Antimicrobial resistance of Streptococcus pneumoniae strains isolated from children following immunization with a 13-valent conjugated vaccine
- Authors: Protasova I.N.1, Feldblium I.V.2, Bakhareva N.V.3, Zinovieva L.V.4, Kulik E.V.5
-
Affiliations:
- Professor V.F. Voino-Yasenetsky Krasnoyarsk State Medical University
- Perm State Medical University named after Academician E.A. Wagner
- Krasnoyarsk Regional Center for Prevention and Control of AIDS
- Krasnoyarsk City Children's Hospital No. 8
- Krasnoyarsk City Children's Clinic No. 2
- Issue: Vol 102, No 4 (2025)
- Pages: 445-455
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18777
- DOI: https://doi.org/10.36233/0372-9311-660
- EDN: https://elibrary.ru/UILZON
- ID: 18777
Cite item
Abstract
Introduction. Monitoring the resistance of Streptococcus pneumoniae to antimicrobials is an important component of epidemiological surveillance of pneumococcal infection, addressing the challenges of improving therapeutic approaches and containing the spread of resistance at regional and national levels.
The aim of the study is to investigate pneumococcal antibiotic resistance in preschool children following immunization with a 13-valent conjugated vaccine within the national immunization schedule.
Materials and methods. From 2016 to 2022, 1250 healthy children under the age of 6 from organized groups were examined. Detection of nasopharyngeal carriage of pneumococcus was performed using the culture method, and serotype was determined by polymerase chain reaction. Antimicrobial susceptibility was investigated using the disk diffusion method, and the minimum inhibitory concentration of antibiotics in resistant isolates was determined using E-tests.
Results. 265 isolates of S. pneumoniae were studied, and resistance to antimicrobials was found in 36.6%. The proportion of resistant isolates was 33.8% in the early post-vaccination period (2016–2018) and 45.3% in the late period (2020–2022) (p = 0.097). Penicillin-resistant pneumococci were detected only in the early post-vaccination period, with a frequency of 11.4% (p = 0.005). The prevalence of macrolide-resistant isolates in the analyzed periods was 27.9% and 35.9% (p = 0.219), and those resistant to 3 or more classes of antibiotics were 23.4% and 17.2%, respectively (p = 0.297). The serotype composition of resistant S. pneumoniae has changed: vaccine serotypes 19F, 6A, 6B, etc. (83.8%) have been replaced by non-vaccine serotypes – 15AF, 23A, etc. The proportion of vaccine serotypes in the late post-vaccination period was only 10.3%.
Conclusion. Against the backdrop of child immunization against pneumococcal infection, there has been a decrease in the prevalence of pneumococci with reduced susceptibility to β-lactams. S. pneumoniae resistance to macrolides remains high (35.9%). Resistant pneumococcal isolates primarily belong to serogroup 15 (15AF) and serotype 23A, which are not included in the 13-valent conjugate vaccine.
Full Text
Introduction
Streptococcus pneumoniae is a conditionally pathogenic microorganism that often colonizes the upper respiratory tract and is the pathogen of a wide range of diseases, including otitis media, sinusitis, purulent bacterial meningitis and community-acquired pneumonia. Over the past two decades, vaccination against pneumococcal infection has been implemented in many countries as part of national programs [1–4]. In Russia, immunization of children and adults in risk groups for infection has been carried out since 2014 in accordance with the National Immunization Schedule [5]. In the context of ongoing specific prevention of pneumococcal infections, research interest is focused not only on changes in the serotype and clonal landscapes of the microbial population under the influence of conjugate vaccines, but also on the dynamics of S. pneumoniae resistance to antimicrobials.
Pneumococcal resistance to antibiotics is characterized by a growing trend and is considered a serious threat to public health and healthcare in many countries [6]. Macrolide-resistant pneumococci are currently on the World Health Organization's list of priority bacterial pathogens (medium priority level)1. Previously, S. pneumoniae non-susceptible to penicillin were included in this list2.
The incorporation of pneumococcal conjugate vaccines (PCV) into routine childhood immunization programs has led to a decrease in the proportion of infections caused by resistant pneumococci in many countries. This occurred due to the partial or complete elimination of S. pneumoniae serotypes belonging to the vaccine variants [1, 4–6]. However, in the future, against the backdrop of the ongoing serotype replacement, an increase in the proportion of resistant pneumococci belonging to non-vaccine variants of the pathogen has been noted in a number of countries. The prevalence of isolates resistant to macrolides, tetracycline, and in some countries, penicillin has sharply increased [6–8]. The serotype landscape and the prevalence of resistant S. pneumoniae serotypes vary significantly not only between continents and countries, but also within a single country, depending on vaccination rates, antibiotic consumption levels, and other factors [7]. In this regard, dynamic assessment of the serotype landscape and S. pneumoniae antimicrobial resistance, conducted both in the early and late post-vaccination periods, is one of the most important components of epidemiological surveillance of pneumococcal infections, serving as the basis for determining the strategy and tactics of specific prevention and empirical therapy for pneumococcal diseases [9]. Microbiological monitoring is particularly important in the preschool age group, where S. pneumoniae carriage occurs with a frequency of 24.4–54.3% [10–12], and the level, structure of resistance, as well as its molecular mechanisms are currently insufficiently studied.
Based on the above, the aim of our study was a comparative analysis of the structure and mechanisms of pneumococcal resistance to antimicrobials in dynamics – in the early (2016–2018) and late (2020–2022) post-vaccination periods in preschool children.
