Dynamics of enzymatic activity in primary culture of Syrian hamster adherent leukocytes ex vivo infected with SARS-CoV-2
- Authors: Abramova S.A.1, Lyapun I.N.1, Drobot E.I.1, Krylova N.V.1,2, Iunikhina O.V.1,2, Lubova V.A.1, Merlov E.K.1, Belov I.A.1,2, Somova L.M.1, Shchelkanov M.Y.1,2
-
Affiliations:
- Somov Institute of Epidemiology and Microbiology
- Far Eastern Federal University
- Issue: Vol 102, No 2 (2025)
- Pages: 168-178
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18825
- DOI: https://doi.org/10.36233/0372-9311-649
- EDN: https://elibrary.ru/KTYMCV
- ID: 18825
Cite item
Abstract
Introduction. The continued epidemic relevance of SARS-CoV-2, even after the end of the associated COVID-19 pandemic in 2023, necessitates further study of the interaction of this virus with the first line of cellular defense, neutrophils.
The aim of the study was to determine the enzymatic activity of peripheral blood leukocytes of Syrian hamsters (Mesocricetus auratus) in the dynamics of ex vivo SARS-CoV-2 infection, which characterizes the microbicidal potential of innate immunity cells.
Materials and methods. The SARS-CoV-2/Vladivostok/R-8726/2021 strain was used at infectious doses of 3 lg (TCID50/mL) and 2 lg (TCID50/mL) (TCID50 is the 50% tissue cytopathic dose for the Vero E6 cell line); the contact time of the infecting virus-containing liquid with the cell culture was 1 h. The number of viable cells in the adherent leukocyte culture was counted using an inverted microscope equipped with a digital camera and MCView program. The specific (per 1 viable cell) activities of adenosine triphosphatase (ATPase), 5′-nucleotidase (adenosine monophosphatase, AMPase), lactate dehydrogenase (LDH), succinate dehydrogenase (SDH), myeloperoxidase (MPO) and cytochrome oxidase (CCO) were determined spectrophotometrically after incubation with specific substrates of infected and uninfected cell cultures 1, 16, 24, 48 h after virus inoculation.
Results. The enzymatic activity of leukocytes 1 h after virus inoculation, compared to uninfected leukocytes, was as follows: specific activity of ATPase, MPO was decreased, activity of AMPase, LDH, SDH was increased; 16 h after virus inoculation, activity of MPO was decreased, activity of AMPase, LDH, SDH was increased, activity of ATPase and CCO was at the initial level, i.e. approximately at the level of the uninfected control; 24 h after virus inoculation, AMPase activity was decreased, ATPase activity was increased, LDH, SDH, MPO, CCO activity was at the initial level; 48 h after virus inoculation, ATPase, LDH, SDH, MPO, CCO activity was increased, AMPase activity was at the initial level. Changes in enzymatic activity depend on the infecting dose and correlate with virus accumulation in the culture medium.
Conclusion. The revealed dynamics of enzymatic activity in the primary culture of adherent leukocytes ex vivo infected with SARS-CoV-2 indicates a decrease in the microbicidal potential of cells of innate immunity in the course of this infection.
Full Text
Introduction
Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) (Nidovirales: Coronaviridae, Betacoronavirus, subgenus Sarbecovirus) is the etiologic agent of the 2019 coronavirus disease (COVID-19), which pandemic (2020–2023) was the longest and one of the deadliest among acute respiratory diseases in recent human history [1]. After the end of the pandemic period, SARS-CoV-2 did not disappear from the human population, but became one of the components in the structure of the seasonal rise in the incidence of acute respiratory diseases [2]. For this reason, the study of the pathogenesis of SARS-CoV-2 infection does not lose its relevance, as a number of aspects of this process are not fully understood. In this regard, the process of virus interaction with peripheral blood cells, especially with cells of innate immunity (neutrophils and monocytes) is of particular interest.
The available literature contains theoretical speculations about the possible ability of SARS-CoV-2 virus to infect neutrophils. Thus, N. Rong et al. reported that the CD147 receptor, an alternative to the ACE2 receptor, which determines virus tropism, is expressed in neutrophils of healthy donors and activated in COVID-19 patients [3]. Another non-canonical receptor is the C-type lectin receptor, which mediates neutrophil extracellular trap formation in COVID-19 [4]. Based on this, it can be inferred that the virus is able to directly affect blood leukocytes.
The previously described morphological changes in peripheral blood leukocytes also indicate their significant involvement in the process of SARS-CoV-2 infection [5–8]. However, the scientific literature lacks detailed information on the nature and dynamics of enzymatic activity of leukocytes during infection with this virus. There are some reports about changes in the activity of myeloperoxidase (MPO) and lactate dehydrogenase (LDH) in the serum of patients diagnosed with COVID-19, and the severity of these changes correlates with the severity of the underlying disease [9–11]. For this reason, it is necessary to study not only morphological, but also morpho-functional changes in complex in order to judge the metabolic processes of innate immunity cells under the influence of SARS-CoV-2.
The aim of this study is to determine the enzymatic activity of peripheral blood leukocytes of Syrian hamsters in the dynamics of ex vivo SARS-CoV-2 infection, characterizing the microbicidal potential of innate immunity cells.
