Study of the protective properties of immunodominant proteins of orthopoxviruses in various methods of immunization
- Authors: Zrelkin D.I.1, Zubkova O.V.1, Ozharovskaia T.A.1, Popova O.1, Voronina D.V.1, Goldovskaya P.P.1, Vavilova I.V.1, Chugunova A.S.1, Dolzhikova I.V.1, Shcherbinin D.N.1, Shcheblyakov D.V.1, Logunov D.Y.1, Gintsburg A.L.1
-
Affiliations:
- National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
- Issue: Vol 102, No 1 (2025)
- Pages: 18-30
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18755
- DOI: https://doi.org/10.36233/0372-9311-643
- EDN: https://elibrary.ru/ONKJVE
- ID: 18755
Cite item
Abstract
Introduction. In 2022, the World Health Organization declared monkeypox a public health emergency. The monkeypox virus (MPV) is part of the Orthopoxvirus genus within the Poxviridae family. During replication, orthopoxviruses produce two distinct forms of viral particles: the extracellular enveloped virion (EEV), released via exocytosis, and the intracellular mature virion (IMV), released through cell lysis. These forms differ in surface proteins composition, influencing their immunogenicity and infectivity.
Aim. To evaluate the immunogenic and protective activity of nine surface antigens of vaccinia virus.
Materials and methods . Recombinant human adenoviruses type 2 (rAd2) carrying surface antigens of vaccinia virus were obtained using homologous recombination in bacteria, followed by adenoviral particle assembly in HEK293 cells. The immunogenic and protective properties of these adenoviruses were tested in BALB/c mice. The presence of antibodies to the vaccinia virus was assessed using ELISA, and survival rates were evaluated in a lethal infection model after intranasal challenge with the vaccinia virus strain Western Reserve.
Results. The most immunogenic and protective antigens of the vaccinia virus within rAd2 were glycoprotein B5 of the EEV and membrane-associated protein H3 of the IMV, both showing 100% protective efficacy after intranasal immunization.
Conclusion. Using a panel of recombinant adenoviruses carrying genes of vaccinia virus surface proteins, it was shown that optimal protection is achieved using a combination of enveloped and mature virion antigens. This method could be used for development of new multivalent preparations against various viral infections.
Full Text
Introduction
Orthopoxviruses belong to the Poxviridae family, Chordopoxvirinae subfamily, Orthopoxvirus genus, which contains 13 species: Orthopoxvirus abatinomacacapox, Orthopoxvirus akhmetapox, Orthopoxvirus camelpox, Orthopoxvirus cowpox, Orthopoxvirus ectromelia, Orthopoxvirus monkeypox, Orthopoxvirus raccoonpox, Orthopoxvirus skunkpox, Orthopoxvirus taterapox, Orthopoxvirus vaccinia, Orthopoxvirus variola, Orthopoxvirus volepox, and the unofficially classified Alaska smallpox virus1. Species are divided into 2 related clades: Old World (Eurasian and African) and North American orthopoxviruses [1]. Many of these viruses can cause severe disease in domestic animals as well as zoonotic infections in humans. Human infection most commonly occurs through livestock rearing, contact with domestic animals, and trade in exotic animals as a result of direct contact with them. When introduced into non-endemic regions, enzootic orthopoxviruses may pose a threat to native and endemic species [2–4].
Orthopoxviruses of different species are antigenically and immunologically close to each other. Vaccination against smallpox, for example, provides cross-protection against other members of the genus [5]. However, after the global eradication of smallpox in 1980, many countries began to phase out routine vaccination against the disease [6]. As a result, a significant proportion of the population currently lacks immune protection to orthopoxviruses. This explains the increasing frequency of outbreaks in various regions of the world, among which monkeypox is particularly notable. The global outbreak that occurred in 2022 was recognized by the World Health Organization (WHO) as a public health emergency of international concern [7]. The ongoing epidemic of monkeypox in African countries may lead to accelerated viral evolution and adaptation to human-to-human transmission of the zoonotic disease [8]. Despite existing vaccines, WHO has recommended the development of less reactive vaccines to improve the efficacy and duration of protection to get the current outbreak under control.
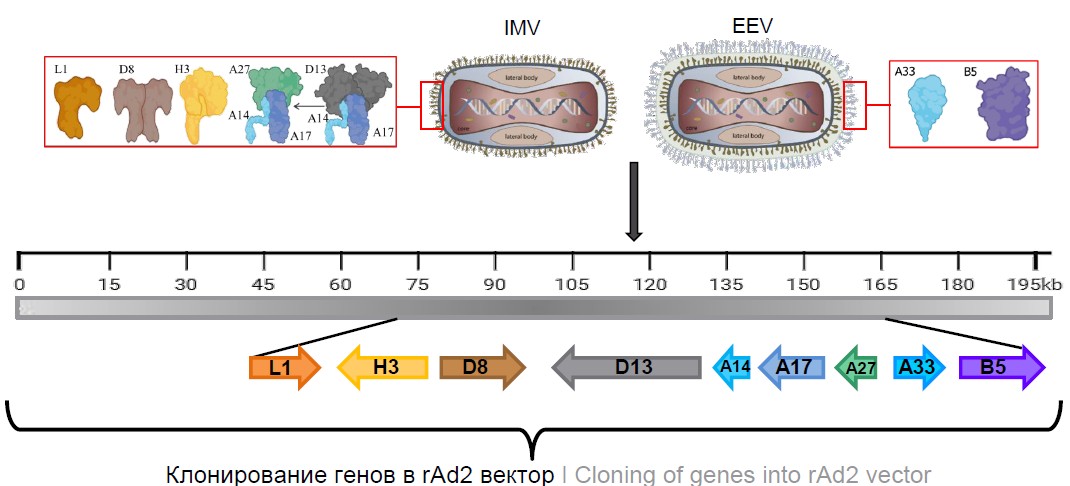
Orthopoxviruses have a large and complex proteome containing more than 200 proteins, of which more than 30 are structural proteins [9, 10]. During infection, the virus exists in two antigenically distinct infectious forms [11–13]. Intracellular mature virions (IMV) are the main infectious viral particles and play a key role in human-to-human transmission. The surface membrane of this form contains at least 11 proteins: A14.5 (~10 kDa), E10 (12 kDa), I5 (13 kDa), A13 (14 kDa), A27 (14 kDa), A9 (~18 kDa), A14 (17–25 kDa), A17 (23–29 kDa), L1 (25–29 kDa), D8 (34 kDa) and H3 (35 kDa) [9–13]. Extracellular enveloped virions (EEVs) are formed from IMVs and have an additional lipoprotein membrane responsible for virus dissemination within the body [12]. EEV and IMV surface proteins ensure the infectivity of orthopoxviruses and serve as major targets for the induction of protective immunity [14, 15]. Because antibodies that neutralize IMV do not neutralize EEV, it is believed that immunization with antigens from both of these forms is necessary for maximal protection [16].
