Monitoring for Clostridioides difficile-associated infection in hospital
- Authors: Gospodarik A.V.1, Prokhorova N.D.1, Kulikova V.K.1, Kalachnuck T.N.1, Khromykh N.I.1, Kudriavtseva J.V.1, Bespyatykh J.A.1
-
Affiliations:
- Lopukhin Federal Research and Clinical Center of Physical-Chemical Medicine
- Issue: Vol 102, No 5 (2025)
- Pages: 605-614
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18779
- DOI: https://doi.org/10.36233/0372-9311-662
- EDN: https://elibrary.ru/WQXHSZ
- ID: 18779
Cite item
Abstract
Introduction. Clostridioides difficile — an anaerobic, spore-forming, Gram-positive bacteria — is a component of the normal intestinal microflora. C. difficile-associated infection develops during its overcolonisation, when vegetative forms produce exotoxins that cause inflammation n the colon wall. Toxigenic strains of C. difficile are the main cause of healthcare-associated infections in hospitals.
The aim of the study is to investigate the frequency of detecting C. difficile (both toxigenic and non-toxigenic strains) in patients admitted to the gastroenterology department of the Yu.M. Lopukhin Federal Scientific Clinical Center for Physical and Chemical Medicine of the Federal Medical Biological Agency of Russia in 2021–2023 with diarrhea syndrome and other established diagnoses.
Materials and methods. The study included 547 patients aged 19–88 years (46.6% male, 53.4% female). Real-time polymerase chain reaction was used to detect C. difficile DNA and its toxin A and B genes, and a bacteriological examination of stool was also performed. Upon detection of clinical signs of bacterial infection, a C-reactive protein (CRP) test was performed.
Results. C. difficile DNA was detected in 65 (11.9%) patients, and toxins A and B genes were found in 32 (5.9%) patients. Non-toxigenic strains were more frequently detected in men (55%) under 40 years, while toxigenic strains were equally frequent in both sexes under 40. CRP analysis indicated that inflammatory processes were more likely in patients over 40 years old. The predominant diagnosis in toxigenic strain carriers with high CRP was C. difficile-associated enterocolitis (ICD-10: A04.7), whereas in the non-toxigenic group, it was ulcerative colitis (ICD-10: K51). Extended bacteriological analysis revealed significant gut microbiota imbalances in all patients.
Conclusion. Over the three-year surveillance period, the prevalence of C. difficile-positive patients increased from 6.6 to 7.9%, and the proportion of samples positive for toxin A/B genes rose from 5.2% to 7.9%. These findings underscore the necessity for enhanced preventive measures to mitigate risk factors for CDI in hospital settings. Therefore, preventive measures are necessary to reduce the impact of risk factors for the development of C. difficile-associated infection in hospitals.
Full Text
Introduction
Ensuring a safe environment for patients and healthcare workers and maintaining quality control of medical care are top priorities for healfcare institutions. Reducing morbidity and mortality associated with nosocomial infections is of particular importance. A key factor in mitigating the risk of healthcare-associated infections (HAIs) is establishing an effective epidemiological surveillance system. Such a system enables the timely detection of infectious diseases in both patients and healthcare workers, thereby improving the safety and quality of medical care. Furthermore, preventive measures aimed at reducing HAIs can help mitigate social risks and the likelihood of insurance claims for patients and healthcare workers1.
The causative agents of HAIs are diverse, including microorganisms, protozoa, helminths, viruses, and prions. Among them, toxigenic strains of Clostridioides difficile are the most common. Numerous factors contribute to the development of C. difficile infection, the primary one being the use of antibacterial therapy and the associated alteration of gastrointestinal microflora. The highest risk is associated with antibacterial drugs from the penicillin, cephalosporin, fluoroquinolone, and clindamycin groups. Other risk factors include age over 65, theuse of proton pump inhibitors in treatment regimens, and comorbidities. The transmission mechanism for C. difficile-associated infection is fecal-oral, occurring through contact with contaminated hands of healthcare personnel, used medical equipment, and surfaces in hospital rooms. C. difficile is part of the normal intestinal microflora, and carriers of its spores can be both patients and healthcare workers. The emergence of vegetative forms can be associated with individual host factors, disruption of the intestinal microflora composition, its stability, and a decrease in the abundance of Firmicutes and Bacteroides.