Materials and methods
1250 healthy children from organized children's groups in Krasnoyarsk were examined. The inclusion criteria were the child's regular attendance at an organized children's group (kindergarten), age 0–6 years inclusive, the presence of informed consent signed by a parent or legal guardian, and the absence of signs of infectious diseases at the time of the examination; the exclusion criterion was the presence of signs of an acute infectious disease at the time of the examination. The vaccination status was assessed based on medical documentation (form No. 112/u).
The average age of the examined children was 4.12 ± 0.97 years. Among those surveyed in 2016–2018, only 1.9% of children completed the full course of immunization (three doses, according to the national immunization schedule), 33.9% were partially vaccinated (received 1 or 2 doses of the vaccine), and 64.2% were not vaccinated. Among those surveyed in 2020–2022, those who received the full course of immunization already accounted for 68.8%, those who received 1 or 2 doses of the vaccine accounted for 26.4%, and those not vaccinated against pneumococcal infections accounted for 4.8%.
All children included in the study had nasopharyngeal swabs taken once using flocked swabs with the liquid Amies transport medium. In all cases, informed consent was obtained from the parents or legal guardians of the children included in the study. S. pneumoniae was cultured on Columbia agar with 5% sheep blood, nalidixic acid and colistin (ready-made agar produced by LLC Sredoff) under capnophilic conditions using Campilogaz gas generator sachets. Pneumococcal identification was performed based on colony morphology, optochin and bile tests, as well as PCR detection of the cpsA and lytA genes3. Serotyping was performed using multiplex PCR4. Cryopreservation of the isolated cultures was performed using Cryoinstant mix colores (Deltalab), stored at –80°C.
To determine the susceptibility of the isolates to antimicrobials, the obtained S. pneumoniae cultures were tested with 8 drugs: oxacillin (1 μg), erythromycin (15 μg), clindamycin (2 μg), tetracycline (30 μg), norfloxacin (10 μg), vancomycin (5 μg), linezolid (10 μg), and rifampicin (5 μg) using the disk diffusion method on Mueller–Hinton 2 agar (bioMerieux) supplemented with 5% defibrinated horse blood and 20 mg/L β-NAD. Bio-Rad disks were used for this process. S. pneumoniae strain ATCC 49619 was used as a control. The interpretation of the results was performed in accordance with the Russian guidelines "Determination of the Susceptibility of Microorganisms to Antimicrobial Drugs" (2025 version).
To exclude β-lactam resistance mechanisms, screening with a 1 μg oxacillin disk was performed. If a zone of growth inhibition around the oxacillin disk was greater than 20 mm, the isolate was considered susceptible to all β-lactams. If the zone diameter was less than 20 mm, the isolate was considered resistant to phenoxymethylpenicillin. The diameter of the zone, 9–19 mm, indicated susceptibility to ampicillin, amoxicillin, piperacillin, cefepime, cefotaxime, ceftaroline, ceftobiprole, ceftriaxone, imipenem and meropenem. For inhibition zone diameters less than 9 mm, the minimum inhibitory concentration (MIC) of all β-lactam antibiotics was required. Accordingly, the MIC of benzylpenicillin was determined for all isolates with a zone diameter of less than 20 mm, and for cultures with a zone diameter of less than 9 mm, the MIC of amoxicillin, ceftriaxone, and imipenem was also determined. E-tests (bioMerieux) were used in this study5. Isolates with an MIC greater than 1 mg/L for penicillin and amoxicillin, and greater than 2 mg/L for ceftriaxone and imipenem (according to criteria for infections other than endocarditis and meningitis) were considered resistant.
An erythromycin disk (15 μg) was used to screen for macrolide resistance: isolates susceptible to it were considered susceptible to azithromycin, clarithromycin, and roxithromycin. Erythromycin-resistant isolates were considered macrolide-resistant. To detect inducible resistance to clindamycin, erythromycin and clindamycin disks were placed 12–16 mm apart, and the result was considered positive if a D-shaped zone of growth inhibition of the tested culture was present.
Pneumococci susceptible to tetracycline were considered susceptible to doxycycline and minocycline; if tetracycline resistance was detected, they were considered resistant to these antibiotics.
A 10 μg norfloxacin disk diffusion test was used to screen for resistance to fluoroquinolones. S. pneumoniae isolates susceptible to norfloxacin were considered susceptible to moxifloxacin and susceptible at increased exposure to levofloxacin.
Upon identifying resistance to antimicrobials in the isolate using phenotypic methods, resistance genes were detected. Erythromycin-resistant pneumococcal isolates were tested for the presence of the ermB and mef genes, which are responsible for resistance to macrolides, lincosamides, and streptogramin B, according to the method described by R.R. Reinert et al. [13]. The tetM gene was identified in tetracycline-resistant isolates [14]. At a benzylpenicillin MIC level exceeding 0.064 mg/L, S. pneumoniae isolates were tested for mutations in the genes encoding penicillin-binding proteins (PBP): pbp1a, pbp2x and pbp2b [15].
Statistical processing of the obtained results was performed using the Statistica v. 10.0.1011 software package. Qualitative characteristics were calculated as proportions (%), and quantitative characteristics were calculated as means and standard deviations. The distribution of the features was assessed using the Shapiro–Wilk test. If the normality of the distribution was confirmed, the Student's t-test was used for group comparisons; if not, the Mann–Whitney test (for quantitative traits) or χ2 (for qualitative traits) was used. The level of statistical significance for the differences was considered to be p < 0.05 in all cases.