The immunodominant proteins to which antibodies are produced, including virus-neutralizing antibodies, are two EEV glycoproteins, A33 and B5, as well as IMV proteins: L1, H3, D8 and a complex of 3 proteins of immature virion A14, A17 and D13 or a complex of A14, A17 and A27 of a mature virion [14, 17–19]. Proteins A33 and B5 play a role in envelope virion formation and subsequent infection [14]. Among the surface proteins of the mature virion, 3 proteins mediate the attachment of the virus to the host cell. Protein D8 forms dimers on the viral membrane and ensures virion infectivity by binding to chondroitin sulfate on a cell membrane [20]. A27 and H3 proteins bind heparan sulfate. A27, a major membrane protein, is required for the formation of the enveloped extracellular virion and is involved in the attachment of the virus to the cell and subsequent fusion of the viral and cell membranes. A27 forms a complex with the transmembrane proteins A14 and A17, which are important structural elements of the mature virion membrane. A17 is also required for virus entry into the cell and serves as an anchor for A27 [21]. H3 binds to the cell surface via heparan sulfates and, like D8 and A27 proteins, is involved in virus entry into the cell. H3 is a major protein in the development of immune response in humans [22]. L1 is a transmembrane protein that is essential for the formation of mature virions and is involved in virus entry into the cell [23].
Although most of these antigens (A27, L1, B5 and A33) have been studied as part of various polyvalent vaccines [18, 24, 25], certain immunodominant proteins were not included in these studies. Furthermore, while orthopoxviruses are highly immunogenic, the antigens themselves have low immunogenicity. Vaccination with purified proteins or DNA encoding proteins of vaccinia virus requires multiple immunizations for induction of a protective immune response [24, 26, 27]. In contrast to DNA vaccines and subunit vaccines, vaccination with recombinant adenoviruses (rAd) has been shown to promote both a robust humoral and cellular immune response and protective immunity after a single immunization [28–30]. Given the importance of rapid induction of protective immunity in a potential outbreak, we studied the possibility of a single immunization with rAd expressing the EEV and IMV antigens of the vaccinia virus.
The aim of this study was to evaluate the immunogenic and protective activity of 9 surface antigens of vaccinia virus. To achieve this goal, rAd carrying genes of surface proteins of mature (D8, H3, L1, A14, A17, A27 and D13) and extracellular (A33 and B5) virions of smallpox virus were constructed. Furthermore, individual immunogenic and protective properties of the obtained adenoviruses were investigated by intranasal and intramuscular methods of immunization. This approach can be used in the development of new polyvalent vaccines against various viral infections.
Materials and methods
Cell lines and viruses
The Vero E6 cell line (green monkey kidney) was cultured in DMEM medium (Cytiva) containing 4% fetal bovine serum (Gibco), 25,000 units of penicillin and 25 mg of streptomycin (Paneco) at 5% CO2. The HEK293 cell line (human embryonic kidney) was cultured in DMEM medium (Cytiva) containing 10% fetal bovine serum (Gibco), 25,000 units of penicillin and 25 mg of streptomycin (Paneco) at 5% CO2.
Western Reserve strain of vaccinia virus (VACV WR) (GenBank #OP584857.1) was grown in Vero E6 cells. Aliquots of virus-containing medium were stored at –80ºC. The biological activity of the virus was determined by standard titration method on cell culture by counting plaques [31].
Production of recombinant adenoviruses
rAd was constructed using the technology described previously [32]. Vaccinia virus antigen genes were amplified from the VACV WR genome (GenBank #OP584857.1) using primers indicated in Table 1 and cloned under the control of the human cytomegalovirus promoter. Plasmid vectors with the human rAd genome of type 2 (rAd2) and the target antigen were obtained by homologous recombination in bacterial cells. The rAds were produced and grown in HEK293 cells. Viruses were purified and concentrated by ultracentrifugation in cesium chloride density gradient according to standard methods [33]. The number of virus particles was determined by the standard spectrophotometric method [34].