The clinical manifestation of C. difficile infection is due to the pathogenic properties of the vegetative forms, which can produce exotoxins: enterotoxin A, cytotoxin B and binary toxin CDT. Certain strains produce both toxins A and B, others only toxin A or binary toxin, and less commonly, only toxin B. The presence of a high titer of antitoxic antibodies and colonization resistance of the normal intestinal microflora can result in clinically asymptomatic forms of the disease.
Etiological identification of C. difficile-associated infection is relevant in the presence of clinical forms of the disease. Laboratory diagnosis should employ multiple methods to improve accuracy. In Russia, according to clinical guidelines2, and considering the ACG clinical guidelines [1], a three-stage algorithm for laboratory confirmation is recommended for diagnosing C. difficile infection:
- Isolation of a toxigenic C. difficile using bacteriological methods and determination its susceptibility to antibacterial drugs.
- Detection of glutamate dehydrogenase in fecal samples using serological methods (immunochromatographic assay, enzyme-linked immunosorbent assay) or polymerase chain reaction (PCR).
- Detection of toxins A and B in fecal samples using serological methods and PCR [2].
The gold standard for establishing disease etiology is the bacteriological method of isolating a pure culture and determining its cytotoxicity on cell culture. However, assesing toxin production by isolated C. difficile strains via cell culture assay is burdensome for practical laboratories in medical organizations, despite its high sensitivity and specificity. Furthermore, the isolation of a pure C. difficile culture from patients with signs of acute intestinal infection is not routinely performed and is not included in the standards of medical care for intestinal infections of unknown etiology.This significantly hinders timely diagnosis when diarrheal syndrome occurs in a hospital setting within 48 hours of admission or when a clinical episode of diarrhea is linked to a hospital stay within the previous 4 weeks. Such cases should be considered healthcare-associated infections (HAIs) regardless of whether the infection is exogenous or endogenous [3, 4].
Enzyme-linked immunosorbent assay (ELISA) for the detecting C. difficile toxins A and B in fecal samples is the most widely used method in multidisciplinary hospitals in Russia.
Real-time PCR (qPCR) is a highly sensitive and prioritized method due to its rapid turnaround time. It is used in medical organizations with PCR capabilities for the simultaneous detection and identification of C. difficile DNA and genes for toxin A and B in biological material (feces). Combining PCR with other methods can increase the predictive value of a positive result [5].
The aim of the study was to investigate the frequency of C. difficile detection (both toxigenic and non-toxigenic strains) in patients admitted to the gastroenterology department of a multidisciplinary hospital.
Materials and methods
A retrospective observational, open, descriptive study was conducted in the gastroenterology department of the Y.M. Lopukhin Federal Scientific Clinical Center of Physical and Chemical Medicine from January 1, 2021 to December 31, 2023.
Inclusion criteria were: male and female patients aged 18–89 years hospitalized in the gastroenterology department, presenting with clinical signs of diarrhea syndrome, and diagnosed with various conditions including irritable bowel syndrome (ICD-10 code: K58), inflammatory bowel diseases (ulcerative colitis (K51), Crohn's disease (K50)), C. difficile-associated enterocolitis (A04.7), pseudomembranous colitis (K52.8) and megacolon (K59.3).
Exclusion criteria were: patients with diarrhea without a confirmed diagnosis; the presence of mental or behavioral disorders preventing proper history taking; immunodeficiency conditions ( HIV, bone marrow or organ transplantation, primary immunodeficiency); a concomitant illness requiring hospitalization/surgery or considered life-threatening within 30 days prior to or at enrollment, as assessed by the investigator; and a history of severe alcohol, drug, or narcotic dependence.
The study was conducted with voluntary informed written consent from all patients. The study protocol was approved by the local ethics committee of the Y.M. Lopukhin Federal Scientific Clinical Center of Physical and Chemical Medicine of the Federal Medical Biological Agency of Russia (protocol No. 2022/05/31 dated May 31, 2022).
All included patients underwent a bacteriological stool examination and PCR detection of C. difficile DNA and the genes encoding toxins A and B (tcdA, tcdB). Patients with clinical signs of bacterial intestinal infection (persistent fever > 3 days despite antipyretic, leukocytosis with neutrophilic shift presence of pathological impurities in the stool — blood, mucus) also had blood test for C-reactive protein (CRP).
Stool analysis for C. difficile DNA and detection of toxin A and B genes
Stool samples for C. difficile testing were collected within the first 24 hours of admission to ensure standardization. DNA extraction from feces was performed using the NK-SORBENT nucleic acid isolation kit (NPF Lytech). Extracted DNA was stored at -20°C prior to PCR.