Results
During the study, 265 pneumococcal isolates were obtained. At the same time, between 2016 and 2018, resistance to one or more antibiotics was detected in 33.8% of the S. pneumoniae isolates (in 68 out of 201), while between 2020 and 2022, it was found in 45.3% (in 29 out of 64). The proportion of resistant pneumococci increased by 11.5% over 7 years (χ2 = 2.76; p = 0.097).
Most of the resistant isolates obtained in the early post-vaccination period were isolated from children who had not been vaccinated against pneumococcal infections, while in the late post-vaccination period, the majority of S. pneumoniae cultures were isolated from fully vaccinated children (Table 1).
Table 1. The structure of resistant nasopharyngeal isolates of S. pneumoniae depending on the vaccination status of the examined children
Isolates | Early post-vaccination period (2016–2018; n = 68) | Late post-vaccination period (2020–2022; n = 29) | Significance of differences |
From fully vaccinated children, n (%) | 5 (7.35) | 22 (75.86) | χ2 = 47.50; p = 0.000 |
From partially vaccinated children, n (%) | 10 (14.71) | 4 (13.79) | χ2 = 0.01; p = 0.907 |
From unvaccinated children, n (%) | 53 (77.94) | 3 (10.34) | χ2 = 34.55; p = 0.000 |
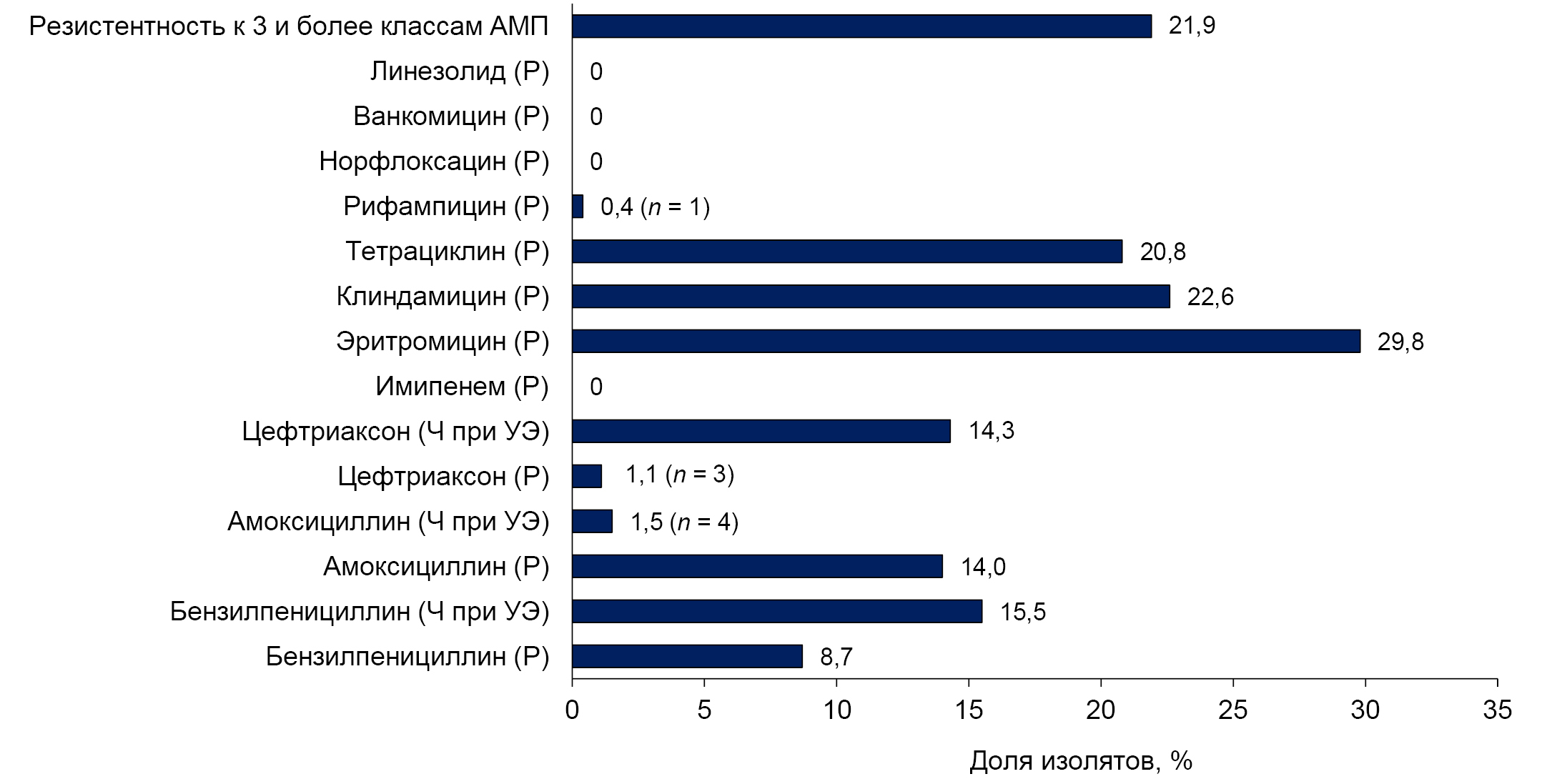
Over the entire analyzed period (2016–2022), 83 isolates were obtained with a growth inhibition zone diameter around the oxacillin disk of less than 20 mm, of which 34.9% had a diameter of 9–19 mm. The MIC range of benzylpenicillin for these pneumococci was 0.016–0.750 mg/L, with an MIC50 level of 0.064 mg/L and an MIC90 of 0.19 mg/L. For isolates with a growth inhibition zone diameter of 0–8 mm (n = 54; 65.1%), the benzylpenicillin MIC50 was 1 mg/L, the MIC90 was 2 mg/L, with an MIC range of 0.094–8 mg/L. Thus, the proportion of penicillin-resistant isolates (according to criteria for infections other than endocarditis and meningitis) during the analyzed period was 8.7% (23 out of 265); and the proportion of those susceptible to penicillin with increased exposure was 15.5% (41 out of 265) (Figs. 1, 2).