Materials and methods
Primary adhesive culture of leukocytes of the Syrian hamster (Mesocricetus auratus) was obtained from the blood of 15 individuals aged 4 months and weighing about 100 g. All procedures with animals were performed strictly in accordance with the requirements of the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes of 18.03.1986. The study protocol was approved by the Ethical Committee of the Somov Research Institute of Epidemiology and Microbiology of Rospotrebnadzor (protocol No. 2 of 16.05.2024).
Blood was collected from the heart into glass tubes with heparin added to each tube at a rate of 5 U/mL. The tubes were placed in a thermostat at 45° and 37°C for 1 h, after which the top layer of plasma was carefully removed, and the leukocyte film was collected, adjusted to a concentration of 2 × 106 cells/mL with nutrient medium 199 (BioloT), and dispensed 100 μL into the wells of a flat-bottom 96-well plate (TFS), which was placed in a thermostat (5% CO2, 37°C) for 40 min; then the medium with non-adherent cells was removed and the wells were washed three times with 150 µL of medium 199.
The number of live adherent cells in the well was determined using a MIB-R inverted microscope (LOMO) equipped with a MC-8.3 C digital camera (LOMO). Using the MCView program (LOMO-Microsystems), the area of the field of view, not including the edge of the well, was set equal to 0.26 mm2, the number (n) of living (attached with an integral outer membrane) cells was counted in it; the cells crossing the outer boundary were counted on the left/upper edges of the square of the field of view and not counted on the right/bottom edges. Since the total area of the well is 35 mm2, the total number of cells in the well (N) was estimated by the formula:
. (1)
The final estimation of the number of live cells in each well was performed by 10 randomly selected fields of view.
Ex vivo infection of the primary culture of adherent leukocytes of Syrian hamster was performed by adding 100 µL of medium 199 with working dilution of supernatant of Vero E6 cell culture supernatant into wells with cell monolayer, infected with SARS-CoV-2 (in control samples — without virus-containing supernatant), followed by incubation for 1 h at 37°C, after which the wells were washed three times and filled with culture medium containing medium 199 with 15% fetal bovine serum (FBS) and 0.004% gentamicin K (BioloT). Two infectious doses were used: 3 lg (TCID50/mL) and 2 lg (TCID50/mL), where TCID50 is the 50% tissue cytopathic dose for the African green monkey (Chlorocebus sabaeus, ♀) kidney cell line (Vero E6).
The SARS-CoV-2/Vladivostok/R-8726/2021 strain was obtained from the Collection of Pathogenic Microorganisms of the Somov Research Institute of Epidemiology and Microbiology. This strain belongs to genotype Delta (AY.121) (VGARus ID: prim000041; GenBank ID: OQ318430; GISAID ID: EPI_ISL_16643370) and was isolated from the nasopharyngeal wash of a COVID-19 patient in December 2021 on the Vero E6 cell line model [2].
SARS-CoV-2 RNA was detected by reverse transcription followed by real-time polymerase chain reaction (RT-qPCR) using the RT-qPCR-SARS-CoV-2 reagent kit (Syntol). RNA was isolated using the M-Sorb-NK reagent kit (Syntol). All manipulations were performed according to the manufacturer’s protocols. The threshold cycle (threshold cycle, CT) of RT-qPCR was considered as a semi-quantitative characteristic of the content of viral particles in the medium: the higher their content, the lower the CT. The absence of virus corresponded to CT ≥ 36.
ATPase and AMPase activity was determined after double washing of adherent leukocytes with growth medium without FCS by adding 50 μL of substrate for ATPase (8 mg/mL adenosine-5′-triphosphate in 10-fold diluted Tris-HCl-buffer, pH 7.8, containing 87 mg NaCl, 28.7 mg KCl, 5.2 mg MgCl2 × 6 H2O) and for AMPase (4 mg/mL adenosine-5′-monophosphate in the same buffer solution containing 87 mg NaCl and 70 mg MgCl2). The samples were left at 37°C for 30 and 60 min, respectively. The reaction was stopped by adding 50 μL of a 1 : 1 mixture of ascorbic acid and molybdic acid. After 20 min, the absorbance of the solutions was measured1 at a wavelength of 620 nm [12].
Myeloperoxidase (MPO) and cytochrome oxidase (CCO) activity was determined after double washing of adherent leukocytes with growth medium without FCS by adding 100 μL of orthophenylenediamine solution (Merck), 0,4 mg/mL for MPO or 3,3′-diaminobenzidine (Merck), 2 mg/mL for CCO in phosphate-citrate buffer pH 5.0 with 0.033% H2O2 and incubated for 10 min at room temperature. The reaction was stopped by adding 100 μL of 10% sulfuric acid solution. Optical density was measured at a wavelength of 492 nm [12].
Viral load in the dynamics of SARS-CoV-2 infection was determined by semi-quantitative method based on the change of threshold cycle in RT-qPCR (CT (0)), after 1 h (CT (1)) — in virus-containing fluid after contact with cells; 16 h (CT (16)), 24 h (CT (24)) and 48 h (CT (48)) — in growth medium of infected cells.