Detection and differentiation of C. difficile DNA and toxins A and B genes were performed using the Fluoroplex Clostridioides difficile + Toxin A + Toxin B reagent kit (NPF Lytech) via real-time PCR on a CFX96 thermocycler (Bio-Rad Laboratories). An internal exogenous control (detected in the HEX channel) was included to monitor DNA extraction quality and prevent false-negative results. PCR conditions were: 80°C for 2 minutes, 95°C for 1 minute 30 seconds, followed by 40 cycles: 95°C for 15 seconds, 60°C for 30 seconds and 72°C for 40 seconds. A total of 547 DNA samples were tested.
Bacteriological stool culture
Analysis was conducted according to the methodological guidelines "Bacteriological Diagnosis of Dysbacteriosis" (approved on April 14, 1977) and OST 91500.11.0004-2003 "Patient Management Protocol." Intestinal dysbacteriosis. One gram of the native stool sample was homogenized in 9 mL of physiological saline (1/10) and left at room temperature for 10–15 minutes. From the original dilution (101), a series of subsequent dilutions were prepared up to 108. The resulting suspension was inoculated onto culture media for cultivating various groups of microorganisms.
Bacteriological analysis was evaluated:
- after 20–22 hours on Endo, blood agar, SS agar and XLD agar;
- after 48 hours on Sabouraud's medium, yolk salt agar and milk-inhibitory;
- after 72 hours on Blaurock, MRS-2 and iron sulfite agar.
Statistical analysis
The sample size was not calculated a priori. Statistical analysis was perfomedusing SPSS Statistics v. 27 (IBM). To analyze the prevalence assessment of toxigenic and non-toxigenic strains, indicators such as the incidence rate and the structure of morbidity (proportion) were used. Quantitative data were assessed for normality. Frequency data within groups are presented as percentage. Descriptive statistics (absolute values, percentages) were used. Given the descriptive nature of the analysis of trends from 2021–2023, the statistical significance of differences was not calculated.
Results
Research subjects (participants)
The study included 547 individuals aged 19–88 years, comprising 255 (46.6%) men and 292 (53.4%) women. The average hospital stay was 9.44 days.
Main research findings
Real-time PCR detected C. difficile DNA in 65 patients (34 male, 31 female), representing 11.9% of the sample. Toxin A and B genes were found in 32 (5.9%) patients. Non-toxigenic C. difficile strains were predominantly found in men (55%) under 40 years old. Toxigenic strains were found with equal frequency in men and women under 40.Analysis from 2021 to 2023 showed an increase in patients with C. difficile from 6.6% to 7.9%. The detection rate of toxigenic C. difficile strains also increased from 5.2% to 7.9% (Table).
Detection rate of toxigenic and non-toxigenic C. difficile strains in patients during the observation period 2021–2023 (n=547)
Analysis | 2021 | 2022 | 2023 | Total patients | |||
number of patients | % | number of patients | % | number of patients | % | ||
Non-toxigenic strains of C. difficile have been identified | 14 | 6.6 | 6* | 3.5 | 13 | 7.9 | 33 |
Toxigenic strains of C. difficile (toxins A and B) were identified | 11* | 5.2 | 8 | 4.7 | 13 | 7.9 | 32 |
C. difficile strains were not identified | 186 | 88.2 | 157 | 91.8 | 139 | 84.2 | 482 |
Total patients | 211 | 171 | 165 | 547 | |||
Note. *One patient was hospitalized twice: the first time in 2021, then independently a second time in 2022.
