Stabilization of tigecycline solutions during susceptibility testing of microorganisms by broth microdilution method
- Authors: Kosilova I.S.1, Domotenko L.V.1, Khramov M.V.1
-
Affiliations:
- State Research Center for Applied Microbiology and Biotechnology
- Issue: Vol 102, No 4 (2025)
- Pages: 474-481
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18821
- DOI: https://doi.org/10.36233/0372-9311-690
- EDN: https://elibrary.ru/YNTISP
- ID: 18821
Cite item
Abstract
Introduction. Tigecycline is widely used in the treatment of infections, leading to the emergence of resistant bacteria. Determining susceptibility by broth microdilution method is recommended to be conducted using freshly prepared Mueller–Hinton broth (MHB) or MHB with antioxidant additives, due to the oxidation of the antibiotic. At the same time, there is no information on the possibility of storing and further using antibiotic solutions.
The aim of the study is to determine the feasibility of stabilizing and rationally using tigecycline solutions to achieve acceptable values of minimum inhibitory concentrations (MIC) when testing control strains by the reference method.
Materials and methods. In the study, the MIC of tigecycline for Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212, and Streptococcus pneumoniae ATCC 49619 was determined using the microdilution method in MHB, which was pre-prepared and stored for 24–48 hours prior to the study. For the study, a tigecycline stock solution was prepared in water with the addition of various concentrations of Оxyrase, which were stored at 2–6°C and –70°C, and then testing was conducted in accordance with GOST R ISO 20776-1-2022.
Results. The stability of the tigecycline stock solutions does not exceed 5 hours at 2–6°C, but with the addition of 5.0–8.0% Оxyrase, it increases to 16 days, allowing for the determination of MIC values for all test strains within the acceptable range. Changing the storage temperature to –70°C increases the stability of the solutions to 43 days, and with the addition of 5.0–8.0% Оxyrase, to at least 48 weeks
Conclusion. The possibility of stabilizing tigecycline solutions stored at negative temperatures (with and without the addition of Оxyrase) has been determined to obtain acceptable MIC values when determining the susceptibility of control strains to antimicrobial agents. The use of these solutions allowed for a reduction in testing costs through the rational use of the antibiotic.
Full Text
Introduction
Microorganisms resistant to antimicrobial agents are one of the greatest threats to global health and food product safety [1]. The rapid increase in the spread of Gram-negative microorganisms, especially those belonging to Enterobacterales and Acinetobacter spp., which exhibit resistance to the first-line carbapenem drugs, threatens to reduce their effectiveness. For the treatment of infections caused by such strains of microorganisms, colistin and tigecycline are mainly used [2, 3]. However, currently, in more than 40 countries, including Russia, the global spread of resistance in the studied strains to colistin, mediated by the mcr gene (mobilized colistin resistance), has been recorded, which significantly reduces its clinical effectiveness [4–7]. Therefore, tigecycline is a critically important drug for medicine1, especially in the treatment of infections caused by multidrug-resistant microorganisms [8].
Tigecycline is an antibiotic of the new class of glycylcyclines, with a broad spectrum of activity, structurally similar to tetracyclines, but more active against most gram-positive and gram-negative strains of microorganisms. However, certain bacteria, such as Morganella spp., Proteus spp., Providencia spp. and Pseudomonas aeruginosa, exhibit natural resistance to tigecycline [9]. Reduced susceptibility of A. baumannii to the antibiotic has also been described [10]. Acquired resistance to tigecycline can develop in Klebsiella pneumoniae, E. aerogenes, E. cloacae and others, due to the overexpression of the non-specific efflux gene ArcAB, which confers resistance to many drugs [11].
For the selection and adjustment of antibacterial therapy, it is recommended to conduct microbiological diagnostics with the identification of the pathogen and determination of its susceptibility to tigecycline.