The enzymatic activity of cells under the influence of SARS-CoV-2 infection was determined 1, 16, 24, 48 h after virus inoculation by comparing the ratios of specific (per 1 cell) enzymatic activities of infected and uninfected cells: for each time point t, the coefficient of change in specific enzymatic activity γ(t) was calculated according to the formula:
, (2)
where the optical density and the number of living cells for uninfected (D(t) and z(t)) and infected (D~(t) and z~(t)) samples, respectively, are taken into account [13, 14]. Of course, there is an a priori equation of:
(3)
Statistical processing of the results was based on the fact that at each time point t for each of the 6 enzymes, measurements were performed in 3 wells with uninfected cells and in 3 wells with infected cells. For this purpose, the maintenance medium was removed at the beginning in order to introduce media with appropriate substrates after washing. The enzymes are numbered in any order using the index f = 1, 2, ...6. Then at each time instant t there are 6 × 3 = 18 samples of virus-containing fluid: CTjf(t), j = 1, 2, 3. Thus CTjf (1) represented the viral load in the samples as a result of virus accumulation in de novo medium: after the initial virus-containing fluid CTjf (0) had been in contact with cells for 1 h; CTjf (16), CTjf (24), and CTjf (24). Thus, for t = 1, 16, 24, 48 h, the sample mean <CT(t)> and the standard deviation of the sample mean mCT are determined using standard formulas as modified as follows:
; (4)
. (5)
The initial sample was a single copy and its viral load was characterized by a single CT(0) value.
After the chemical reactions catalyzed by the studied enzymes, the optical density was measured in 3 wells with uninfected cells (Di(t), i = 1, 2, 3) and in 3 wells with infected cells (D~j(t), j = 1, 2, 3). Prior to this, the number of live cells was measured in each well: zi(t) (i = 1, 2, 3) and z~j(t) (j = 1, 2, 3). Each value of zi(t) and z~j(t) was evaluated by 10 fields of view according to (1)2: zik(t), k = 1, 2, ...10 and z~hk(t), h = 1, 2, ...10. Thus all measurements Di(t), zik(t), D~j(t), z~jh(t) are independent and equal at any values of the coefficients. There are 30 values of the fraction D~j(t)/z~jh(t), 30 values of the fraction zik(t)/Di(t) and 900 variants of their products of the form (2), i.e., the sample consists of 900 values of γ(t). Therefore, the sample mean <γ(t)> and the standard deviation of the sample mean mγ(t) were calculated using standard formulas modified for this case:
(6)
(7)
The significance of differences between samples of 900 values for the time values, γ(t1) and γ(t2), and between samples of 18 values for the time values CT (t1) and CT (t2), where t1 = 1, 16, 24, 48 h, t2 = 1, 16, 24, 48 h, t1 ≠ t2, was assessed using the Mann–Whitney–Wilcoxon test. This nonparametric test does not require a priori assumptions about the distribution function of random variables, the realization of which are the values of γ(t) and CT (t). The assessment was considered reliable when the probability of realization of the alternative hypothesis was p ≤ 0.05.
Results
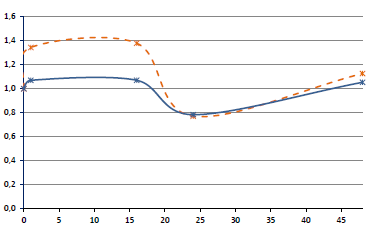
The content of SARS-CoV-2 in the growth medium of adherent leukocyte culture is shown in Fig. 1 (hereinafter, it should be kept in mind that a higher CT value corresponds to a lower virus content in the test sample). During the 1st hour after virus inoculation, when the virus-containing fluid was in contact with the cells, the cells were infected. After removal of the virus-containing fluid, new particles in the medium accumulated as a result of virus replication in the infected cells. Taking into account the fact that these are different stages of the infection process, a break was made in the dynamic curves presented in Fig. 1.
Fig. 1. Dynamics of viral load: lower CT values correspond to higher virion concentration values, and vice versa. During the 1st hour after virus inoculation, the concentration of virions decreases due to their penetration into target cells. After that, the medium changes and de novo accumulation of progeny virions produced by infected cells begins (*p ≤ 0.05 when compared to the CT value at the previous time point).
One hour after virus inoculation (toward the end of the infection process), the ATPase activity of adherent leukocytes under SARS-CoV-2 infection ex vivo decreased relative to the uninfected control in a dose-dependent manner (γ(1) < 1), but then began to increase also in a dose-dependent manner: γ(16) ~ 1; γ(24) ≈ 1.2; γ(48) ≈ 1.6 (Fig. 2, a). The increase in ATPase activity between 16 and 48 h was nearly linear with a slight but reproducible excess of activity for a dose of 3 lg(TCID50) compared with 2 lg(TCID50).
Fig. 2. Changes in enzyme activities as a result of SARS-CoV-2 infection: ATPase (a); AMPase (5'-nucleotidase) (b); LDH (c); SDH (d); MPO (e); CCO (f). *p ≤ 0.05 when compared with the γ value at the previous time point
AMPase activity changed differently from ATPase activity during infection (Fig. 2, b). During the initial period of infection, the level of 5′-nucleotidase increased rapidly compared with the uninfected control and remained at this level for at least 16 h after virus inoculation, then decreased by 24 h (γ(24) ≈ 0.8 for both infecting doses) and increased slowly over the following 24 h (γ(48) ~ 1).