Table 1. Primer sequences for amplification of vaccinia virus genes
Name | Sequence |
A33-F | CACCGGCGGTCGACAGATCTGCCACCATGATGACACCAGAAAACGACG |
A33-R | GATATCTCTAGATTAACAAAAATACTTTCTAACTTCTTGTG |
H3-F | ACTAAGCTTATATGGCGGCGGCGAAAACT |
H3-R | ATCTAGATATCTG TTAGATAAATGCGGTAAC |
B5-F | CACCGGCGGTCGACAGATCTGCCACCATGAAAACGATTTCCGTTGTTACG |
B5-R | TCTAGATTACGGTAGCAATTTATGGAACTTATA |
D8-F | CACCGGCGGTCGACAGATCTGCCACCATGCCGCAACAACTATCTCCTATTAA |
D8-R | GATATCGCTAGCTTACTAGTTTTGTTTTTCTCGCGAATATCG |
A14-F | CACCGGCGGTCGACAGATCTGCCACCATGGACATGATGCTTATGATTGG |
A14-R | GATATCTCTAGATTATTAGTTCATGGAAATATCGCTATG |
A27-F | CACCGGCGGTCGACAGATCTGCCACCATGGACGGAACTCTTTTCCC |
A27-R | TCTAGATTATTACTCATATGGGCGCCGTC |
L1-F | CACCGGCGGTCGACAGATCTGCCACCATGGGTGCCGCGGCAAGCAT |
L1-R | GATATCTCTAGATTATCAGTTTTGCATATCCGTGGTAGC |
A17-F | CACCGGCGGTCGACAGATCTGCCACCATGAGTTATTTAAGATATTACAATATG |
A17-R | GATATCTCTAGATTATTAATAATCGTCAGTATTTAAACTG |
D13-F | CACCGGCGGTCGACAGATCTGCCACCATGAATAATACTATCATTAATTCTTTG |
D13-R | ACTAGTTTATTAGTTATTATCTCCCATAATCTTG |
Animal models
Mice of the inbred line BALB/c, males and females, body weight 16–18 g, were obtained from the nursery of the Pushchino Nursery of laboratory animals, branch of the Institute of Bioorganic Chemistry of RAS. The animals were kept in the vivarium of N.F. Gamaleya NRCEM in accordance with the requirements for keeping laboratory animals and had free access to food and water. The authors confirm compliance with institutional and national standards for the use of laboratory animals in accordance with the “Consensus Author Guidelines for Animal Use” (IAVES, 23.07.2010). The study protocol was approved by the Biomedical Ethics Committee of the N.F. Gamaleya NRCEM of the Ministry of Health of Russia (protocol # 34 from 16.01.2023).
Immunization and infection of mice
Five-week-old BALB/c mice were immunized intranasally under mild inhalation anesthesia or intramuscularly with rAd at a dose of 2 × 1010 viral particles. The same adenoviruses were re-administrated at the same dose 3 weeks later. Serum samples were collected on day 28 after the 1st immunization. Immunized mice were subjected to intranasal infection with vaccinia virus strain WR at a dose of 16 LD50 (5 × 104 PFU) 35 days after the 1st immunization. During 14 days after infection, the animals were examined daily and clinical symptoms of smallpox infection (decreased locomotor activity, hunched, ruffled hair, conjunctivitis), changes in body weight, and death of mice were recorded. Animals were sacrificed if they lost more than 25% of their body weight.
Enzyme immunoassay
The titer of specific IgG antibodies to vaccinia virus in serum samples was determined by enzyme-linked immunosorbent assay. 96-well plates were coated with vaccinia virus WR (105 PFU/plate) diluted in carbonate-bicarbonate buffer (pH 9.6) overnight at 4ºC. Serum samples were serially diluted twice to 1 : 102,400, added to blocked 96-well plates and incubated at 37ºC for 1 h. The plates were then washed and secondary antibodies conjugated to a complex of recombinant streptavidin with horseradish peroxidase were added to each well. The plates were incubated for 1 h at 37ºC. Thereafter, tetramethylbenzidine hydrochloride was added to the plates. The reaction was stopped by adding H2SO4 and the absorbance (450 nm) was read using a Multiskan FC tablet photometer (ThermoFisher). The highest serum dilution with an optical density value 2 or more times higher than the value in the corresponding serum dilution of intact (unimmunized) mice was taken as the final titer.
Statistical processing of data
Statistical processing of data and construction of diagrams were performed in the GraphPad Prism 8 program. Statistical significance of differences between the studied groups was determined using the Wilcoxon T-test for dependent samples or Mann–Whitney U-test for independent samples. Differences were considered reliable at p < 0.05.
Results
Design and production of rAd expressing surface antigen genes
The main immunodominant proteins of the vaccinia virus are 2 glycoproteins of the enveloped extracellular virion, A33 and B5, as well as proteins of the mature intracellular virion: D8, H3, L1, A14, A17, A27 and D13. These antigens have a high degree of homology between different orthopoxvirus species (Table 2) [35–37].
Table 2. Homology between proteins of different orthopoxvirus species
VACV antigen | Antigen (% homology) | ||
Orthopoxvirus monkeypox | Orthopoxvirus cowpox | Orthopoxvirus variola | |
L1 | M1 (98,4%) | CPVX099 (98,4%) | M1 (99,2%) |
H3 | H3 (93,83%) | CPVX112 (94,46%) | I3 (93,85%) |
D8 | E8 (94,41%) | CPVX125 (97,37%) | F8 (93,09%) |
D13 | E13 (98,91%) | CPVX131 (98,73%) | N3 (98,91%) |
A14 | А15 (100%) | CPVX146 (100%) | A14 (97,78%) |
A17 | А18 (97,55%) | CPVX150 (95,59%) | A17 (97,55%) |
A27 | А29 94,55%) | CPVX162 (95,45%) | A30 (93,64%) |
A33 | А35 (92,47%) | CPVX168 (91,49%) | A36 (89,25%) |
B5 | В6 (96,53%) | CPVX199 (93,69%) | B7 (92,74%) |
The genes of the proteins described above were obtained from the genome of vaccinia virus and cloned into the rAd2 genome under the control of the human cytomegalovirus promoter (Fig. 1). The rAd2 genome carries a deletion of the E1 region that renders the adenovirus replication-defective (an expression cassette with the target gene is cloned into this deletion site). To increase the packaging capacity, the E3 region was also deleted.