The reference method for determining susceptibility is the broth microdilution method, which allows for the determination of the minimum inhibitory concentrations (MIC) of the tested antibiotics. During the establishment of acceptable values for the MIC of tigecycline for control strains, discrepancies in MIC values were identified, which were associated with the storage period of the Mueller–Hinton broth (MHB). The results of several studies showed that when testing tigecycline in freshly prepared (less than 12 hours before testing) MHB, it was 2–3 times more active against 3 control strains (E. coli ATCC 25922, S. aureus ATCC 29213, and E. faecalis ATCC 29212) than in stored medium (MIC 0.03–0.25 and 0.12–0.50 µg/mL, respectively) [12–14]. In this regard, international standards CLSI2, EUCAST3 and Russian recommendations4 for determining the MIC values of tigecycline using the broth microdilution method recommend using only freshly prepared MHB.
The instability of tigecycline in aqueous solutions is related to its chemical structure, as it can be subjected to two chemical processes that lead to the formation of pharmacologically inactive products. On one hand, the phenolic group of tigecycline makes it susceptibility to oxidation, especially at pH values exceeding 7.0; on the other hand, at lower pH values, tigecycline is more prone to non-enzymatic epimerization5.
For therapeutic purposes, tigecycline is released in the form of a lyophilized powder6, containing excipients such as lactose to stabilize the drug against epimerization, and hydrochloric acid or sodium hydroxide to adjust the pH in the range of 4.5–5.5 and to prevent oxidation [10, 15].
Since the pH of the MHB does not change over time, the discrepancies in the MIC results are attributed to the accelerated oxidative degradation of tigecycline caused by the increased amount of dissolved oxygen in the MHB during storage. To stabilize tigecycline, various antioxidant additives in MHB were investigated, such as: ascorbic acid, sodium pyruvate, sodium thioglycolate, L-cysteine, catalase, as well as anaerobic storage conditions of MHB [12, 15, 16]. The addition of ascorbic acid and pyruvate at concentrations of 0.3% and 6%, respectively, contributed to the stabilization of tigecycline for up to 7 days. However, the study [16] showed that ascorbic acid causes rapid degradation of tigecycline and leads to a loss of antibacterial activity. In several publications, the possibility of using Oxyrase — an enzyme that helps reduce oxygen concentration in MHB—has been investigated [12, 14, 16]. The results of the studies showed that tigecycline activity was maintained in a 2% Oxyrase solution for up to 7 days at 4–6°C.
The study [12] describes the freezing of MHB containing tigecycline and dispensed into 96-well plates at –20°C. Testing the susceptibility of test strains after thawing showed that the antibacterial activity was preserved for up to 6 weeks, and the test results matched those obtained with freshly prepared MHB.
Analysis of publications showed that all manipulations in the studies (antioxidant additives, storage in frozen state, in anaerobic conditions) were carried out with MHB, therefore, it seems important to study the possibility of storing specifically aqueous solutions of the antibiotic.
In accordance with the requirements of GOST R ISO 20776-1-20227, which regulates the procedure for performing the broth microdilution method, antibiotic stock solutions are used for testing, a portion of which, as a rule, remains unused. According to the requirements of GOST R ISO 20776-1-2022, the storage of stable antibiotic solutions is permitted, while the storage of unstable ones, such as aqueous tigecycline solutions, is not regulated in any way. It is only specified that if there is no information on the stability of the solutions under certain storage conditions, a fresh stock solution should be prepared for each batch being tested.
The aim of the study is to determine the possibility of stabilizing and rationally using tigecycline solutions to achieve acceptable MIC values when testing control strains by the reference method.