Changes in LDH (Fig. 2, c) and SDH (Fig. 2, d) activity during infection were similar: first a small sharp increase (γ(1) ≈ γ(16) ≈ 1.2), then a return to the activity value of the uninfected control (γ(24) ~ 1) and an increase over the following 24 h (γ(48) ≈ 1.8). The decrease in dehydrogenase activity 24 h after infection is reproduced in all cases and is likely to be dose-dependent (most pronounced for ADH).
MPO activity in infected cells decreased rapidly in a dose-dependent manner compared with the uninfected control already within 1 h after virus inoculation (Fig. 2, e) and recovered to the previous level after 24 h (γ(24) ~ 1), and then it increased (γ(48) ≈ 1.4).
CHO activity initially decreased in a dose-dependent manner (Fig. 2, e) but then returned to the level of the uninfected control as early as 16 h after virus inoculation (γ(16) ≈ γ(24) ≈ 1.0), and then it increased to γ(48) ≈ 1.2 for dose 2 lg(TCID50) and to γ(48) ≈ 1.4 for dose 3 lg(TCID50).
Discussion
Syrian hamsters (Mesocricetus auratus) are a convenient experimental model for reproducing SARS-CoV-2 coronavirus infection [1, 16, 17]. In this work, we used ex vivo infection of adherent leukocyte culture, which contains the main fraction of neutrophils undergoing a complex of morphofunctional changes upon contact with infectious agents [18]. Neutrophils, a very important innate immune cells, are rather short-lived leukocytes, and after 48 h their adhesive population is rapidly depleted (this, in particular, determines the chosen duration of the experiment).
It is known that AMPase (5′-nucleotidase) and ATPase are actively involved in the process of spatial transformation of the neutrophil plasma membrane during chemotaxis [18, 19]. In particular, 5′-nucleotidase is a regulator of the level of cyclic AMPase, which ensures the transmission of signals from the plasmalemma inside the cell and regulates the formation of extracellular adenosine, which mediates cytoprotection and a variety of physiological effects (suppression of inflammation, vasodilation, inhibition of thrombosis, anti-adrenergic, etc.) through specific receptors. [20]. When cells are damaged, they increase AMP and decrease ATP [21, 22]. Accordingly, an increase in AMPase activity and a decrease in ATPase activity were recorded during the first 16 h after virus inoculation. The early stages of coronavirus damage to target cells are associated with receptor-mediated fusion of virus-cell membranes and formation of special cisternae in the rough endoplasmic reticulum of the infected cells, in which virions are assembled [23, 24]. The monotonic increase in ATPase activity, starting at about 16 h after virus inoculation, is associated with active synthesis of viral proteins (both structural and regulatory) and virus-specific RNA. The repeated increase in AMPase activity later than 24 h after virus inoculation (Fig. 2, a, b) apparently reflects the process of secondary infection of leukocytes (including as a result of syncytium formation).
LDH is a zinc-containing intracellular enzyme that catalyzes the oxidation of lactic acid into pyruvate, takes part in glucose metabolism, is found in almost all cells of the body and is released when they are damaged [25]. Therefore, the level of serum LDH reliably marks the level of adverse effects of inflammatory reactions and other pathological processes. In particular, serum LDH levels have been found to be informative for assessing clinical severity and monitoring response to treatment in pneumonia in COVID-19 patients [26]. LDH belongs to coenzyme-independent flavoproteins and is part of the membrane-bound respiratory chain of membranes. The flavin group of this enzyme contains 4 iron atoms and is covalently bound to the protein, and the enzymatic activity of SDH depends on SH groups [25]. Mammalian SDH is not only involved in energy generation in mitochondria, but also plays a role in the oxygen sensitivity of the cells [27]. Dehydrogenase activity of infected cells first increases due to virus stimulation of replication processes and then decreases as a result of virus-induced cytodestruction: in the model of human immunodeficiency virus type 1 (Ortervirales: Retroviridae, Lentivirus) and immortalized cell lines of different origin, it was shown that the magnitude and rate of such dehydrogenase shift are proportional to the infecting dose and the level of pathogenicity of a particular strain (at the same infecting dose) [13, 14]. Under the conditions of the experiment described in this article, the dehydrogenase activity of SARS-CoV-2-infected primary culture of adherent leukocytes of the Syrian hamster (Fig. 2, c, d) has two maxima: on the 1st day, which is associated with virus entry into the cell, and after the 1st day – due to de novo virus production (Fig. 1, 2). Another explanation (related to the previous one): the first peak of dehydrogenase activity is associated with neutrophil viability, and the second peak with longer-lived monocytes (but the peak of the maximum was not reached due to the fact that the aim of the experiment was to study primarily the biochemistry of infected neutrophils).