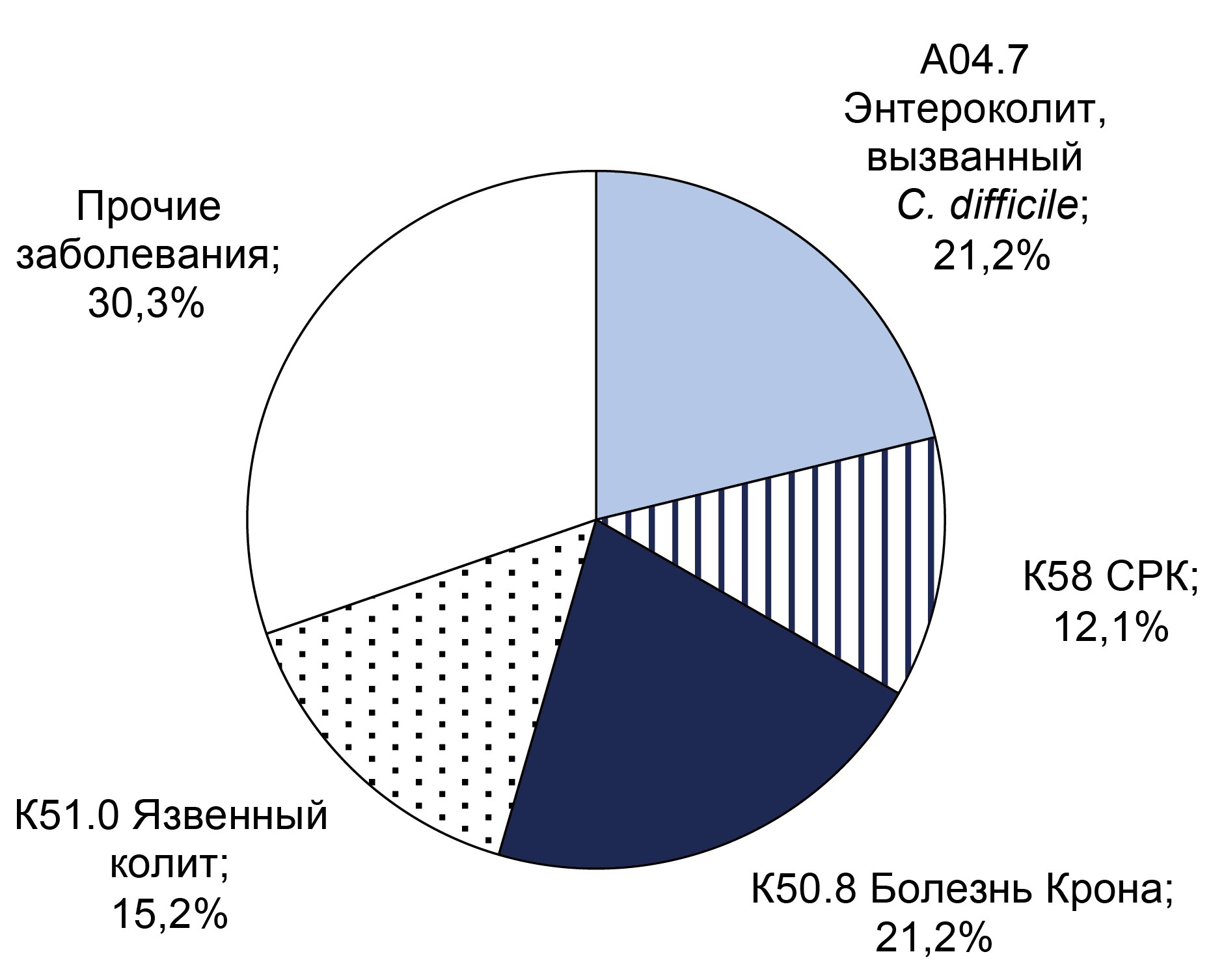
The frequency of various diseases among patients with confirmed C. difficile infection is shown in the Figure.
Distribution of confirmed diagnoses in patients with diagnosed non-toxigenic (a) and toxigenic (b) C. difficile strains.
Non-toxigenic C. difficile strains (n = 33) were identified in patients with C. difficile-associated enterocolitis (7, 21.2%), Crohn's disease (5,15.2%), ulcerative colitis (5, 15.2%), and irritable bowel syndrome (4, 12.1%). In 10 (30.3%) cases, non-toxigenic strains were detected in patients with gastrointestinal diseases (e.g., chronic pancreatitis, steatohepatitis, stomach ulcers).
Toxigenic C. difficile strains (n = 32) were detected in patients diagnosed with: C. difficile-associated enterocolitis (17, 53.1%), Crohn's disease (5, 15.6%), ulcerative colitis (4,12.5%), and other ulcerative lesions in the large intestine (6,18.8%).
Blood biochemistry parameters
CRP were measured in 49 of the 65 patients with detected C. difficile strains. CRP levels were elevated (>5 mg/L) in 22 patients (group with toxigenic C. difficile strains — 11 people, including 8 men and 3 women; group with non-toxigenic C. difficile strains — 11 people, including 6 men and 5 women), ranging from 6.53 to 240.9 mg/L. High CRP levels were more common in male patients. Among men with toxigenic strains, the predominant diagnosis (5 out of 11, 45%) was C. difficile-associated enterocolitis (all over 40). In the non-toxigenic group, the largest proportion of men (4 out of 11, 36.4%) had ulcerative colitis (aged 40–60). These results suggest a higher likelihood of inflammatory processes in patients over 40 with various forms of colitis.
