells: involvement of dephosphorylation of epidermal growth factor receptor. Cancer Res. 2001;61(16):6151–7.
- Hytönen J., Haataja S., Gerlach D., et al. The SpeB virulence factor of Streptococcus pyogenes, a multifunctional secreted and cell surface molecule with strepadhesin, laminin-binding and cysteine protease activity. Mol. Microbiol. 2001;39(2):512–9. DOI: https://doi.org/10.1046/j.1365-2958.2001.02269.x
- Singh N., Baby D., Rajguru J.P., et al. Inflammation and cancer. Ann. Afr. Med. 2019;18(3):121–6. DOI: https://doi.org/10.4103/aam.aam_56_18
- Fukui H., Reynolds C.W. Antitumor activity of a Streptococcus pyogenes preparation (OK-432). II. Analysis of the cytotoxic lymphocytes induced by OK-432 injection into tumor-bearing F344 rats. J. Natl. Cancer Inst. 1987;79(5):1019–24.
- Ryoma Y., Moriya Y., Okamoto M. Biological effect of OK-432 (picibanil) and possible application to dendritic cell therapy. Anticancer Res. 2004;24(5C):3295–301.
- Henderson E.A., Ivey A., Choi S.J., et al. Group A streptococcal collagen-like protein 1 restricts tumor growth in murine pancreatic adenocarcinoma and inhibits cancer-promoting neutrophil extracellular traps. Front. Immunol. 2024;15:1363962. DOI: https://doi.org/10.3389/fimmu.2024.1363962
- Marks L.R., Mashburn-Warren L., Federle M.J., et al. Streptococcus pyogenes biofilm growth in vitro and in vivo and its role in colonization, virulence, and genetic exchange. J. Infect. Dis. 2014;210(1):25–34. DOI: https://doi.org/10.1093/infdis/jiu058
- Howell L.M., Forbes N.S. Bacteria-based immune therapies for cancer treatment. Semin. Cancer Biol. 2022;86(Pt. 2):1163–78. DOI: https://doi.org/10.1016/j.semcancer.2021.09.006
- Iwai T., Oebisu N., Hoshi M., et al. Promising abscopal effect of combination therapy with thermal tumour ablation and intratumoural OK-432 injection in the rat osteosarcoma model. Sci. Rep. 2020;10(1):9679. DOI: https://doi.org/10.1038/s41598-020-66934-6
- Oba M.S., Teramukai S., Ohashi Y., et al. The efficacy of adjuvant immunochemotherapy with OK-432 after curative resection of gastric cancer: an individual patient data meta-analysis of randomized controlled trials. Gastric Cancer. 2016;19(2):616–24. DOI: https://doi.org/10.1007/s10120-015-0489-9, DOI: https://doi.org/10.1097/00002371-200209000-00004
- Rebuffini E., Zuccarino L., Grecchi E., et al. Picibanil (OK-432) in the treatment of head and neck lymphangiomas in children. Dent. Res. J. (Isfahan). 2012;9(Suppl. 2):S192–6. DOI: https://doi.org/10.4103/1735-3327.109752
- Kong D., Zhang D., Cui Q., et al. Sapylin (OK-432) alters inflammation and angiogenesis in vivo and vitro. Biomed. Pharmacother. 2019;113:108706. DOI: https://doi.org/10.1016/j.biopha.2019.108706
- Happonen L., Collin M. Immunomodulating enzymes from Streptococcus pyogenes — in pathogenesis, as biotechnological tools, and as biological drugs. Microorganisms. 2024;12:200. DOI: https://doi.org/10.3390/microorganisms12010200
Supplementary files