MPO is a hemoprotein present in the azurophilic granules of neutrophils, released upon cell activation into the phagolysosome [28]. This enzyme is involved in the conversion of superoxide anion radical to hypochlorous acid, realizing the cell's defense against excessive reactive oxygen species mediators [29]. After phagocyte activation, degranulation occurs and MPO is secreted into the phagosome or into the extracellular space. MPO is an important part of antimicrobial activity of phagocytes, providing innate nonspecific immunity. In vivo, MPO is released into the extracellular fluid (specifically, into the blood) if for any reason the neutrophil is unable to phagocytize a pathogen, during cell lysis, or when the neutrophil is exposed to various soluble factors [28].
When using automated cytochemical blood cell counters in patients diagnosed with COVID-19, a decrease in MPO activity was observed [9]. At the same time, when neutrophil extracellular traps forming one of the lines of defense against pathogens (together with viruses, including SARS-CoV-2) are formed, an increase in MPO activity in the extracellular space is detected [30–32]. The decrease in MPO content in adherent leukocyte culture within 1 day after virus inoculation (Fig. 1, e) can be explained by the fact that under the influence of SARS-CoV-2 infection neutrophils excrete MPO into the extracellular space and form neutrophil-like extracellular trap structures ex vivo.
CCO localizes mainly on the inner mitochondrial membrane, where it captures protons from the intramitochondrial matrix and, by transferring electrons from cytochrome c to oxygen, reduces O2 to H2O. This enzyme plays an important role in the functioning of the aerobic link of the respiratory chain and energy production in eukaryotic cells [25]. Therefore, the decrease in CCO activity correlates with the decrease in ATPase activity during the first hours after virus inoculation (see Fig. 2, a, e). Furthermore, in leukocytes, the activity of CCO serves as a reliable indicator of the level of oxidative metabolism, and its activity increases during cell death [25] — this is the effect observed in the culture of adherent leukocytes by the end of the 1st day after virus inoculation (Fig. 2, e).
The observed changes in the enzymatic spectrum of SARS-CoV-2-infected leukocytes are dose-dependent (Fig. 2): the modulus of such changes is proportional to the infecting dose of the virus. When analyzing the dynamics of enzymatic activity, it should be taken into account that by the end of the 1st day after virus inoculation, the cellular composition of the adherent leukocyte culture decreases at the expense of short-lived neutrophils, but at the same time, longer-lived cells remain in the culture.
Of course, we cannot exclude that SARS-CoV-2 virus-infected Vero E6 cells produce soluble exogenous factors that could affect cell physiology during infection, since virus-containing Vero E6 cell culture supernatant was used. However, it is known that Vero and Vero E6 cell lines do not produce type I interferon due to loss of the type I interferon gene cluster [33, 34] and are defective in the production of interferons-α-1/13, α-2, α-4, α-6, α-8, α-14, α-17, α-21, β-1 and ω-1 [33]. As for the remaining virus-containing soluble factors, they may be the subject of further studies.
Conclusion
The observed changes in enzymatic activity in neutrophils ex vivo infected with SARS-CoV-2 indicate a decrease in the microbicidal potential of these innate immunity cells, which is one of the causes of immune system dysfunction in COVID-19.
1 Hereinafter, photometric measurements were performed using a Multiscan RC spectrophotometer (LabSystems). Blanking was performed on a solution of equal amounts of medium without the corresponding substrates and cells.
2 The counting of viable cells in suspension cultures is most easily performed in a Goryaev chamber, extracting a small volume of growth medium with cell suspension. This method is more convenient for a single measurement (which does not give sufficient statistical accuracy), but is difficult in case of several repetitions; moreover, the reliability of MTT-test results when working with suspension cell cultures is additionally reduced by artifactual capture of cells during washing, which has to be compensated by the use of pipette tips of special design (S-tips) [15].
About the authors
Svetlana A. Abramova
Somov Institute of Epidemiology and Microbiology
Author for correspondence.