Fig. 1. MIC of benzylpenicillin in S. pneumoniae isolates that screened positive with 1 μg of oxacillin (n = 83).
Fig. 2. Prevalence of antimicrobial resistant S. pneumoniae isolates.
Here and in Fig. 3: R — resistant; S — susceptible at normal dosing; I — susceptible at increased exposure.
When genotyping S. pneumoniae cultures resistant and susceptible to increased penicillin exposure, mutations in the genes encoding PBP were identified in 100% of cases. Mutations in the PBP genes were detected in 11.4% of penicillin-susceptible pneumococci and were absent in 88.6%.
Erythromycin resistance was detected in 29.8% (79 out of 265) of the isolates (Fig. 2), with macrolide resistance genes present in 98.7%. In 41.8% of cases, only the ermB gene, which encodes 23S rRNA methylation, was detected, and in 20.3%, only the macrolide efflux mef genes were found. The combination of these genes was detected in 38% of the isolates. Macrolide resistance genes were not detected in erythromycin-susceptible pneumococci.
S. pneumoniae resistant to clindamycin accounted for 22.6% (60 out of 265), and 3 (1.1%) isolates exhibited inducible resistance. In 61.7% of cases, the ermB gene was found in clindamycin-resistant isolates, and in 38.3%, a combination of the ermB and mef genes.
One-fifth — 20.8% (55 out of 265) of the S. pneumoniae isolates were found to be resistant to tetracycline (Fig. 2). The ribosomal protection tetM gene was present in 98.2% of these isolates and was not detected in tetracycline-susceptible pneumococci.
S. pneumoniae resistant to 3 or more classes of antibiotics were found in 21.9% of cases (Fig. 2).
Rifampicin resistance was detected in only 1 case. All isolates tested were susceptible to fluoroquinolones, vancomycin and linezolid.
Differences were identified in the susceptibility of S. pneumoniae to β-lactams depending on the observation period. In the early post-vaccination period, 23 isolates were penicillin-resistant, 37 were amoxicillin-resistant, and 3 isolates were resistant to ceftriaxone (according to criteria for infections other than endocarditis and meningitis) (Fig. 3). The proportions of isolates susceptible to penicillin, amoxicillin, and ceftriaxone at increased exposure were 12.9%, 2.0% and 18.9%, respectively.
Fig. 3. Prevalence of antimicrobial resistant S. pneumoniae isolates.
*Statistically significant differences.
In the late post-vaccination period, isolates resistant to penicillin, amoxicillin and ceftriaxone were not detected (χ2 = 8.02; p = 0.005; χ2 = 13.69, p = 0.000; and χ2 = 0.97; p = 0.326, respectively) (Fig. 2). The proportions of isolates susceptible to penicillin, amoxicillin, and ceftriaxone at increased exposure were 23.4% (χ2 = 4.09; p = 0.044), 4.7% (χ2 = 1.37; p = 0.242) and 0% (χ2 = 14.13; p = 0.000), respectively.
The prevalence of S. pneumoniae resistant to macrolides, clindamycin, and tetracycline did not change significantly during the periods presented (χ2 = 1.51; p = 0.219; χ2 = 0.26; p = 0.610 and χ2 = 0.21; p = 0.650, respectively; Fig. 3). The proportion of pneumococci with inducible resistance to clindamycin was 1% (2 isolates) in the early post-vaccination period and 1.6% (1 isolate) in the late post-vaccination period.
The proportion of S. pneumoniae exhibiting resistance to 3 or more classes of antimicrobials did not differ during the observation periods (χ2 = 1.09; p = 0.297; Fig. 3). In the early post-vaccination period, the majority of isolates showed simultaneous resistance to β-lactams, macrolides, lincosamides and tetracyclines (69.6%), while in the late period, the phenotype of all pneumococci resistant to 3 or more classes of antimicrobials (100%) was characterized by simultaneous resistance to macrolides, lincosamides and tetracyclines.
Significant differences were found in the serotype landscape of resistant S. pneumoniae in the early and late post-vaccination periods (Fig. 4). In 2016–2018, serotypes belonging to the vaccine serotypes included in the 13-valent conjugated vaccine used were predominant: 19F (χ2 = 10.07; p = 0.002), 6A and 6B (χ2 = 12.13; p = 0.000). In 2020–2022, non-vaccine serotypes/serogroups of pneumococcus predominated: 15AF (χ2 = 22.04; p = 0.000), 23A (χ2 = 11.22; p = 0.000), and 35B (χ2 = 7.25; p = 0.008). During this period, S. pneumoniae of serogroups 11 and 15 (15BC and 11AD) were also detected, which are additionally included in the 20- and 23-valent PCV.
Fig. 4. Dynamics of the prevalence of S. pneumoniae serotypes (serogroups) resistant to antimicrobials.
N/t (non-typed) – isolates whose serotype has not been determined (not included in the standard serotyping scheme). PPSV23 is a 23-valent pneumococcal polysaccharide vaccine.*Statistically significant differences.