Fig. 1. Scheme for the production of recombinant adenoviruses expressing surface antigens of the vaccinia virus.
L1 — myristoylated protein; H3 — membrane-associated protein p35 IMV; D8 — transmembrane carbonic anhydrase-like protein; D13 — IMV membrane protein; A14 — structural transmembrane protein p16 IMV; A17 — transmembrane protein IMV (morphogenesis factor); A27 — membrane protein IMV; B5 — type I membrane glycoprotein; A33 — type II membrane glycoprotein.
Thus, we obtained 9 rAd2, 2 of which carried surface antigens of the enveloped virion (rAd2-B5 and rAd2-A33) and 7 of which carried antigens of the mature virion (rAd2-H3, rAd2-L1, rAd2-D8, rAd2-D13, rAd2-A14, rAd2-A17, and rAd2-A27).
rAd2 vectors induce humoral immune response in vivo
To evaluate the immunogenicity of the obtained rAd (rAd2-A33, rAd2-B5, rAd2-H3, rAd2-L1, rAd2-D8, rAd2-D13, rAd2-A14, rAd2-A17, rAd2-A27), mice were immunized intramuscularly or intranasally with adenoviruses at a dose of 2 × 1010 viral particles once or twice. Titers of specific IgG antibodies to vaccinia virus were determined by enzyme-linked immunosorbent assay on the 28th day after immunization (Figs. 2, 3).
Fig. 2. Immunogenicity of recombinant adenoviruses expressing surface antigens of the vaccinia virus after single (a) and double (b) intramuscular immunization.
The figures show the confidence at p < 0.05; *p < 0.0001. C — control.
Fig. 3. Immunogenicity of recombinant adenoviruses expressing surface antigens of the vaccinia virus after two intranasal immunizations.
The figure shows the confidence at p < 0.05; *p < 0.0001. C– — control.
A single intramuscular immunization resulted in the formation of specific IgG antibodies in mice that received rAd2-B5, rAd2-H3, rAd2-D8, rAd2-D13, and rAd2-A14. The titer values in these groups had significant differences with the control group, where titers were less than 1 : 50 (Fig. 2, a). High levels of IgG antibodies were found in the group of mice immunized with rAd2-H3 (geometric mean titers (GMT) 1 : 3676). Administration of rAd2-B5 resulted in the induction of IgG-antibodies with GMT = 1 : 348.2. Minimal levels of IgG antibodies were observed for rAd2-D8 (GMT = 1 : 100), rAd2-A14 (GMT = 1 : 107.2) and rAd2-D13 (GMT = 1 : 57.43) groups with no significant differences between groups. Double immunization resulted in a significant increase in IgG antibody levels compared to single immunization only for the rAd2-H3 (GMT = 1 : 14703) and rAd2-B5 (GMT = 1 : 2111) groups (Fig. 2, b). No significant differences were found between the other groups.
After single intranasal immunization, specific IgG antibodies in serum were not detected in any group. After double intranasal immunization, specific IgG antibodies were detected in the serum of animals immunized with rAd2-B5 and rAd2-H3. The titer values in these groups had significant differences with the control group, where titers were less than 1 : 50 (Fig. 3). High antibody levels were found in the group of mice immunized with rAd2-H3 (GMT = 1 : 1902.73). Administration of rAd2-B5 resulted in induction of IgG antibodies with GMT = 1 : 183.4.
Protective efficacy of rAd2 in vivo
The next step was to test the protective efficacy of the antigens. After 35 days from the start of double immunization, mice were infected with a pathogenic WR strain at a dose of 16 LD50 (5 × 104 PFU). The infectious dose was chosen based on preliminary experiments to determine the LD50 in vivo [38]. Survival and body weight changes were recorded for 14 days after infection.
As expected, in the control group, pronounced clinical signs of the disease with a body weight loss of more than 13% were observed from day 4. As shown in Fig. 4, all control mice rapidly lost weight and died on the 5th–8th day after the infection.
Fig. 4. The protective efficacy of recombinant adenovirus vectors expressing the surface antigens of the vaccinia virus after intramuscular (a, b) or intranasal (c, d) immunization in a mouse model of lethal infection.
a, c — weight dynamics (the graph shows the average value and the standard error of the average value); b, d — survival. C– — control.
After intramuscular immunization with rAd2-B5 expressing the B5 EEV glycoprotein gene, we observed 20% protection of animals from lethal infection (Fig. 4, a, b). Double intramuscular immunization with rAd2-D8 expressing the D8 IMV glycoprotein gene resulted in 40% survival of animals. Animals immunized with rAd2 expressing other surface antigens lost 25% of their initial weight within 5–7 days of infection. The results of the study concluded that when using the intramuscular route of administration, a single antigen is not sufficient for protection.
Interestingly, intranasal immunization significantly reduced the severity of infection and protected the animals from both lethality and weight loss (Fig. 4, c, d).
In particular, during immunization with rAd2-B5 we observed 100% protective efficacy. It should be noted that in the group of animals immunized with rAd2-B5, insignificant weight loss (no more than 3%) on days 3-5 was observed in only half of the animals. In the rAd2-H3 group, the survival rate was 87.5% (out of the 8 mice, 1 did not survive). Furthermore, 1 mouse had a weight loss of about 15% on day 5 with gradual recovery by day 14. In the rAd2-A33, rAd2-L1, rAd2-D8 and rAd2-D13 groups, the survival rate was 75%. Temporary weight loss by day 5 for the groups did not exceed 25%. The mice in the rAd2-A17 and rAd2-A27 groups had a 50% lethality with a weight loss of 15% or less. The mice immunized with rAd2-A14 lost 21% of their initial weight within 5 days of infection. The survival rate in this group was only 25%.