Email: svetochey99@mail.ru
ORCID iD: 0000-0002-2428-3186
junior researcher, Pathology laboratory
Russian Federation, VladivostokIrina N. Lyapun
Somov Institute of Epidemiology and Microbiology
Email: irina-lyapun@list.ru
ORCID iD: 0000-0002-5290-3864
Cand. Sci. (Biol.), senior researcher, Pathology laboratory
Russian Federation, VladivostokElena I. Drobot
Somov Institute of Epidemiology and Microbiology
Email: eidrobot@mail.ru
ORCID iD: 0000-0001-7672-1582
Cand. Sci. (Biol.), senior researcher, Pathology laboratory
Russian Federation, VladivostokNatalia V. Krylova
Somov Institute of Epidemiology and Microbiology; Far Eastern Federal University
Email: krylovanatalya@gmail.com
ORCID iD: 0000-0002-9048-6803
Sci. (Biol.), leading researcher, Head, Laboratory of respiratory infections, Assistant Professor, Department of epidemiology, microbiology and parasitology with the International scientific and educational center for biological safety of Rospotrebnadzor, School of Life Sciences and Biomedicine
Russian Federation, Vladivostok; VladivostokOlga V. Iunikhina
Somov Institute of Epidemiology and Microbiology; Far Eastern Federal University
Email: olga_iun@inbox.ru
ORCID iD: 0000-0002-6723-582X
Sci. (Biol.), leading researcher, Head, Laboratory of respiratory infections, Assistant Professor, Department of epidemiology, microbiology and parasitology with the International scientific and educational center for biological safety of Rospotrebnadzor, School of Life Sciences and Biomedicine
Russian Federation, Vladivostok; VladivostokValeria A. Lubova
Somov Institute of Epidemiology and Microbiology
Email: valeri_priority@mail.ru
ORCID iD: 0000-0002-4290-6164
researcher, Laboratory of natural focal infections
Russian Federation, VladivostokEvgeniy K. Merlov
Somov Institute of Epidemiology and Microbiology
Email: zhenya.merlov.2000@mail.ru
ORCID iD: 0000-0003-1515-3221
junior researcher, Department of experimental biomedicine
Russian Federation, VladivostokIurii A. Belov
Somov Institute of Epidemiology and Microbiology; Far Eastern Federal University
Email: bornley@yandex.ru
ORCID iD: 0000-0001-8313-5610
junior researcher, Head, Center of molecular diagnostics, assistant, Department of epidemiology, microbiology and parasitology with the International scientific and educational center for biological safety of Rospotrebnadzor, School of Life Sciences and Biomedicine
Russian Federation, Vladivostok; VladivostokLarisa M. Somova
Somov Institute of Epidemiology and Microbiology
Email: l_somova@mail.ru
ORCID iD: 0000-0003-2023-1503
Dr. Sci. (Med.), Professor, chief researcher, Head, Pathology laboratory
Russian Federation, VladivostokMikhail Yu. Shchelkanov
Somov Institute of Epidemiology and Microbiology; Far Eastern Federal University
Email: adorob@mail.ru
ORCID iD: 0000-0001-8610-7623
Sci. (Biol.), Director, Head, Department of epidemiology, microbiology and parasitology with the International scientific and educational center for biological safety of Rospotrebnadzor, School of Life Sciences and Biomedicine
Russian Federation, Vladivostok; VladivostokReferences
- Щелканов М.Ю. Этиология COVID-19. В кн.: COVID-19: от этиологии до вакцинопрофилактики. Руководство для врачей. М.;2023:11–53. Shchelkanov M.Yu. Etiology of COVID-19. In: COVID-19: from Etiology to Vaccination. A Guide for Doctors. Moscow; 2023:11–53. EDN: https://elibrary.ru/itytdm
- Попова А.Ю., Щелканов М.Ю., Крылова Н.В. и др. Генотипический портрет SARS-CoV-2 на территории Приморского края в период пандемии COVID-19. Журнал микробиологии, эпидемиологии и иммунобиологии. 2024;101(1):19–35. Popova A.Yu., Shchelkanov M.Y., Krylova N.V., et al. Genotypic portrait of SARS-CoV-2 in Primorsky krai during the COVID-19 pandemic. Journal of Microbiology, Epidemiology and Immunobiology. 2024;101(1):19-35. DOI: https://doi.org/10.36233/0372-9311-497, EDN: https://elibrary.ru/pujffa
- Rong N., Wei X., Liu J. The role of neutrophil in COVID-19: positive or negative. J. Innate Immun. 2024;16(1):80–95. DOI: https://doi.org/10.1159/000535541
- Zhu Y., Chen X., Liu X. NETosis and neutrophil extracellular traps in COVID-19: Immunothrombosis and beyond. Front. Immunol. 2022;13:838011. DOI: https://doi.org/10.3389/fimmu.2022.838011
- Сомова Л.М., Коцюрбий Е.А., Дробот Е.И. и др. Клинико-морфологические проявления дисфункции иммунной системы при новой коронавирусной инфекции COVID-19. Клиническая и экспериментальная морфология. 2021;10(1):11–20. Somova L.M., Kotsyurbiy E.A., Drobot E.I., et al. Clinical and morphological manifestations of immune system dysfunction in new coronavirus infection (COVID-19). Clinical and Experimental Morphology. 2021;10(1):11–20. DOI: https://doi.org/10.31088/CEM2021.10.1.11-20 EDN: https://elibrary.ru/upppqm