Materials and methods
In the study, the chemical substances tigecycline (Sigma) and Oxyrase (Sigma) were used. Determination of the MIC values of tigecycline for the control (test) strains E. coli ATCC 25922, S. aureus ATCC 29213, E. faecalis ATCC 29212, S. pneumoniae ATCC 49619 was conducted using the microdilution method in MHB (SRCAMB), which was prepared in advance and stored for 24–48 hours at room temperature before the study. When working with the test strain S. pneumoniae ATCC 49619, 5% lysed horse blood and 20 mg/L β-NAD (Sigma) were added to the broth. All strains used in the study were obtained in lyophilized form from the State Collection of Pathogenic Microorganisms of SCPM-Obolensk.
Lysed horse blood was prepared from defibrinated blood (ECOlab). In order to carry out the preparation, sterile deionized water was added to the defibrinated horse blood in a 1:1 ratio, and it was placed in a freezer for 7–8 hours at –20°C. Then, the thawed blood at room temperature was subjected to repeated freeze-thaw cycles, repeating this process 4 times until the complete lysis of the blood cells. After that, the lysed horse blood was clarified by centrifugation at 7000 rpm for 30 minutes on an Eppendorf Centrifuge 5702 (Eppendorf).
Testing was conducted thrice in accordance with the requirements of GOST R ISO 20776-1-2022. To carry this process out, a stock solution of tigecycline with a concentration of 512 mg/L was prepared in water and in water with the addition of various concentrations of Oxyrase (2.0, 3.0, 5.0, and 8.0%), stored at 2–6°C in a household refrigerator and at –70°C in an MDF-U33V low-temperature freezer (Sanyo). Every hour, individual aliquots of the solution were removed from the storage chamber, thawed at room temperature for 20–30 minutes, prepared as working (twofold) dilutions in distilled water in the concentration range of 0.016–1.000 mg/L, and filled into a 96-well plate.
Inoculates of all test strains were prepared in a saline solution with a concentration of 1–2 × 108 CFU/mL and 0.1 mL was added to 9.9 mL of 2-fold concentrated MHB. Inoculates in MHB were added in a 1:1 ratio to prepared plates, and were incubated at 35 ± 1°C for 18 ± 2 hours, with plates containing S. pneumoniae ATCC 49619 incubated in an atmosphere of 4–6% CO2. The results were recorded according to the EUCAST methodology.
The results were processed using the MS Excel software package. The reliability of various averages was assessed using the Student's t-test. The two-tailed Fisher criterion was used for comparative analysis. Differences were considered significant at p < 0.05.
Results
The MIC of tigecycline for all test strains using stock solutions stored at 2–6°C for up to 3 hours corresponded to the target values, and with further storage up to 5 hours, they fell within the acceptable range (Table 1). Further, with an increase in storage time, the MIC values exceeded the acceptable range.
Table 1. Effect of storage time at 2–6°C of the tigecycline stock solution on the MIC value, mg/L
Storage time of the tigecycline stock solution | E. соli АТСС 25922 | S. aureus АТСС 29213 | E. faecalis ATCC 29212 | S. pneumoniae ATCC 49619 |
0 h (control) | 0.06–0.12 | 0.06–0.12 | 0.03–0.06 | 0.03–0.06 |
1–2 h | 0.06–0.12 | 0.06–0.12 | 0.03–0.06 | 0.03–0.06 |
3 h | 0.06–0.12 | 0.06–0.12 | 0.03–0.06 | 0.03–0.06 |
4 h | 0.12–0.25 | 0.12–0.25 | 0.03–0.12 | 0.06–0.12 |
5 h | 0.12–0.25 | 0.12–0.25 | 0.06–0.12 | 0.06–0.12 |
6 h | 0.5–1.0 | 0.5–1.0 | 0.5–1.0 | 0.5–1.0 |
7 h | 0.5–1.0 | 0.5–1.0 | 0.5–1.0 | 0.5–1.0 |
8–10 h | ≥ 1.0 | ≥ 1.0 | ≥ 1.0 | ≥ 1.0 |
Acceptable range, mg/L | 0.03–0.25 | 0.03–0.25 | 0.03–0.12 | 0.016–0.12 |
Target values, mg/L | 0.06–0.12 | 0.06–0.12 | 0.06 | 0.03–0.06 |
The MIC values of tigecycline for all test strains using antibiotic stock solutions stored at -70°C for up to 31 days corresponded to the target values, and with further storage up to 43 days, it fell within acceptable ranges (Table 2). Longer storage (≥ 44 days) resulted in MIC values for all 4 test strains exceeding the acceptable ranges.