The resistance phenotypes of S. pneumoniae of different serotypes and serogroups varied: most serogroup 19 pneumococci (19F and 19A) were found to be resistant to 3 or more classes of antimicrobials, with the high proportion of penicillin-resistant isolates being noteworthy (Table 2). A lower prevalence of S. pneumoniae resistant to 3 or more classes of antimicrobials was found among members of serogroup 6 – 6A and 6B. All serotype 23A pneumococci were also resistant to 3 or more classes of antimicrobials, while penicillin resistance has not been established in representatives of this serotype.
Table 2. Phenotypes and genetic mechanisms of resistance in pneumococci belonging to serotypes prevalent in the early and late post-vaccination periods
Serotype / Serogroup | Amount of isolates | Percentage of resistant isolates, % or absolute number when the amount of isolates is less than 10 | Identified resistance mechanisms, % or absolute number when the amount of isolates is less than 10 | ||||||||
benzylpenicillin * | amoxicillin | ceftriaxone | erythromycin | clindamycin | tetracycline | mutations in PBP genes | ermB gene | mef gene | tet gene | ||
Early post-vaccination period | |||||||||||
23F | 3 | R — 0 I — 3 | 0 | 0 | 0 | 0 | 0 | pbp1a + pbp2b — 2; pbp1a + pbp2b + pbp2x — 1 | 0 | 0 | 0 |
6АВ | 22 | R — 9.1% I — 91.9% | 9.1 | 0.0 | 95.5 | 90.1 | 59.1 | pbp1a + pbp2b + pbp2x — 22.7; pbp2x + pbp2b — 68.2 | 95.5 | 22.7 | 95.5 |
19F | 24 | R — 70.8% I — 29.2% | 95.8 | 8.3 | 100.0 | 83.3 | 54.2 | pbp1a + pbp2b + pbp2x — 95.8; pbp2x — 4.2 | 83.3 | 83.3 | 87.5 |
19А | 8 | R — 3 I — 5 | 6 | 1 | 8 | 8 | 8 | pbp1a + pbp2b + pbp2x — 8 | 8 | 8 | 8 |
34 | 5 | R — 0 I — 5 | 4 | 0 | 0 | 0 | 0 | pbp1a + pbp2b+ pbp2x — 5 | 0 | 0 | 0 |
Late post-vaccination period | |||||||||||
15AF | 11 | R — 0.0% I — 36.4% | 0.0 | 0.0 | 81.8 | 36.4 | 27.3 | pbp1a + pbp2b + pbp2x — 45.5 | 36.4 | 45.5 | 27.3 |
23А | 7 | R — 0 I — 4 | 0 | 0 | 7 | 7 | 6 | pbp2x + pbp2b — 6 | 7 | 0 | 6 |
39 | 2 | R — 0 I — 2 | 1 | 0 | 0 | 0 | 0 | pbp1a + pbp2b + pbp2x — 2 | 0 | 0 | 0 |
35В | 3 | R — 0 I — 3 | 0 | 0 | 3 | 0 | 0 | pbp1a + pbp2b + pbp2x — 3 | 0 | 3 | 0 |
Note. *For benzylpenicillin, the percentage of resistant isolates (R), as well as susceptible at increased exposure isolates (I), is indicated.
Among pneumococci belonging to serogroup 15AF, macrolide-resistant isolates predominated. All S. pneumoniae serotypes 23F, 34, and 35B showed susceptibility with increased exposure to penicillin. All serotype 35B isolates were macrolide-resistant and had mef genes (Table 2).
Discussion
This paper presents the results of an analysis of a population of pneumococci isolated from the nasopharynx of healthy preschool children in 2016–2022, against the backdrop of PCV13 use in accordance with the Russian National Immunization Schedule. At the same time, resistant S. pneumoniae isolates obtained in 2016–2018 (the early post-vaccination period) were predominantly isolated from children who had not been vaccinated against pneumococcal infection (77.9%), while those obtained in 2020–2022 (the late post-vaccination period) were mainly isolated from children who had been fully vaccinated (three times), accounting for 75.9%. Accordingly, the proportion of fully vaccinated children also increased over the past period, from 1.9% to 68.8% (χ2 = 691.47; p = 0.000), indicating a significant increase in immunization coverage.
The dynamics of antimicrobial resistance in the bacterial population depend on many factors, one of which, in the case of S. pneumoniae, is vaccination. It has been proven that the use of conjugate vaccines leads to a decrease in the frequency and duration of pneumococcal colonization of the upper respiratory tract — primarily by the serotypes included in the vaccine [6–8]. However, data on changes in the serotype composition and resistance of S. pneumoniae following population immunization with conjugate vaccines are contradictory [16–21]. For instance, the results of a study conducted in Japan, along with the detection of changes in the serotype landscape and new serotypes 15A and 35B, showed a significant increase in the resistance of S. pneumoniae to β-lactam antibiotics — penicillin and meropenem — following the use of PCV13 [16]. The increase in resistance levels is also evidenced by a study conducted in Portugal: following the immunization of children within the national PCV13 program, the proportion of penicillin- and macrolide-resistant pneumococci increased from 9.3% and 13.4% to 20% due to the prevalence of non-vaccine serotypes 11A, 15BC, 24F, 15A and 21 [17].
The data in this study also indicate a change in the serotype composition of the resistant pneumococcal population: the serotypes that were predominant in the early post-vaccination period — 19F, 6A and 6B — have been replaced by non-vaccine variants such as 15AF, 23A and others. Accordingly, if 83.8% of resistant pneumococci belonged to the vaccine serotypes included in PCV13 in the early post-vaccination period, the proportion of such isolates was only 10.3% in the late post-vaccination period (χ2 = 46.52; p = 0.000).