Bacteriological stool analysis
A comprehensive bacteriological analysis for intestinal dysbiosis was performed on 36 (55.4%) patients with positive PCR for C. difficile based on clinical indications. All exhibited significant disturbances in intestinal microflora (normoflora): reduction in obligate anaerobes (bifidobacteria and lactobacilli were significantly reduced in some cases), a shift in the ratio of lactose-positive and lactose-negative Escherichia coli toward the latter, the appearance of hemolytic Escherichia coli, and a decrease in the total number of enterococci. Contamination with sulfite-reducing Clostridium spp. above normal (≤ 106) was detected in 20 (55.6%) patients. In 18 (50%) patients, increased colonization with opportunistic microflora was detected, including Proteus spp., Klebsiella spp., Citrobacter spp., Enterobacter spp., Acinetobacter spp., and Staphylococcus aureus. At the same time, increased presence of Candida fungi was detected in 13 (36%) patients, indicating imbalances in the intestinal microflora, caused in part by antibiotic therapy.
Treatment of C. difficile-associated infection
Among the 65 PCR-positive patients, 24 (36.9%) received first-line antibiotic therapy with oral vancomycin (1 g/day for 10–14 days). This group included 18 patients with toxigenic strains and 6 with non-toxigenic strains.
For recurrent C. difficile infection, 6 patients (4 women, 2 men under 60 years old) diagnosed with C. difficile-associated enterocolitis underwent fecal microbiota transplantation (FMT) into the terminal ileum via an endoscopic instrument channel.
Recurrences (relapses of the disease)
Re-hospitalization within the 2021-2023 period for patients with previously detected C. difficile DNA was recorded in 9 (13.8%; 6 women, 3 men) of the 65 patients. Initial detection was non-toxigenic strains in 5 patients and toxigenic in 4. Re-hospitalization occurred in patients with Crohn's disease (3 non-toxigenic, 1 toxigenic), ulcerative colitis (2 non-toxigenic, 2 toxigenic), and enterocolitis from a toxigenic strain (1 patient).
Discussion
Summary of Research Findings
Including gastroenterology patients with diarrhea syndrome allowed us to cohort with signs of active C. difficile infection. This approach aligns with international hospital testing practices for C. difficile infection. According to ACG guidelines, testing should be considered for individuals with ≥3 loose stools in 24 hours, those with high comorbidity indices (oncology, hematology, transplant patients), and patients with signs of intestinal obstruction [6, 7]. Our results indicate an increase in C. difficile infections in the gastroenterology department from 2021 (6.6%) to 2023 (7.9%), including an increase in toxin-positive from 5.2% to 7.9%. This rise may be linked to the COVID-19 pandemic. T. Zuo et al. concluded that COVID-19 adversely affects gut microbiota, reducing colonization resistance against C. difficile [8].
The inclusion of C. difficile in the ESCAPE pathogen group and its ability to form hospital strains (clones) is concerning. Besides primary C. difficile diseases (enterocolitis, pseudomembranous colitis, megacolon), superinfection can complicate other conditions like Crohn's disease and ulcerative colitis [9]. Therefore, epidemiological and microbiological monitoring for C. difficile in gastroenterology department, both in symptomatic patients and those with risk factors is highly relevant. The development of C. difficile infection in patients can be associated with endogenous causes (the development of clinical signs of the disease in asymptomatic carriers due to excessive colonization with C. difficile and toxin production), as well as with medical care [10]. M. Sartelli et al. report an increase in community-acquired cases over the past 20 years (up to 25–28% of C. difficile-associated infections) [11].
Identification of toxigenic and non-toxigenic strains
As part of the normal gut flora, C. difficile may not cause disease. In this study, since all patients had diarrhea, the identification of only non-toxigenic C. difficile strains (without toxin genes) was interpreted as the diarrheal episode likely not being caused by C. difficile, but rather by other factors (other infectious agents, exacerbation of the underlying disease, drug effects, etc.). While detection of non-toxigenic strains is not always associated with the presence of an infectious disease, but there is evidence of asymptomatic carriage of C. difficile as a predisposing factor for the development of clinically manifest C. difficile infection [12]. Geographic variation in asymptomatic C. difficile carriage rates among hospitalized adults exists, ranging from 4.4% to 23.2% [13]. Detection of toxigenic C. difficile strains upon admission is associated with an almost 6-fold higher risk of developing clinical infection. The detection rate of toxigenic C. difficile strains in hospitalized patients in a multicenter study by I.M. Zacharioudakis et al. was 8.1% [14]. In our study, the rate in the gastroenterology department in 2023 was 7.9%.