- Сомова Л.М., Дробот Е.И., Пустовалов Е.В. и др. Морфология лейкоцитов периферической крови у больных новой коронавирусной инфекцией COVID-19. Клиническая и экспериментальная морфология. 2023;12(3):41–9. Somova L.M., Drobot E.I., Pustovalov E.V., et al. Morphology of peripheral blood leucocytes in patients with new coronavirus infection (COVID-19). Clinical and Experimental Morphology. 2023;12(3):41–9. DOI: https://doi.org/10.31088/CEM2023.12.3.41-49, EDN: https://elibrary.ru/vcexry
- Сомова Л.М., Абрамова С.А., Дробот Е.И. и др. Нейтрофильные синцитии в периферической крови пациентов с коронавирусной инфекцией (COVID-19). Клиническая экспериментальная морфология. 2024;13(3):26–33. Somova L.M., Abramova S.A., Drobot E.I., et al. Neutrophil syncytia in the peripheral blood of patients with coronavirus infection (COVID-19). Clinical Experimental Morphology. 2024;13(3):26–33. DOI: https://doi.org/10.31088/CEM2024.13.3.26-33, EDN: https://elibrary.ru/soelfa
- Абрамова С.А., Дробот Е.И., Пустовалов Е.В. и др. Ультраструктурная патология клеток врождённого иммунитета при COVID-19. Дальневосточный журнал инфекционной патологии. 2023;(45):109–11. Abramova S.A., Drobot E.I., Pustovalov E.V., et al. Ultrastructure pathology of innate immunity cells during COVID-19. The Far Eastern Journal of Infectious Pathology. 2023;(45):109–11. EDN: https://elibrary.ru/jtwvjg
- Han Y., Zhang H., Mu S., et al. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: a retrospective and observational study. Aging (Albany NY). 2020;12(12):11245–58. DOI: https://doi.org/10.18632/aging.103372
- Poggiali E., Zaino D., Immovilli P., et al. Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in COVID-19 patients. Clin. Chim. Acta. 2020;509:135–8. DOI: https://doi.org/10.1016/j.cca.2020.06.012
- Zini G., d'Onofrio G. Coronavirus disease 2019 (COVID-19): Focus on peripheral blood cell morphology. Br. J. Haematol. 2023;200(4):404–19. DOI: https://doi.org/10.1111/bjh.18489
- Сомова Л.М., Плехова Н.Г., Кондрашова Н.М., Запорожец Т.С. Определение функциональной активности лейкоцитов периферической крови в качестве показателя неспецифической защиты организма: Методические рекомендации. Владивосток;2005. Somova L.M., Plekhova N.G., Kondrashova N.M., Zaporozhets T.S. Determination of the functional activity of peripheral blood leukocytes as an indicator of nonspecific body protection: Methodological recommendations. Vladivostok;2005.
- Щелканов М.Ю., Сахурия И.Б., Бурунова В.В. и др. Дегидрогеназная активность ВИЧ-инфицированных клеток при анализе результатов МТТ-теста. Иммунология. 1999;20(1):37–41. Shchelkanov M.Yu., Sakhuria I.B., Burunova V.V., et al. HIV-infected cell dehydrogenase activity in the evaluation of anti-HIV compound efficiency. Immunologiya. 1999;20(1):37–41. EDN: https://elibrary.ru/lqohkg
- Щелканов М.Ю., Ерёмин В.Ф., Сахурия И.Б. и др. Дегидрогеназная активность инфицированных клеток и биологические свойства различных вариантов ВИЧ-1. Биохимия. 1999;64(4):513–9. Shchelkanov M.Yu., Eremin V.F., Sakhuriya I.B., et al. Dehydrogenase activity of infected cells and biological properties of HIV-1 variants. Biochemistry (Moscow). 1999;64(4):431–6. EDN: https://elibrary.ru/lfiulj
- Щелканов М.Ю., Сахурия И.Б., Полякова Е.Б. и др. Повышение качества МТТ-метода с помощью микродозаторных наконечников специальной конструкции. Иммунология. 1998;19(4):57–9. Shchelkanov M.Yu., Sakhuriya I.B., Polyakova E.B., et al. Improvement of the MTT-based assay by modification of pipette tips. Immunology (Moscow). 1998;19(4):57–9. EDN: https://elibrary.ru/mpawbj
- Грачева А.В., Дроков А.О., Смирнова Д.И. и др. Вирулентность и тканевая специфичность разных эпидемически значимых вариантов SARS-CoV-2 для золотистых сирийских хомячков. Журнал эпидемиологии, микробиологии и иммунобиологии. 2024;101(4):470–82. Gracheva A.V., Drokov A.O., Smirnova D.I., et al. Virulence and tissue tropism of different epidemiologically significant SARS-CoV-2 variants for golden Syrian hamsters. Journal of Epidemiology, Microbiology and Immunobiology. 2024;101(4):470–82. DOI: https://doi.org/10.36233/0372-9311-528 EDN: https://elibrary.ru/jukmca
- Фоменко Е.П., Гапека А.В., Милованкин П.Г. и др. Эффективные животные модели для изучения SARS-CoV-2-инфекции. Дальневосточный журнал инфекционной патологии. 2024;(47):36–8. Fomenko E.P., Gapeka A.V., Milovankin P.G., et al. Effective animal models for the study of SARS-CoV-2 infection. The Far Eastern Journal of Infectious Pathology. 2024;47(47):36–8. DOI: https://doi.org/10.62963/2073-2899-2024-47-36-38 EDN: https://elibrary.ru/rfdeaj