Data on the circulation of resistant S. pneumoniae in Russia are mainly presented in the results of studies, including multicenter ones, involving isolates obtained from both adult and child carriers and patients with various nosological forms of pneumococcal infections [18–23].
For instance, in the study by Z.A. Alacheva et al., among isolates obtained from children in 2017–2022, the resistance level to erythromycin was 33%, to tetracycline — 26%, to trimethoprim/sulfamethoxazole – 25%, and to clindamycin — 19% [18]. Most isolates were susceptible to penicillin, and 27% were susceptible with increased exposure. Resistance to 3 or more classes of antibiotics was detected in 24.2% of pneumococci, the majority of which belonged to serotype 19F [18]. According to the multicenter SPECTRUM study, which included S. pneumoniae isolates obtained from adults, resistance to erythromycin in pneumococci isolated from carriers was 23.2%, and to tetracycline was 25.5%. Strains susceptible to penicillin at increased exposure were found in 16.3% of cases [19].
The results that were obtained in this study during a similar period (2016–2022) are comparable to the data presented above: the proportion of erythromycin-resistant pneumococcal isolates was 29.8%, tetracycline-resistant isolates was 20.8%, and clindamycin-resistant isolates was 22.6%. Isolates susceptible to penicillin at increased exposure accounted for 15.5%, while resistant ones made up 8.7%. Pneumococci resistant to 3 or more classes of antimicrobials were found in 21.9% of cases. At the same time, all S. pneumoniae isolates showing resistance, as well as susceptibility at increased exposure to penicillin, were found to have altered genes encoding PBP. In macrolide-resistant pneumococcal isolates, the ermB gene was detected in 41.8% of cases, the mef macrolide efflux genes in 20.3%, and a combination of these genes in 38%. Resistance to clindamycin was associated with the presence of the ermB gene in 61.7% of cases, and with a combination of the ermB and mef genes in 38.3%. Tetracycline-resistant S. pneumoniae were found to have the tetM ribosomal protection gene in 98.2% of cases.
A comparative analysis of the prevalence of resistant S. pneumoniae in 2016–2018 and 2020–2022 revealed the absence of isolates resistant to β-lactams — penicillin (χ2 = 8.02; p = 0.005), amoxicillin (χ2 = 13.69; p = 0.000), and ceftriaxone (χ2 = 0.97; p = 0.326) — in the late post-vaccination period, while in the early post-vaccination period, the proportion of pneumococci resistant to these drugs was 11.4%, 18.4%, and 1.5%, respectively. Also, in the late post-vaccination period, no isolates susceptible to ceftriaxone at increased exposure were detected, and in 2016–2018, the proportion of such isolates was 18.9% (χ2 = 14.13; p = 0.000). Similar data were obtained in studies by H. Dabaja-Younis et al. [21] and K. Andrejko et al. [22], which demonstrated a significant decrease in the prevalence of penicillin-resistant pneumococci in the pediatric population following PCV vaccination.
The proportion of macrolide-resistant pneumococci remained high despite immunization and tended to increase, rising from 27.9% to 35.9%. At the same time, the proportion of S. pneumoniae resistant to clindamycin and tetracycline among the identified resistant isolates significantly decreased – from 69.1% to 44.8% (χ2 = 5.08; p = 0.025) and from 63.2% to 41.4% (χ2 = 3.96; p = 0.047), respectively. However, the decrease was insignificant when calculated for the total pneumococcal population. The prevalence of pneumococci resistant to 3 or more classes of antibiotics among preschool children did not change significantly, being 23.4% and 17.2% in the early and late post-vaccination periods, respectively. Throughout the analyzed period, antibiotic resistance of vaccine serotypes of S. pneumoniae was generally higher than that of non-vaccine serotypes, which correlates with data from the PEGAS study (2015–2020) [23].
Conclusion
Following routine PCV13 immunization in children, there has been a decrease in the prevalence of pneumococci with reduced susceptibility to β-lactams: amoxicillin by 14.2% (χ2 = 7.50; p = 0.007) and ceftriaxone by 20.4% (χ2 = 15.44; p = 0.000), as well as a trend toward a decrease in the prevalence of isolates resistant to lincosamides and tetracyclines. S. pneumoniae resistance to macrolides remains high in the late post-vaccination period, at 35.9%.
Resistant S. pneumoniae isolates mainly belong to serogroup 15 (15AF) and serotype 23A, which are not included in the PCV13 used.
1 WHO Bacterial Priority Pathogens List, 2024: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. Geneva;2024. URL: https://who.int/publications/i/item/9789240093461
2 WHO. Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis. Geneva;2017.
URL: https://who.int/publications/i/item/WHO-EMP-IAU-2017.12
3 Laboratory diagnostics of community-acquired pneumonia of pneumococcal etiology: Methodological recommendations MR 4.2.0114-16. Moscow; 2017. 64 p.
4 Centers for Disease Control and Prevention. Streptococcus pneumoniae detection and serotyping using PCR. URL: https://www.cdc.gov/strep-lab/php/pneumococcus/serotyping-using-pcr.html?CDC_AAref_Val=https://www.cdc.gov/streplab/pneumococcus/resources.html (дата обращения 22.02.2025).
5 Biomérieux. Etest. Тестирование антимикробной чувствительности. URL: https://omb.ru/upload/iblock/4c4/e_test_amoxicillinclavulanic_acid_21_xl_amoksitsillinklavulanovaya_kislota_256.pdf (дата обращения 22.02.2025).