CRP level test
Using standard laboratory tests like CRP, a systemic inflammatory response marker, in diagnosing C. difficile infection and assessing severity helps identify patients at risk for severe disease and poor prognosis.
Bacteriological stool analysis
This study involved patients with an adult gut microbiome, characterized by diversity, stability, and resilience. Significant reductions in diversity and stability along with decreased abundance of obligate members (Firmicutes, Bacteroidetes, Actinobacteria), have been reported in C. difficile infection [15, 16]. A predominance of Proteobacteria (especially Enterobacteriaceae) is also noted. Our bacteriological findings confirmed intestinal dysbiosis, characterized by an imbalance with a predominance of opportunistic pathogens like Proteus spp., Klebsiella spp., Citrobacter spp., Enterobacter spp., Acinetobacter spp. and Staphylococcus aureus.
Treatment of C. difficile-associated infection in the hospital
The choice of anticlostridial therapy according to Russian clinical guidelines depends on disease severity. For a first mild episode in patients with a favorable prognosis (young age, no immunodeficiency/chronic diseases), metronidazole (500 mg three times daily for 10 days.) can be used. For other patient groups with a first mild episode of C. difficile infection, vancomycin (250 mg four times daily for 10 days) is recommended.
FMT currently has a low evidence level and weak recommendation, but is suggested for severe/ fulminant C. difficile infection refractory to standard antibiotic therapy. Observational study results support the use of FMT protocols [17]. For example, if severe/fulminant infection is refractory to vancomycin combined with IV metronidazole after 5 days, FMT via colonoscopy is indicated. Results from a randomized clinical trial by G. Ianiro et al. show effective use of this algorithm combined with a 14-day vancomycin course, achieving cure rates of 75% after one FMT and 100% after multiple procedures [18]. However, FMT equires access to a bank of frozen fecal microbiota.
In our study, 24 (36.9%) out of 65 patients PCR-positive patients received vancomycin (1 g/day orally). In the absence of positive clinical and laboratory dynamics, FMT was performed on 6 patients for the treatment of recurrent C. difficile infection. Clinical effect was achieved in all patients, and there were no readmissions to the inpatient department. Our data confirm FMT as a highly promising method for treating C. difficile infections, consistent with the literature [19–21].
Conclusion
The identification of C. difficile infection across various patients group underscores the need to test all patients with diarrhea syndrome for C. difficile. To improve diagnostic accuracy and establish a definitive etiology, a combination of identification methods should be employed. PCR, being highly sensitive and rapid, is currently the preferred standalone method for diagnosing C. difficile infection. Incorporating PCR into clinical guidelines and quality criteria for managing C. difficile infection will facilitate timely diagnosis, appropriate selection of anticlostridial therapy, and reduce the overall incidence and mortality from HAIs.
A separate issue for epidemiological and microbiological monitoring in medical organizations is screening healthcare workers for asymptomatic carriage or latent infection, as well as testing environmental surfaces (diagnostic and treatment equipment, feeding tubes, etc.) for C. difficile contamination. This is particularly relevant for gastroenterology departments and will be a primary focus of our future, more detailed research on the epidemiology and prevention of C. difficile infection in hospitals.
Eliminating risk factors for C. difficile-associated infection, primarily through the rational use of antibiotic, is the main preventive measure. Supporting the host’s dispositional properties, particularly by enhancing the colonization resistance of the colon, is the primary strategy for preventing recurrence.
1 Methodological recommendations "Epidemiological diagnosis of infectious diseases related to the provision of medical care, based on standard definitions of the case." 2024.
URL: https://nasci.confreg.org/libs/files/mr_sos.pdf (data of access: 08.06.2025). (In Russ.)
2 Clinical recommendations "Clostridial infection: diagnosis, treatment and prevention". 2022. URL: https://www.rnmot.ru/public/uploads/RNMOT/clinical/2022/ФИНАЛЬНЫЕ%20КЛИНИЧЕСКИЕ%20РЕКОМЕНДАЦИИ%20Клостридиальная%20инфекция%209112022.pdf (data of access: 08.06.2025). (In Russ.)