Table 2. Effect of storage time at –70°C of tigecycline stock solutions on the MIC value, mg/L
Storage time of tigecycline solutions | E. соli АТСС 25922 | S. aureus АТСС 29213 | E. faecalis ATCC 29212 | S. pneumoniae ATCC 49619 |
1–18 days | 0.06–0.12 | 0.06–0.12 | 0.03–0.06 | 0.03–0.06 |
19–31 days | 0.06–0.12 | 0.06–0.12 | 0.03–0.06 | 0.03–0.06 |
32–43 days | 0.12–0.25 | 0.12–0.25 | 0.06–0.12 | 0.06–0.12 |
44–56 days | 0.5–1.0 | 0.5–1.0 | 0.25–0.50 | 0.25–0.50 |
57–68 days | 0.5–1.0 | 0.5–1.0 | 0.25–0.50 | 0.25–0.50 |
69–80 days | 0.5–1.0 | 0.5–1.0 | 0.5–1.0 | 0.5–1.0 |
≥ 81 days | ≥ 1.0 | ≥ 1.0 | ≥ 1.0 | ≥ 1.0 |
Acceptable range, mg/L | 0.03–0.25 | 0.03–0.25 | 0.03–0.12 | 0.016–0.120 |
Target values, mg/L | 0.06–0.12 | 0.06–0.12 | 0.06 | 0.03–0.06 |
The results obtained in the first stage of the study showed that the activity of the stock solutions of tigecycline was maintained for a longer period at –70°C (up to 43 days) than at 2–6°C (no more than 5 hours), which ensured the determination of the minimum inhibitory concentration (MIC) of tigecycline for all test strains within acceptable value ranges.
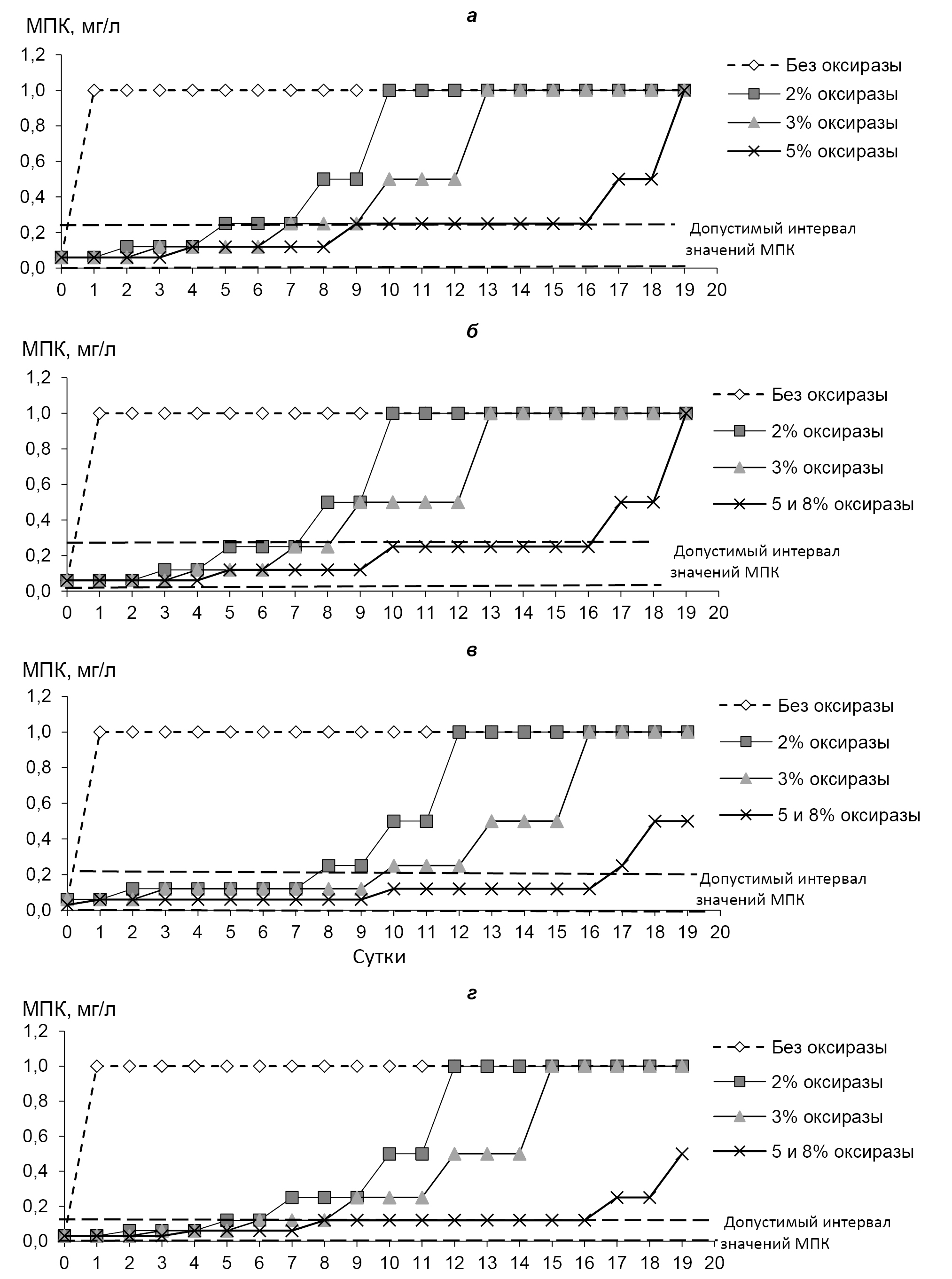
The second stage of the study is dedicated to examining the effect of adding Oxyrase on the MIC of tigecycline for 4 test strains. Increasing the concentration of Oxyrase contributed to the longer storage of tigecycline stock solutions at –2–6°C compared to solutions without a stabilizer, as the