These data indicate that rAd2-B5 and rAd2-H3 are optimal vectors for protection against intranasal vaccinia virus infection, demonstrating the importance of B5 and H3 as protective antigens in this model of orthopoxvirus infection. Therefore, we further tested the efficacy of combined immunization (intramuscular + intranasal) for induction of both systemic and local response.
Mice were injected intramuscularly with rAd2-B5 or rAd2-H3 at a dose of 2 × 1010 viral particles; 21 days later, the same adenoviral vector was administered intranasally. After 35 days from the start of immunization, animals were infected with the pathogenic WR strain at a dose of 16 LD50 (5 × 104 PFU). Survival and body weight changes were recorded for 14 days after infection (Fig. 5).
Fig. 5. The protective efficacy of recombinant adenovirus vectors rAd2-B5 and rAd2-H3 after combined immunization (im+in) in a mouse model of lethal infection.
a — weight dynamics; b — survival. C — control.
Combined immunization with rAd2-B5 or rAd2-H3 provided 100% protection of animals against lethal infection. No weight loss was recorded in the group of animals intramuscularly and intranasally immunized with rAd2-B5. However, in the rAd2-H3 group, weight loss was about 23% on day 6 in 2 out of 8 mice with gradual recovery by day 14.
Discussion
Since the use of vaccinia virus in the last century, concomitant vaccination is no longer available. Despite the eradication of smallpox, the world continues to face orthopoxvirus infections that require active actions. Serious side effects following smallpox virus vaccination have emphasized the need to develop safer vaccine formulations to combat current orthopoxvirus infections such as monkeypox.
Russian scientists have made a great contribution to the development of smallpox vaccines [39–41]. Currently, the least reactogenic and safe vaccine is the recently developed OrthopoxVac vaccine from the Vector Research Center of Rospotrebnadzor for the prevention of orthopoxvirus infections, which is an L-IVP strain of the vaccinia virus with 6 disrupted virulence genes [42].
A high degree of homology in the central region of the genome of monkeypox and vaccinia virus, amounting to 96.3%, indicates their genetic similarity [35–37]. In particular, the MPV genes A35, B6, M1, E8, H3, A15, A18, A29 and E13 show significant conservation with orthologous orthopoxvirus genes, including smallpox and vaccinia viruses [24].
Several technologies have been used to develop orthopoxvirus vaccines: live vaccine, attenuated replication-defective virus, DNA-based vaccine and subunit vaccines. Each of them has its own disadvantages, such as serious adverse events with live vaccine, lower immunogenicity in attenuated non-replicating vaccines and DNA vaccines, and a long development cycle for recombinant proteins [24, 26, 43, 44].
rAd possess a number of key properties that make them good candidates for vector vaccine development. rAd are physically and genetically stable and effectively induce innate and adaptive immune response by various routes of administration, including delivery via mucosal surfaces [45]. The latter is a significant advantage over other types of vaccines, since the effectiveness of vaccination depends on the site of administration and the recruitment of antigen-presenting cells [46]. Intramuscular immunization leads to stimulation of the systemic immune response, but does not provide effective barrier immunity. Meanwhile, intranasal immunization can induce humoral and cellular immunity both on mucosal membranes and systemically [47]. Thus, the development of mucosal vaccines will help to meet the need for more effective protection against pathogens that penetrate through mucous membranes.
Adenovirus technology provides a versatile platform for the rapid development and deployment of vaccines to combat viral infections, including those with pandemic potential. In this study, we developed a panel of recombinant replication-defective vectors based on human adenovirus type 2 encoding genes of surface proteins of vaccinia virus. Two proteins of the extracellular enveloped virion (A33 and B5) and 7 proteins of the intracellular mature virion (L1, D8, H3, A14, A17, A27, D13) were selected as target genes for cloning into the rAd2 genome. We evaluated the immunogenicity of 9 EEV and IMV surface proteins, most of which have been shown to be effective in previous studies [15, 17, 19, 24, 26, 27]. However, in our study, of the 9 surface antigens, only B5 and H3 induced an immune response in mice upon systemic and intranasal administration. We hypothesize that this may be due to the fact that both B5 and H3 proteins are glycoproteins. Glycoproteins contain epitopes that are recognized by immune system cells more efficiently than epitopes of other types of proteins. According to the literature sources, in silico predicted B- and T-cell epitopes for immunodominant MPV proteins (M1, H3, E8, A29, A35 and B6) have been reported [48].
In experiments to study the protective efficacy of rAds, we demonstrated the efficacy of intranasal immunization. Intranasal immunization with rAd2-B5 expressing the B5 EEV glycoprotein gene provided the best protection (100%) with the least weight loss and fastest recovery. Intranasal immunization with rAd2-H3 expressing IMV glycoprotein H3 gene provided 87.5% protection. Other surface proteins: EEV A33, IMV L1, D8 and D13 protected animals from lethal infection by 75%. Administration of rAds with genes of IMV antigens A17, A27 and A14 provided only partial protection against VACV by 25-50% depending on the antigen. The efficacy of combined immunization (intramuscular + intranasal) with rAd2-B5 or rAd2-H3 was 100%. Our data are consistent with the results of other studies; in which it was shown that specific immunity to EEV surface antigens is most important for protection against intranasal infection [49]. Antibodies specific to B5 play an important role against pulmonary or intracerebral infection [50]. H3 protein activates dendritic cells, which leads to the secretion of cytokines such as interleukins-12p70, -10, -6 and tumor necrosis factor-α, which further induces proliferation of CD8+ T-lymphocytes, thereby destroying virus-infected cells [51].