- Плехова Н.Г., Сомова Л.М., Слонова Р.А. и др. Метаболическая активность макрофагов, зараженных Hantaviruses — возбудителями геморрагической лихорадки с почечным синдромом. Биохимия. 2005;70(9):1198–208. Plekhova N.G., Somova L.M., Slonova R.A., et al. Metabolic activity of macrophages infected with hantavirus, an agent of hemorrhagic fever with renal syndrome. Biochemistry (Moscow). 2005;70(9): 990–7. DOI: https://doi.org/10.1007/s10541-005-0214-0, EDN: https://elibrary.ru/ljdewp
- Плехова Н.Г., Сомова Л.М. Роль моноцитов/макрофагов в патогенезе вирусных инфекций. Тихоокеанский медицинский журнал. 2010;(3):5–9. Plekhova N.G., Somova L.M. The role of monocytes/macrophages in pathogenesis of viral infections. Pacific Medical Journal. 2010;(3):5–9. EDN: https://elibrary.ru/oiheqp
- Zukowska P., Kutryb-Zajac В., Toczek М., et al. The role of ecto-5′-nucleotidase in endothelial dysfunction and vascular pathologies. Pharmacol. Rep. 2015;67(4):675–81. DOI: https://doi.org/10.1016/j.pharep.2015.05.002, EDN: https://elibrary.ru/xouavl
- Бра М., Квинан Б., Сузин С.А. Митохондрии в программированной гибели клетки: различные механизмы гибели (обзор). Биохимия. 2005;70(2):284–93. Bras M., Queenan B., Susin S.A. Programmed cell death via mitochondria: different modes of dying. Biochemistry (Moscow). 2005;70(2):231–9. DOI: https://doi.org/10.1007/s10541-005-0105-4, EDN: https://elibrary.ru/mhrivj
- Осколок Л.Н., Порядин Г.В. Основные механизмы повреждения клеток. М.;2016. Oskolok L.N., Poryadin G.V. The Main Mechanisms of Cell Damage. Moscow;2016. EDN: https://elibrary.ru/xglrxt
- Щелканов М.Ю., Колобухина Л.В., Бургасова О.А. и др. COVID-19: этиология, клиника, лечение. Инфекция и иммунитет. 2020;10(3):421–45. Shchelkanov M.Yu., Kolobukhina L.V., Burgasova O.A., et al. COVID-19: Etiology, Clinical Picture, Treatment. Russian Journal of Infection and Immunity. 2020;10(3):421–45. DOI: https://doi.org/10.15789/2220-7619-CEC-1473, EDN: https://elibrary.ru/imaadb
- Щелканов М.Ю., Попова А.Ю., Дедков В.Г. и др. История изучения и современная классификация коронавирусов (Nidovirales: Coronaviridae). Инфекция и иммунитет. 2020;10(2):221–46. Shchelkanov M.Yu., Popova A.Yu., Dedkov V.G., et al. History of investigation and current classification of coronaviruses (Nidovirales: Coronaviridae). Russian Journal of Infection and Immunity. 2020;10(2):221–46. DOI: https://doi.org/10.15789/2220-7619-HOI-1412, EDN: https://elibrary.ru/kziwrq
- Кольман Я., Рём К.Г. Наглядная биохимия. Пер. с нем. М.;2004. Koolman J., Röhm K.H. Taschenatlas der Biochemie. New York;1998. EDN: https://elibrary.ru/qkmqbj
- Wu M.Y., Yao L., Wang Y.I., et al. Clinical evaluation of potential usefulness of serum lactate dehydrogenase (LDH) in 2019 novel coronavirus (COVID-19) pneumonia. Respir. Res. 2020;21(1):171. DOI: https://doi.org/10.1186/s12931-020-01427-8, EDN: https://elibrary.ru/ojobmo
- Bardella C., Pollard P.J., Tomlinson I. SDH mutations in cancer. Biochim. Biophys. Acta. 2011;1807(11):1432–43. DOI: https://doi.org/10.1016/j.bbabio.2011.07.003, EDN: https://elibrary.ru/phvbwx
- Klebanoff S.J. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77(5):598–625. DOI: https://doi.org/10.1189/jlb.1204697 EDN: https://elibrary.ru/mfbqgj
- Witko-Sarsat V., Rieu P., Descamps-Latscha B., et al. Neutrophils: molecules, functions and pathophysiological aspects. Lab. Invest. 2000;80(5):617–53. DOI: https://doi.org/10.1038/labinvest.3780067
- Ashar H.K., Mueller N.C., Rudd J.M., et al. The role of extracellular histones in influenza virus pathogenesis. Am. J. Pathol. 2018;188(1):135–48. DOI: https://doi.org/10.1016/j.ajpath.2017.09.014, EDN: https://elibrary.ru/yfdncp
- Bader S.M., Cooney J.P., Pellegrini M., Doerflinger M. Programmed cell death: the pathways to severe COVID-19? Biochem. J. 2022;479(5):609–28. DOI: https://doi.org/10.1042/bcj20210602, EDN: https://elibrary.ru/kcewfq
- Kapoor S., Mihalovičová L., Pisareva E., et al. Association of vascular netosis with COVID-19 severity in asymptomatic and symptomatic patients. iScience. 2024;27(5):109573. DOI: https://doi.org/10.1016/j.isci.2024.109573, EDN: https://elibrary.ru/rvfsne
- Osada N., Kohara A., Yamaji T., et al. The genome landscape of the African green monkey kidney-derived vero cell line. DNA Res. 2014;21(6):673–83. DOI: https://doi.org/10.1093/dnares/dsu029
- Konishi K., Yamaji T., Sakuma C., et al. Whole-genome sequencing of Vero E6 (Vero C1008) and comparative analysis of four Vero cell sublines. Front. Genet. 2022;13:801382. DOI: https://doi.org/10.3389/fgene.2022.801382
Supplementary files