obtained MIC values for the tested strains corresponded to acceptable values for a longer period (Figure, Table 3). The addition of Oxyrase at concentrations of 5% and 8% to the tigecycline stock solution increased its storage time at 2–6°C from 4–5 hours to 16 days and ensured the attainment of acceptable MIC values for 4 test strains.
Table 3. Storage periods (days) of tigecycline stock solutions with different Oxyrase concentrations at 2–6°C while maintaining acceptable MIC values
Test strains | Without Oxyrase | Oxyrase concentration, % | |||
2 | 3 | 5 | 8 | ||
E. соli АТСС 25922 | 5 ч | 7 | 9 | 16 | 16 |
S. aureus АТСС 29213 | 5 ч | 7 | 8 | 16 | 16 |
E. faecalis ATCC 29212 | 4 ч | 7 | 9 | 16 | 16 |
S. pneumoniae ATCC 49619 | 5 ч | 6 | 8 | 16 | 16 |
The dependence of tigecycline MIC for E. coli ATCC 25922 (a), S. aureus ATCC 29213 (b), E. faecalis ATCC 29212 (c), and S. pneumoniae ATCC 49619 (d) on the concentration of Oxyrase added to the antibiotic stock solutions and their storage periods at 2–6°C.
The results of the tigecycline MIC measurements for test strains, obtained using stabilized Oxyrase antibiotic stock solutions during storage at –70°C, are presented in Table 4. For convenience, the MIC results are presented in arbitrary units: average MIC values from 3 measurements within the acceptable range were marked as C (Correct); average values from 3 measurements exceeding the acceptable range were marked as HE (High error).