These results demonstrate the protective potential of the selected antigens and provide valuable information for the subsequent development of effective and safe polyvalent orthopoxvirus vaccines. Our study confirms that B5 and H3 have the highest protective potential, and in combination with other surface proteins of the mature virion can provide maximum efficacy. Further study of the immunogenic and protective potential of different antigen combinations is required. This study lays a solid foundation for the subsequent optimization of vector vaccines by confirming the feasibility of combining different EEV and IMV surface antigens to achieve maximum protection against orthopoxviruses, including monkeypox virus.
Conclusion
In this study, a panel of rAds carrying genes of vaccinia virus surface proteins was created and their immunogenicity and efficacy were evaluated. In our study, we found that in a lethal infection model induced by the WR vaccinia virus strain, glycoproteins B5 and H3 showed the greatest protective activity when immunized intranasally. Furthermore, combination with other antigens can not only enhance the immune response but also generate cross-immunity to other members of the Orthopoxvirus genus causing infections in humans. These results provide insight into the protective mechanism of polyvalent vector vaccines and a basis for further development and introduction of such vaccines aimed at enhancing protection against orthopoxviruses.
1 ICTV. Subfamily: Chordopoxvirinae. Genus: Orthopoxvirus.
About the authors
Denis I. Zrelkin
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: aleza4striker@yandex.ru
ORCID iD: 0000-0003-0899-8357
junior researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowOlga V. Zubkova
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Author for correspondence.
Email: olga-zubkova@yandex.ru
ORCID iD: 0000-0001-7893-8419
Cand. Sci. (Biol.), leading researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowTatiana A. Ozharovskaia
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: t.ozh@yandex.ru
ORCID iD: 0000-0001-7147-1553
Cand. Sci. (Biol.), senior researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowOlga Popova
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: olga.popova31@yandex.ru
ORCID iD: 0000-0003-3248-1227
junior researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowDaria V. Voronina
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: daryavoronin2009@yandex.ru
ORCID iD: 0000-0001-6629-744X
junior researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowPolina P. Goldovskaya
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: goldovskaya00@mail.ru
ORCID iD: 0009-0000-1965-0482
laboratory assistant researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowIrina V. Vavilova
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: vavilovairinav@yandex.ru
ORCID iD: 0009-0008-6272-0368
junior researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowAnastasia S. Chugunova
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: 170297_97@mail.ru
ORCID iD: 0009-0009-5964-6045
laboratory assistant researcher, Laboratory of immunobiotechnology
Russian Federation, MoscowInna V. Dolzhikova
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0003-2548-6142
Cand. Sci. (Biol.), Head, Laboratory of the State Virus Collection
Russian Federation, MoscowDmitry N. Shcherbinin
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dim284@inbox.ru
ORCID iD: 0000-0002-8518-1669
Cand. Sci. (Biol.), Laboratory of molecular biotechnology
Russian Federation, MoscowDmitry V. Shcheblyakov
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: sdmitriyv@mail.ru
ORCID iD: 0000-0002-1289-3411
Cand. Sci. (Biol.), leading researcher, Head, Laboratory of immunobiotechnology
Russian Federation, MoscowDenis Y. Logunov
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: logunov@gamaleya.org
ORCID iD: 0000-0003-4035-6581
Dr. Sci. (Biol.), RAS academician, Head, Laboratory of cellular microbiology
Russian Federation, MoscowAlexander L. Gintsburg
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: gintsburg@gamaleya.org
ORCID iD: 0000-0003-1769-5059
Dr. Sci. (Biol.), RAS Academician, Director
Russian Federation, MoscowReferences
- Emerson G.L., Li Y., Frace M.A., et al. The phylogenetics and ecology of the orthopoxviruses endemic to North America. PLoS One. 2009;4(10):e7666. DOI: https://doi.org/10.1371/journal.pone.0007666
- McInnes C.J., Wood A.R., Thomas K., et al. Genomic characterization of a novel poxvirus contributing to the decline of the red squirrel (Sciurus vulgaris) in the UK. J. Gen. Virol. 2006;87(Pt. 8):2115–25. DOI: https://doi.org/10.1099/vir.0.81966-0
- Smits J.E., Tella J.L., Carrete M., et al. An epizootic of avian pox in endemic short-toed larks (Calandrella rufescens) and Berthelot’s pipits (Anthus berthelotti) in the Canary Islands, Spain. Vet. Pathol. 2005;42:59–65. DOI: https://doi.org/10.1354/vp.42-1-59
- Thiel T., Whiteman N.K., Tirapé A., et al. Characterization of canarypox-like viruses infecting endemic birds in the Galápagos islands. J. Wildl. Dis. 2005;41(2):342–53. DOI: https://doi.org/10.7589/0090-3558-41.2.342
- Voigt E.A., Kennedy R.B., Poland G.A. Defending against smallpox: a focus on vaccines. Expert Rev. Vaccines. 2016;15(9):1197–211. DOI: https://doi.org/10.1080/14760584.2016.1175305
- Henderson D.A. The eradication of smallpox – an overview of the past, present, and future. Vaccine. 2011;29(Suppl. 4):D7–9. DOI: https://doi.org/10.1016/j.vaccine.2011.06.080
- Nuzzo J.B., Borio L.L., Gostin L.O. The WHO declaration of monkeypox as a global public health emergency. JAMA. 2022; 328(7): 615–7. DOI: https://doi.org/10.1001/jama.2022.12513
- WHO. Executive board, 154th session. Provisional agenda item 18 "Smallpox eradication: destruction of variola virus stocks"; 2024.