About the authors
Irina N. Protasova
Professor V.F. Voino-Yasenetsky Krasnoyarsk State Medical University
Author for correspondence.
Email: ovsyanka802@gmail.com
ORCID iD: 0000-0001-6521-8615
Dr. Sci. (Med.), Associate Professor, Department of microbiology named after Associate Professor B.M. Zelmanovich
Russian Federation, KrasnoyarskIrina V. Feldblium
Perm State Medical University named after Academician E.A. Wagner
Email: irinablum@mail.ru
ORCID iD: 0000-0003-4398-5703
Dr. Sci. (Med.), Professor, Head, Epidemiology department
Russian Federation, PermNatalya V. Bakhareva
Krasnoyarsk Regional Center for Prevention and Control of AIDS
Email: bakhareva@kraszdrav.ru
ORCID iD: 0009-0009-8203-328X
Epidemiologist
Russian Federation, KrasnoyarskLudmila V. Zinovieva
Krasnoyarsk City Children's Hospital No. 8
Email: lzinovieva@gdb8.ru
ORCID iD: 0009-0005-5176-6190
Deputy chief physician
Russian Federation, KrasnoyarskElena V. Kulik
Krasnoyarsk City Children's Clinic No. 2
Email: kulik@gdp2krsk.ru
ORCID iD: 0009-0006-4140-4812
Head, Department of medical care for children in educational institutions
Russian Federation, KrasnoyarskReferences
- O'Reilly R., Yokoyama S., Boyle J., et al. The impact of acute pneumococcal disease on health state utility values: a systematic review. Qual. Life Res. 2022;31(2):375–88. DOI: https://doi.org/10.1007/s11136-021-02941-y
- Tantawichien T., Hsu L.Y., Zaidi O., et al. Systematic literature review of the disease burden and vaccination of pneumococcal disease among adults in select Asia-Рacific areas. Expert. Rev. Vaccines. 2022;21(2):215–26. DOI: https://doi.org/10.1080/14760584.2022.2016399
- Navarro-Torné A., Montuori E.A., Kossyvaki V., Méndez C. Burden of pneumococcal disease among adults in Southern Europe (Spain, Portugal, Italy, and Greece): a systematic review and meta-analysis. Hum. Vaccin. Immunother. 2021;17(10): 3670–86. DOI: https://doi.org/10.1080/21645515.2021.1923348
- Micoli F., Romano M.R., Carboni F., et al. Strengths and weaknesses of pneumococcal conjugate vaccines. Glycoconj. J. 2023;40(2):135–48. DOI: https://doi.org/10.1007/s10719-023-10100-3
- Авдеев С.Н., Алыева М.Х., Баранов А.А. и др. Вакцинопрофилактика пневмококковой инфекции у детей и взрослых. Методические рекомендации. Профилактическая медицина. 2023;26(9-2):3–23. Avdeev S.N., Alyeva M.H., Baranov A.A., et al. Federal clinical guidelines on vaccination of pneumococcal infection in children and adults. Russian Journal of Preventive Medicine. 2023;26(9-2):3–23. DOI: https://doi.org/10.17116/profmed2023260923 EDN: https://elibrary.ru/ufufle
- Li L., Ma J., Yu Z., et al. Epidemiological characteristics and antibiotic resistance mechanisms of Streptococcus pneumoniae: An updated review. Microbiol. Res. 2023;266:127221. DOI: https://doi.org/10.1016/j.micres.2022.127221
- Watkins E.R., Kalizang'Oma A., Gori A., et al. Factors affecting antimicrobial Resistance in Streptococcus pneumoniae following vaccination introduction. Trends Microbiol. 2022;30(12):1135–45. DOI: https://doi.org/10.1016/j.tim.2022.06.001
- Knupp-Pereira P.A., Cabral A.S., Dolores Í.M., et al. Antimicrobial resistance in Streptococcus pneumoniae before and after the introduction of pneumococcal conjugate vaccines in Brazil: a systematic review. Antibiotics. 2024;13(1):66. DOI: https://doi.org/10.3390/antibiotics13010066
- Исаева Г.Ш., Цветкова И.А., Никитина Е.В. и др. Молекулярно-генетическая характеристика Streptococcus pneumoniae серогрупп 15 и 11, циркулирующих в России, и их связь с глобальными генетическими линиями. Журнал микробиологии, эпидемиологии и иммунобиологии. 2024;101(4):483–501. Isaeva G.Sh., Tsvetkova I.A., Nikitina E.V., et al. Molecular genetic characteristics of Streptococcus pneumoniae serogroups 15 and 11 representatives circulating in Russia and their relationship with global genetic lineages. Journal of Microbiology, Epidemiology and Immunobiology. 2024;101(4):483–501. DOI: https://doi.org/10.36233/0372-9311-498 EDN: https://elibrary.ru/gciets
- Ben Ayed N., Ktari S., Jdidi J., et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Tunisian healthy under-five children during a three-year survey period (2020 to 2022). Vaccines. 2024;12(4):393. DOI: https://doi.org/10.3390/vaccines12040393
- Исаева Г.Ш., Зарипова А.З., Баязитова Л.Т. и др. Характеристика бактерионосительства Streptococcus pneumoniae в детской популяции. Журнал микробиологии, эпидемиологии и иммунобиологии. 2024;101(1):89–99. Isaeva G.Sh., Zaripova A.Z., Bayazitova L.T., et al. Characteristics of Streptococcus pneumoniae carriage in the pediatric population. Journal of Microbiology, Epidemiology and Immunobiology. 2024;101(1):89–99. DOI: https://doi.org/10.36233/0372-9311-445 EDN: https://elibrary.ru/wqbjrf