Table 4. Conditional tigecycline MIC values for test strains Oxyrase concentration in antibiotic stock solutions and storage duration at –70°C
Storage time of stock solutions, weeks | E. соli АТСС 25922 | S. aureus АТСС 29213 | E. faecalis ATCC 29212 | S. pneumoniae ATCC 49619 | ||||||||||||
2% | 3% | 5% | 8% | 2% | 3% | 5% | 8% | 2% | 3% | 5% | 8% | 2% | 3% | 5% | 8% | |
0 | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C |
12 | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C |
18 | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C | C |
28 | HE | C | C | C | HE | C | C | C | HE | C | C | C | HE | C | C | C |
48 | HE | HE | C | C | HE | HE | C | C | HE | HE | C | C | HE | HE | C | C |
Зависимость значений МПК тигециклина для E. coli ATCC 25922 (а), S. aureus ATCC 29213 (б), E. faecalis ATCC 29212 (в) и S. pneumoniae ATCC 49619 (г) от концентрации оксиразы, добавленной в базовые растворы антибиотика, и сроков их хранения при 2–6°С.
As seen from Table 4, the use of Oxyrase as a stabilizer for tigecycline stock solutions showed that the stability of these solutions is maintained up to 18 weeks with the addition of 2% Oxyrase, up to 28 weeks with the addition of 3% Oxyrase, and up to 48 weeks with the addition of 5 or 8% Oxyrase, resulting in MIC values for tigecycline for all tested strains corresponding to acceptable levels (C).
Discussion
Currently, tigecycline is the drug of choice for treating many severe infections caused by multidrug-resistant microorganisms. Due to its broad-spectrum antibacterial activity, tigecycline is often used empirically, which leads to the emergence of resistant bacteria [17].
Therefore, the treatment of infectious diseases, including tigecycline, should be based on the results of pathogen identification and antimicrobial susceptibility testing.
Determining susceptibility to tigecycline using broth microdilution methods is associated with the problem of its instability in aqueous solutions. To obtain reliable test results according to CLSI and EUCAST standards, it is recommended to use freshly prepared MHB.
This study examined the effect of various methods for stabilizing basic aqueous solutions of tigecycline: by altering storage temperature and using Oxyrase stabilizer in different concentrations for 4 strains, including a fastidious strain S. pneumoniae ATCC 49619.
In the course of the study, pre-prepared MHB was used and stored for 24–48 hours. It has been determined that at 2–6°C, the stability of tigecycline stock solutions without Oxyrase is maintained for no more than 5 hours, while with 5% and 8% Oxyrase, it increases to 16 days. When these solutions are stored without Oxyrase at –70°C, their stability increases to 43 days, and with the addition of 5% and 8% Oxyrase, it increases to at least 48 weeks. The obtained results correlate with published data [12, 14, 16], which show that the activity of tigecycline in MHB is maintained for up to 6 weeks (42 days) at –17–18°C, and at 4–6°C with the addition of 2% Oxyrase solutions, it is maintained for up to 7 days (see Table 3).
In several publications, the authors note that Oxyrase and chemically pure tigecycline are quite expensive products [12–14]. The cost calculations showed that from 5 mg of chemically pure tigecycline (priced at an average of 45,000 rubles/5 mg), more than 200 plates can be prepared with a working concentration range of tigecycline (0.06–4.00) mg/L. At the same time, the cost of preparing one plates will be approximately 270 rubles (including the cost of the plates itself)8. The broth microdilution method is a labor-intensive process, so not every bacteriological laboratory can use such a large number of plates in a single experiment. Unused tigecycline stock solution can be aliquoted into cryovials and stored at negative temperatures until the next use, and the addition of Oxyrase will further extend the storage time and the possibility of using MHB stored for 24–48 hours. The cost of the same number of plates with the addition of 5% Oxyrase increases slightly (on average by 7–10%).