- Rothenburg S., Yang Z., Beard P., et al. Monkeypox emergency: Urgent questions and perspectives. Cell. 2022;185(18):3279–81. DOI: https://doi.org/10.1016/j.cell.2022.08.002
- Zhang R.R., Wang Z.J., Zhu Y.L., et al. Rational development of multicomponent mRNA vaccine candidates against mpox. Emerg. Microbes Infect. 2023;12(1):2192815. DOI: https://doi.org/10.1080/22221751.2023.2192815
- Galinski M.S. Paramyxoviridae: transcription and replication. Adv. Virus Res. 1991;39:129–62. DOI: https://doi.org/10.1016/S0065-3527(08)60794-0
- Curran J., Kolakofsky D. Replication of paramyxoviruses. Adv. Virus Res. 1999;54:403–22. DOI: https://doi.org/10.1016/S0065-3527(08)60373-5
- Law M., Smith G.L. Antibody neutralization of the extracellular enveloped form of vaccinia virus. Virology. 2001;280(1):132–42. DOI: https://doi.org/10.1006/viro.2000.0750
- Riccardo V., Pablo G.C. Neutralization determinants on poxviruses. Viruses. 2023;15(12):2396. DOI: https://doi.org/10.3390/v15122396
- Moss B. Smallpox vaccines: targets of protective immunity. Immunol. Rev. 2011;239(1):8–26. DOI: https://doi.org/10.1111/j.1600-065X.2010.00975.x
- Lustig S., Fogg C., Whitbeck J.C., et al. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J. Virol. 2005;79(21):13454–62. DOI: https://doi.org/10.1128/JVI.79.21.13454-13462.2005
- Mucker E.M., Golden J.W., Hammerbeck C.D., et al. A nucleic acid-based orthopoxvirus vaccine targeting the vaccinia virus L1, A27, B5, and A33 proteins protects rabbits against lethal rabbitpox virus aerosol challenge. J. Virol. 2022;96(3):e0150421. DOI: https://doi.org/10.1128/JVI.01504-21
- Gilchuk I., Gilchuk P., Sapparapu G., et al. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell. 2016;167(3):684–94.e9. DOI: https://doi.org/10.1016/j.cell.2016.09.049
- Sakhatskyy P., Wang S., Chou T.W., Lu S. Immunogenicity and protection efficacy of monovalent and polyvalent poxvirus vaccines that include the D8 antigen. Virology. 2006;355(2):164–74. DOI: https://doi.org/10.1016/j.virol.2006.07.017.
- Niles E.G., Seto J. Vaccinia virus gene D8 encodes a virion transmembrane protein. J. Virol. 1988;62(10):3772–8. DOI: https://doi.org/10.1128/jvi.62.10.3772-3778.1988
- Takahashi T., Oie M., Ichihashi Y. N-terminal amino acid sequences of vaccinia virus structural proteins. Virology. 1994;202(2): 844–52. DOI: https://doi.org/10.1006/viro.1994.1406
- Chertov O.Yu., Telezhinskaya I.N., Zaitseva E.V., et al. Amino acid sequence determination of vaccinia virus immunodominant protein p35 and identification of the gene. Biomed. Sci. 1991;2(2):151–4.
- Franke C.A., Wilson E.M., Hruby D.E. Use of a cell-free system to identify the vaccinia virus L1R gene product as the major late myristylated virion protein M25. J. Virol. 1990;64(12):5988–96. DOI: https://doi.org/10.1128/jvi.64.12.5988-5996.1990
- Hooper J., Custer D., Thompson E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology. 2003;306(1):181–95. DOI: https://doi.org/10.1016/S0042-6822(02)00038-7
- Golden J.W., Zaitseva M., Kapnick S., et al. Polyclonal antibody cocktails generated using DNA vaccine technology protect in murine models of orthopoxvirus disease. Virol. J. 2011;8:441. DOI: https://doi.org/10.1186/1743-422X-8-441
- Berhanu A., Wilson R.L., Kirkwood-Watts D.L., et al. Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J. Virol. 2008;82(7):3517–29. DOI: https://doi.org/10.1128/JVI.01854-07
- Fogg C., Lustig S., Whitbeck J.C., et al. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 2004;78(19):10230–7. DOI: https://doi.org/10.1128/JVI.78.19.10230-10237.2004
- Logunov D.Y., Dolzhikova I.V., Zubkova O.V., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–97. DOI: https://doi.org/10.1016/S0140-6736(20)31866-3
- Dolzhikova I.V., Zubkova O.V., Tukhvatulin A.I., et al. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: An open phase I/II trial in healthy adults in Russia. Hum. Vaccin. Immunother. 2017;13(3):613–20. DOI: https://doi.org/10.1080/21645515.2016.1238535
- Tukhvatulin A.I., Dolzhikova I.V., Shcheblyakov D.V., et al. An open, non-randomised, phase 1/2 trial on the safety, tolerability, and immunogenicity of single-dose vaccine «Sputnik Light» for prevention of coronavirus infection in healthy adults. Lancet Reg. Health Eur. 2021;11:100241. DOI: https://doi.org/10.1016/j.lanepe.2021.100241
- Yakimovich A., Mercer J. High-content analyses of vaccinia plaque formation. In: Mercer J., eds. Vaccinia Virus. Methods in Molecular Biology, Volume 2023. New York;2019:237–53. DOI: https://doi.org/10.1007/978-1-4939-9593-6_15
- Ожаровская Т.А., Попова О., Зубкова О.В. и др. Разработка и характеристика векторной системы на основе аденовируса обезьян 25-го серотипа. Вестник РГМУ. 2023;(1):4–11. DOI: https://doi.org/10.24075/vrgmu.2023.006 Ozharovskaia T.A., Popova O., Zubkova O.V., et al. Development and characterization of a vector system based on the simian adenovirus type 25. Bulletin of RSMU. 2023;(1):4–11. DOI: https://doi.org/10.24075/brsmu.2023.006