About the authors
Alina V. Gospodarik
Lopukhin Federal Research and Clinical Center of Physical-Chemical Medicine
Author for correspondence.
Email: alina.gospodaryk@rcpcm.org
ORCID iD: 0009-0003-7870-1106
Cand. Sci. (Biol.), researcher, Center for molecular medicine and diagnostics
Russian Federation, MoscowNatalya D. Prokhorova
Lopukhin Federal Research and Clinical Center of Physical-Chemical Medicine
Email: yfnecz510@yandex.ru
ORCID iD: 0000-0001-6485-1056
junior researcher, Center for molecular medicine and diagnostics
Russian Federation, MoscowViсtoria K. Kulikova
Lopukhin Federal Research and Clinical Center of Physical-Chemical Medicine
Email: v.k.kulikova@gmail.com
ORCID iD: 0009-0002-7592-9108
laboratory researcher, Center for molecular medicine and diagnostic
Russian Federation, MoscowTatiana N. Kalachnuck
Lopukhin Federal Research and Clinical Center of Physical-Chemical Medicine
Email: ktn-25@mail.ru
ORCID iD: 0009-0002-6953-6818
Cand. Sci. (Med.), Head, Department of gastroenterology
Russian Federation, MoscowNikolay I. Khromykh
Lopukhin Federal Research and Clinical Center of Physical-Chemical Medicine
Email: khromykh.nikolya@mail.ru
ORCID iD: 0009-0001-6806-3548
biologist, Center for molecular medicine and diagnostics
Russian Federation, MoscowJulia V. Kudriavtseva
Lopukhin Federal Research and Clinical Center of Physical-Chemical Medicine
Email: vitolga10@mail.ru
ORCID iD: 0009-0001-6713-5140
laboratory researcher, Center for molecular medicine and diagnostics
Russian Federation, MoscowJulia A. Bespyatykh
Lopukhin Federal Research and Clinical Center of Physical-Chemical Medicine
Email: juliabes@rcpcm.org
ORCID iD: 0000-0002-4408-503X
Cand. Sci. (Biol.), Associate Professor; Head, Laboratory of molecular medicine, Head Center for molecular medicine and diagnostics
Russian Federation, MoscowReferences
- Kelly C.R., Fischer M., Allegretti J.R., et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Сlostridioides difficile infections. Am. J. Gastroenterol. 2021;116(6): 1124–47. DOI: https://doi.org/10.14309/ajg.0000000000001278
- Khuvis J., Alsoubani M., Mae Rodday A., Doron S. The impact of diagnostic stewardship interventions on Clostridiodes difficile test ordering practices and results. Clin. Biochem. 2023;117: 23–9. DOI: https://doi.org/10.1016/j.clinbiochem.2022.03.009
- Solanky D., Juang D.K., Johns S.T., et al. Using diagnostic stewardship to reduce rates, healthcare expenditures and accurately identify cases of hospital-onset Clostridioides difficile infection. Infect. Control Hosp. Epidemiol. 2021;42(1):51–6. DOI: https://doi.org/10.1017/ice.2020.375
- Doll M., Marra A.R., Apisarnthanarak A., et al. Prevention of Clostridioides difficile in hospitals: а position paper of the International Society for Infectious Diseases. Int. J. Infect. Dis. 2021;102:188–95. DOI: https://doi.org/10.1016/j.ijid.2020.10.039
- Johnson S., Lavergne V., Skinner A.M., et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin. Infect. Dis. 2021;73(5):e1029–44. DOI: https://doi.org/10.1093/cid/ciab549
- Kelly C.R., Fischer M., Allegretti J.R., et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am. J. Gastroenterol. 2021;116(6):1124–47. DOI: https://doi.org/10.14309/ajg.0000000000001278
- Захаренко С.М. Clostridioides difficile: название новое, проблемы диагностики и терапии прежние. Альманах клинической медицины. 2022;50(6):377–91. Zakharenko S.M. Clostridioides difficile – a new name, the old problems with diagnosis and treatment. Almanac of Clinical Medicine. 2022;50(6):377–91. DOI: https://doi.org/10.18786/2072-0505-2022-50-048 EDN: https://elibrary.ru/dqvpmx
- Zuo T. et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–55.e8. DOI: https://doi.org/10.1053/j.gastro.2020.05.048