Conclusion
The possibility of stabilizing tigecycline solutions stored at negative temperatures (with and without the addition of Oxyrase) has been determined to obtain acceptable MIC values when determining the susceptibility of control strains to antimicrobial agents. The use of these solutions made it possible to reduce testing costs through the rational use of the antibiotic. In the future, work will continue on studying the possibility of using these tigecycline solutions in the testing of clinical strains.
1 WHO. Critically important antimicrobials for human medicine (6th revision ed.) URL: https://www.who.int/publications/i/item/9789241515528 (data of access: 03.03.2025).
2 Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Thirty-third Informational Supplement. CLSI document M100, 33rd Edition. USA; 2022. 402 p.
3 European Committee for Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters (Version 15.0). URL: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_15.0_Breakpoint_Tables.pdf (data of access: 12.03.2025).
4 Russian recommendations "Determination of the sensitivity of microorganisms to antimicrobial drugs" (version 2024-02). URL: https://www.antibiotic.ru/files/334/ocmap2024.pdf
5 Fawzi M.B., Zhu T., Shah S.M. Tigecycline compositions and methods of preparation (patent). United States US-8975242-B2. 2011. URL: https://patentimages.storage.googleapis.com/c2/25/07/73a12f6c15cdfa/US8975242.pdf (data of access: 07.03.2025).
6 Directory of medicines. Tigacil. Instructions for use. 2024. (In Russ.) URL: https://www.vidal.ru/drugs/tigacil__23094 (data of access: 12.03.2025).
7 GOST R ISO 20776-1-2022 Study of the sensitivity of infectious agents and evaluation of the functional characteristics of products for the study of sensitivity to antimicrobial agents. Part 1. A reference method of microdilution in broth for laboratory investigation of the activity of antimicrobial agents against fast-growing aerobic bacteria that cause infectious diseases. 2022. 20 p. (In Russ.)
8 Merck. URL: https://www.sigmaaldrich.com/RU/en/search/pz0021-5mg?focus=products&page=1&perpage=30&sort=relevance&term=PZ0021-5MG&type=product (data of access: 10.03.2025).
About the authors
Irina S. Kosilova
State Research Center for Applied Microbiology and Biotechnology
Author for correspondence.
Email: kosilova.irina@gmail.com
ORCID iD: 0000-0003-4020-0894
Cand. Sci. (Biol.), senior researcher, Nutrient medium development laboratory
Russian Federation, Obolensk, Moscow RegionLyubov V. Domotenko
State Research Center for Applied Microbiology and Biotechnology
Email: domotenko@obolensk.org
ORCID iD: 0000-0002-4785-6418
Cand. Sci. (Chem.), leading researcher, Nutrient medium development laboratory
Russian Federation, Obolensk, Moscow RegionMikhail V. Khramov
State Research Center for Applied Microbiology and Biotechnology
Email: khramov@obolensk.org
ORCID iD: 0000-0002-4553-3826
Cand. Sci. (Med.), Deputy director for quality and development
Russian Federation, Obolensk, Moscow RegionReferences
- Laxminarayan R., Sridhar D., Blaser M., et al. Achieving global targets for antimicrobial resistance. Science. 2016; 353(6302): 874–9. DOI: https://doi.org/10.1126/science.aaf9286
- Karageorgopoulos D.E., Falagas M.E. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect. Dis. 2008;8(12):751–62. DOI: https://doi.org/10.1016/s1473-3099(08)70279-2
- Rodríguez-Baño J., Gutiérrez-Gutiérrez B., Machuca I., et al. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin. Microbiol. Rev. 2018;31(2):e00079-17. DOI: https://doi.org/10.1128/cmr.00079-17
- IACG Discussion Paper. Interagency Coordination Group on Antimicrobial Resistance. Reduce unintentional exposure and the need for antimicrobials, and optimize their use;2018.