- Su Q., Sena-Esteves M., Gao G. Purification of the recombinant adenovirus by cesium chloride gradient centrifugation. Cold Spring Harb. Protoc. 2019;2019(5). DOI: https://doi.org/10.1101/pdb.prot095547
- Maizel J.V., White D.O., Scharff M.D. The polypeptides of adenovirus: I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968;36(1):115–25. DOI: https://doi.org/10.1016/0042-6822(68)90121-9
- Shchelkunov S.N., Totmenin A.V., Safronov P.F., et al. Analysis of the monkeypox virus genome. Virology. 2002;297(2):172–94. DOI: https://doi.org/10.1006/viro.2002.1446
- Antoine G., Scheiflinger F., Dorner F., Falkner F.G. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998;244(2): 365–96. DOI: https://doi.org/10.1006/viro.1998.9123
- Diaz-Cánova D., Mavian C., Brinkmann A., et al. Genomic sequencing and phylogenomics of cowpox virus. Viruses. 2022;14(10):2134. DOI: https://doi.org/10.3390/v14102134
- Zrelkin D.I., Goldovskaia P.P., Kan V.Y. Determination of threshold value of protective level of antibodies to one of members of orthopoxvirus genus. In: Scientific Research of the SCO Countries: Synergy and Integration. Beijing;2024. DOI: https://doi.org/10.34660/INF.2024.87.21.085
- Максютов Р.A., Якубицкий С.Н., Колосова И.В., Щелкунов С.Н. Сравнение кандидатных вакцин нового поколения против ортопоксвирусных инфекций человека. Acta Naturae. 2017;9(2):93–99. Maksyutov R.A., Yakubitskyi S.N., Kolosova I.V., Shchelkunov S.N. Comparing new-generation candidate vaccines against human orthopoxvirus infections. Acta Naturae. 2017;9(2):88–93. DOI: https://doi.org/10.32607/20758251-2017-9-2-88-93 EDN: https://elibrary.ru/yyylkl
- Стовба Л.Ф., Чухраля О.В., Черникова Н.К. и др. Безопасность и иммуногенность вакцины третьего поколения IMVAMUNE® на основе вируса вакцины, штамм MVA. БИОпрепараты. Профилактика, диагностика, лечение. 2023;23(1):26–41. Stovba L.F., Chukhralya O.V., Chernikova N.K., et al. Safety and immunogenicity of IMVAMUNE®, a third-generation vaccine based on the modified vaccinia Ankara (MVA) strain. Biological Products. Prevention, Diagnosis, Treatment. 2023;23(1):26–41. DOI: https://doi.org/10.30895/2221-996X-2023-23-1-26-41 EDN: https://elibrary.ru/tjwrhx
- Щелкунов С.Н., Сергеев А.А., Титова К.А. и др. Увеличение протективности противооспенной вакцины. Медицинская иммунология. 2022;24(1):201–6. Shchelkunov S.N., Sergeev A.A., Titova K.A., et al. Increasing protectivity of the smallpox vaccine. Medical Immunology (Russia). 2022;24(1):201–6. DOI: https://doi.org/10.15789/1563-0625-IPO-2203 EDN: https://elibrary.ru/asgrmi
- Щелкунов С.Н., Якубицкий С.Н., Титова К.А. и др. Аттенуированный и высокоиммуногенный вариант вируса осповакцины. Acta Naturae. 2024;16(2):82–9. Shchelkunov S.N., Yakubitskiy S.N., Titova K.A., et al. An attenuated and highly immunogenic variant of the vaccinia virus. Acta Naturae. 2024;16(2):82–9. DOI: https://doi.org/10.32607/actanaturae.27384 EDN: https://elibrary.ru/kcsbnp
- Payne L.G., Kristensson K. Extracellular release of enveloped vaccinia virus from mouse nasal epithelial cells in vivo. J. Gen. Virol. 1985;66(Pt. 3):643–6. DOI: https://doi.org/10.1099/0022-1317-66-3-643
- Kenner J., Cameron F., Empig C., et al. LC16m8: аn attenuated smallpox vaccine. Vaccine. 2006;24(47-48):7009–22. DOI: https://doi.org/10.1016/j.vaccine.2006.03.087
- Gao J., Mese K., Bunz O., Ehrhardt A. State‐of‐the‐art human adenovirus vectorology for therapeutic approaches. FEBS Lett. 2019;593(24):3609–22. DOI: https://doi.org/10.1002/1873-3468.13691
- Hervouet C., Luci C., Bekri S., et al. Antigen-bearing dendritic cells from the sublingual mucosa recirculate to distant systemic lymphoid organs to prime mucosal CD8 T cells. Mucosal Immunol. 2014;7(2):280–91. DOI: https://doi.org/10.1038/mi.2013.45
- Holmgren J., Czerkinsky C. Mucosal immunity and vaccines. Nat. Med. 2005;11(4 Suppl.):S45–53. DOI: https://doi.org/10.1038/nm1213
- Wang Y., Yang K., Zhou H. Immunogenic proteins and potential delivery platforms for mpox virus vaccine development: а rapid review. Int. J. Biol. Macromol. 2023;245:125515. DOI: https://doi.org/10.1016/j.ijbiomac.2023.125515
- Kaufman D.R., Goudsmit J., Holterman L., et al. Differential antigen requirements for protection against systemic and intranasal vaccinia virus challenges in mice. J. Virol. 2008;82(14):6829–37. DOI: https://doi.org/10.1128/JVI.00353-08
- Agrawal S., Gupta S., Agrawal A. Vaccinia virus proteins activate human dendritic cells to induce T cell responses in vitro. Vaccine. 2009;27(1):88–92. DOI: https://doi.org/10.1016/j.vaccine.2008.10.031
- Monath T.P., Caldwell J.R., Mundt W., et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain) — a second-generation smallpox vaccine for biological defense. Int. J. Infect. Dis. 2004;8(Suppl. 2):S31–44. DOI: https://doi.org/10.1016/j.ijid.2004.09.002
Supplementary files