- Zaver H.B., Moktan V.P., Harper E.P., et al. Reduction in health care facility-onset Clostridioides difficile infection: a quality improvement initiative. Mayo Clin. Proc. Innov. Qual. Outcomes. 2021;5(6):1066–74. DOI: https://doi.org/10.1016/j.mayocpiqo.2021.09.004
- Волчкова Е.В., Белоусова Е.А., Макарчук П.А. и др. Частота выявления инфекции Clostridium difficile в больничных условиях. Альманах клинической медицины. 2014;(33):71–6. Volchkova E.V., Belousova E.A., Makarchuk P.A., et al. Frequency of detection of Clostridium difficile infection in hospital conditions. Almanac of Clinical Medicine. 2014;(33):71–6. EDN: https://elibrary.ru/sxyjwn
- Sartelli M., Di Bella S., McFarland L.V., et al. 2019 update of the WSES guidelines for management of Clostridioides (Clostridium) difficile infection in surgical patients. World J. Emerg. Surg. 2019;14:8. DOI: https://doi.org/10.1186/s13017-019-0228-3
- Solbach P., Chhatwal P., Woltemate S., et al. Microbiota-associated risk factors for Clostridioides difficile acquisition in hospitalized patients: a prospective, multicentric study. Clin. Infect. Dis. 2021;73(9):e2625–34. DOI: https://doi.org/10.1093/cid/ciaa871
- Hung Y.P., Lee J.C., Lin H.J., et al. Clinical impact of Clostridium difficile colonization. J. Microbiol. Immunol. Infect. 2015;48(3):241–8. DOI: https://doi.org/10.1016/j.jmii.2014.04.011
- Zacharioudakis I.M., Zervou F.N., Pliakos E.E., et al. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am. J. Gastroenterol. 2015;110(3):381–90. DOI: https://doi.org/10.1038/ajg.2015.22
- Amrane S., Hocquart M., Afouda P., et al. Metagenomic and culturomic analysis of gut microbiota dysbiosis during Clostridium difficile infection. Sci. Rep. 2019;9(1):12807. DOI: https://doi.org/10.1038/s41598-019-49189-8
- Magne F., Gotteland M., Gauthier L., et al. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12(5):1474. DOI: https://doi.org/10.3390/nu12051474
- Fischer M., Kao D., Kassam Z., et al. Stool donor body mass index does not affect recipient weight after a single fecal microbiota transplantation for Clostridium difficile infection. Clin. Gastroenterol. Hepatol. 2018;16(8):1351–3. DOI: https://doi.org/10.1016/j.cgh.2017.12.007
- Ianiro G., Masucci L., Quaranta G., et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy plus vancomycin for the treatment of severe refractory Clostridium difficile infection-single versus multiple infusions. Aliment. Pharmacol. Ther. 2018;48(2):152–9. DOI: https://doi.org/10.1111/apt.14816
- Акиньшина А.И., Смирнова Д.В., Загайнова А.В. и др. Перспективы использования методов коррекции микробиоты при терапии воспалительных заболеваний кишечника. Российский журнал гастроэнтерологии, гепатологии, колопроктологии. 2019;29(2):12–22. Akinshina A.I., Smirnova D.V., Zagainova A.V., et al. Prospects of using microbiota correction methods in the treatment of inflammatory bowel disease. Russian Journal of Gastroenterology, Hepatology, Coloproctology. 2019;29(2):12–22. DOI: https://doi.org/10.22416/1382-4376-2019-29-2-12-22 EDN: https://elibrary.ru/zgxuip
- Щербаков П.Л., Белова Н.Д., Генерозов Э.В. и др. Применение фекальной трансплантации в лечении заболеваний пищеварительного тракта (первый клинический опыт). Доктор.Ру. 2019;(3):40–6. Scherbakov P.L., Belova N.D., Generozov E.V., et al. Faecal transplant in git treatment (pilot clinical experience). Doctor.Ru. 2019;(3):40–6. DOI: https://doi.org/10.31550/1727-2378-2019-158-3-40-46 EDN: https://elibrary.ru/bsrtsj
- Woodworth M.H., Carpentieri C., Sitchenko K.L., Kraft C.S. Challenges in fecal donor selection and screening for fecal microbiota transplantation: a review. Gut Microbes. 2017;8(3): 225–37. DOI: https://doi.org/10.1080/19490976.2017.1286006
Supplementary files