- Шедько Е.Д., Тимошина О.Ю., Азизов И.С. Молекулярная эпидемиология генов группы mcr. Клиническая микробиология и антимикробная химиотерапия. 2020;22(4): 287–300. Shedko E.D., Timoshina O.Yu., Azizov I.S. Molecular epidemiology of mcr group genes. Clinical Microbiology and Antimicrobial Chemotherapy. 2020;22(4):287–300. DOI: https://doi.org/10.36488/cmac.2020.4.287-300 EDN: https://elibrary.ru/ycsxgu
- Partridge S.R., Pilato V.D., Doi Y., et al. Proposal for assignment of allele numbers for mobile colistin resistance (mcr) genes. J. Antimicrob. Chemother. 2018;73(10):2625–30. DOI: https://doi.org/10.1093/jac/dky262
- Cheng Y., Li Y., Yu R., et al. Identification of novel tet(X3) variants resistant to tigecycline in Acinetobacter species. Microbiol. Spectr. 2022;10(6):e0133322. DOI: https://doi.org/10.1128/spectrum.01333-22
- Tasina E., Haidich A.B., Kokkali S., et al. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect. Dis. 2011;11(11): 834–44. DOI: https://doi.org/10.1016/s1473-3099(11)70177-3
- Korczak L., Majewski P., Iwaniuk D., et al. Molecular mechanisms of tigecycline-resistance among Enterobacterales. Front. Cell. Infect. Microbiol. 2024;14:1289396. DOI: https://doi.org/10.3389/fcimb.2024.1289396
- He T., Wang R., Liu D., et al. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019;4(9):1450–6. DOI: https://doi.org/10.1038/s41564-019-0445-2
- Liu C., Liu J., Lu Q., et al. The mechanism of tigecycline resistance in Acinetobacter baumannii under sub-minimal inhibitory concentrations of tigecycline. Int. J. Mol. Sci. 2024;25(3):1819. DOI: https://doi.org/10.3390/ijms25031819
- Bradford P.A., Petersen P.J., Young M., et al. Tigecycline MIC testing by broth dilution requires use of fresh medium or addition of the biocatalytic oxygen-reducing reagent Oxyrase to standardize the test method. Antimicrob. Agents Chemother. 2005;49(9):3903–9. DOI: https://doi.org/10.1128/aac.49.9.3903-3909.2005
- Brown S.D., Traczewski M.M. Comparative in vitro antimicrobial activity of tigecycline, a new glycylcycline compound, in freshly prepared medium and quality control. J. Clin. Microbiol. 2007;45(7):2173–9. DOI: https://doi.org/10.1128/jcm.02351-06
- Petersen P.J., Bradford P.A. Effect of medium age and supplementation with the biocatalytic oxygen-reducing reagent Oxyrase on in vitro activities of tigecycline against recent clinical isolates. Antimicrob. Agents Chemother. 2005;49(9):3910–8. DOI: https://doi.org/10.1128/aac.49.9.3910-3918.2005
- Jitkova Y., Gronda M., Hurren R., et al. A novel formulation of tigecycline has enhanced stability and sustained antibacterial and antileukemic activity. PLoS One. 2014;9(5):e95281. DOI: https://doi.org/10.1371/journal.pone.0095281
- Amann L.F., Vicente E.R., Rathke M., et al. Stability studies with tigecycline in bacterial growth medium and impact of stabilizing agents. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40(1):215–8. DOI: https://doi.org/10.1007/s10096-020-03970-0
- Zhou H., Sun X., Lyu S., et al. Evaluation of tigecycline utilization and trends in antibacterial resistance from 2018 to 2021 in a comprehensive teaching hospital in China. Infect. Drug Resist. 2023;16:879–89. DOI: https://doi.org/10.2147/idr.s395158
Supplementary files