- Гирина А.А., Петровский Ф.И., Петровская Ю.А., Заплатников А.Л. Частота носительства S. pneumoniae у организованных детей г. Ханты-Мансийска. РМЖ. Мать и дитя. 2023;6(2):164–8. Girina A.A., Petrovsky F.I., Petrovskaya Yu.A., Zaplatnikov A.L. Frequency of S. pneumoniae carriage in organized children of Khanty-Mansiysk. Russian Journal of Woman and Child Health. 2023;6(2):164–8. DOI: https://doi.org/10.32364/2618-8430-2023-6-2-164-168 EDN: https://elibrary.ru/ivnfhy
- Reinert R.R., Filimonova O.Y., Al-Lahham A., et al. Mechanisms of macrolide resistance among Streptococcus pneumoniae isolates from Russia. Antimicrob. Agents Chemother. 2008;52(6):2260–2. DOI: https://doi.org/10.1128/aac.01270-07
- Poyart C., Jardy L., Quesne G., et al. Genetic basis of antibiotic resistance in Streptococcus agalactiae strains isolated in a French hospital. Antimicrob. Agents Chemother. 2003;47(2):794–7. DOI: https://doi.org/10.1128/aac.47.2.794-797.2003
- Knupp-Pereira P.A., Cabral A.S., Dolores Í.M., et al. Antimicrobial resistance in Streptococcus pneumoniae before and after the introduction of pneumococcal conjugate vaccines in Brazil: a systematic review. Antibiotics (Basel). 2024;13(1):66. DOI: https://doi.org/10.1093/jac/48.6.915
- Ono T., Watanabe M., Hashimoto K., et al. Serotypes and antibiotic resistance of Streptococcus pneumoniae before and after the introduction of the 13-valent pneumococcal conjugate vaccine for adults and children in a rural area in Japan. Pathogens. 2023;12(3):493. DOI: https://doi.org/10.3390/pathogens12030493
- Candeias C., Almeida S.T., Paulo A.C., et al. Streptococcus pneumoniae carriage, serotypes, genotypes, and antimicrobial resistance trends among children in Portugal, after introduction of PCV13 in National Immunization Program: a cross-sectional study. Vaccine. 2024;42(22):126219. DOI: https://doi.org/10.1016/j.vaccine.2024.126219
- Алачева З.А., Алябьева Н.М., Комягина Т.М., Тряпочкина А.С. Серотиповой состав и антибиотикорезистентность Streptococcus pneumoniae, выделенных у детей. Российский педиатрический журнал. 2024;27(S1):11–2. Alacheva Z.A., Alyabieva N.M., Komyagina T.M., Tryapochkina A.S. Serotypes and antibiotic resistance of Streptococcus pneumoniae isolated from children. Russian Pediatric Journal. 2024;27(S1):11–2. EDN: https://elibrary.ru/zjpehw
- Куркова А.А., Муравьев А.А., Козлов Р.С. Современное состояние антимикробной резистентности Streptococcus pneumoniae и специфической вакцинопрофилактики пневмококковой инфекции. Пульмонология. 2023;33(4):534–41. Kurkova A.A., Muraviov A.A., Kozlov R.S. The current status of antimicrobial resistance of Streptococcus pneumoniae and specific vaccine prevention of pneumococcal infection. Pulmonologiya. 2023;33(4):534–41. DOI: https://doi.org/10.18093/0869-0189-2022-3655 EDN: https://elibrary.ru/astwbo
- Филимонова О.Ю., Сафонова Т.Б., Золотарева Л.В. и др. Динамика и клиническая значимость резистентности Streptococcus pneumoniae к антибактериальным препаратам. Бактериология. 2023;8(4):7–13. Filimonova O.Yu., Safonova T.B., Zolotareva L.V., et al. Dynamics and clinical significance of resistance Streptococcus pneumoniae to antibacterial drugs. Bacteriology. 2023;8(4):7–13. EDN: https://elibrary.ru/gqvzst
- Dabaja-Younis H., Geller D., Geffen Y., et al. The impact of pneumococcal conjugate vaccine-13 on the incidence of pediatric community-acquired bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40(7):1433–9. DOI: https://doi.org/10.1007/s10096-021-04167-9
- Andrejko K., Ratnasiri B., Hausdorff W.P., et al. Antimicrobial resistance in paediatric Streptococcus pneumoniae isolates amid global implementation of pneumococcal conjugate vaccines: a systematic review and meta-regression analysis. Lancet Microbe. 2021;2(9):e450–60. DOI: https://doi.org/10.1016/s2666-5247(21)00064-1
- Чагарян А.Н., Иванчик Н.В., Кузьменков А.Ю. и др. Молекулярно-биологическая характеристика изолятов Streptococcus pneumoniae, выделенных от больных пневмококковым менингитом. Журнал микробиологии, эпидемиологии и иммунобиологии. 2025;102(2):150–61. Chagaryan A.N., Ivanchik N.V., Kuzmenkov A.Yu., et al. Molecular and biological characterization of Streptococcus pneumoniae isolates from patients with pneumococcal meningitis. Journal of Microbiology, Epidemiology and Immunobiology. 2025;102(2): 150–61. DOI: https://doi.org/10.36233/0372-9311-614 EDN: https://elibrary.ru/nraeks
Supplementary files