A clinical study of the immunogenicity and protective potency of a live recombinant GamLPV vaccine for intranasal use for the prevention of whooping cough in adult volunteers
- Authors: Lidzhieva A.A.1, Medkova A.Y.1, Kulikov S.V.1, Sinyashina L.N.1, Sioundioukova R.A.1, Markov A.P.1, Verveda A.B.2,3, Abaeva N.E.4, Chernyshova I.N.4, Gavrilova M.V.1,4, Bushkova K.K.4, Dyakov I.N.1,4, Karataev G.I.1
-
Affiliations:
- N.F. Gamaleya National Research Center for Epidemiology and Microbiology
- Research Institute of Industrial and Maritime Medicine
- Eco-Safety Research Center LLC
- I.I. Mechnikov Research Institute for Vaccines and Sera
- Issue: Vol 101, No 6 (2024)
- Pages: 779-793
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18724
- DOI: https://doi.org/10.36233/0372-9311-585
- EDN: https://elibrary.ru/vzqpli
- ID: 18724
Cite item
Abstract
Introduction. The increase in incidence rate of pertussis worldwide, short-term insufficient immunity induced by acellular pertussis vaccines (TDaP) and their failure to provide antibacterial protection and to prevent transmission of infection in human population dictate the development of new pertussis vaccines. A new live recombinant pertussis vaccine for intranasal use (GamLPV) has completed preclinical studies in experiments on nonhuman primates and 2 phases of clinical trials involving adult healthy volunteers, in which the safety, immunogenicity and protective activity of the GamLPV were proven. Method and scheme of vaccine administration have been worked out.
Aim. Confirmation of the immunogenicity and protective antibacterial potency of GamLPV in a randomized multicenter clinical trial on adult volunteers.
Materials and methods. In this multicenter, clinical, randomized, placebo-controlled, double-blind study 260 healthy adults aged 18–65 years were divided into 2 groups: G1 — 210 volunteers (GamLPV) and G2 — 50 volunteers (placebo). 0.25 ml GamLPV delivered to each nostril (5 × 109 CFU) 60 days apart. Levels of Bordetella pertussis-specific IgG, IgA antibodies in blood serum and levels of B. pertussis-specific secretory IgA antibodies in nasopharyngeal aspirates were measured by ELISA method and agglutination test. The dynamics of elimination of attenuated B. pertussis bacteria after the first and second intranasal administration of GamLPV to volunteers was estimated by using qPCR.
Results. Significant seroconversion of B. pertussis-specific IgG and IgA antibodies and growth of B. pertussis-specific secretory IgA antibody levels in nasal aspirates of volunteers were demonstrated. The dynamics of changes in the levels of IgG and IgA antibodies indicates a booster effect after second vaccination. Attenuated B. pertussis bacteria persist in the nose/oropharynx of vaccinated volunteers. The period of elimination after second vaccination is more than 2 times shorter than the period after the first one. The number of persistent B. pertussis bacteria after the second vaccination is less than 3% of the values after the first vaccination.
Conclusion. High immunogenicity and the formation of antibacterial protection after single and double intranasal vaccination of GamLPV have been proven.
Full Text
Introduction
Pertussis is a highly contagious, airborne infectious disease controlled by vaccine prophylaxis. Vaccination against pertussis is included in the mandatory vaccination calendars of most countries of the world, starting from early childhood. According to the World Health Organization, 42 countries currently use tetanus-diphtheria-acellular pertussis vaccines (TDaP) containing an acellular pertussis component (aPC) and 143 countries use an adsorbed tetanus-diphtheria-pertussis vaccine. However, since the 2000s, the mass use of pertussis vaccine in economically developed countries has led to an increase in the incidence of pertussis in these countries, approaching in some years the level of the pre-vaccine period [1]. The age structure has changed towards an increase in the percentage of incidence among adolescents and adults [2]. The increasing incidence of pertussis among older children and adults has led to the realization of the necessity for revaccination. The feasibility of vaccination of pregnant women [3], mothers and close relatives to form a familial immunity to prevent infection of infants is being studied [4]. Low efficacy and short duration of immunity after aPC vaccination, as well as their inability to form antimicrobial immunity, are considered as one of the reasons for the increase in morbidity [5]. The reactogenicity of the pertussis corpuscular component and the low efficacy of aPC stimulate the development of new preparations capable of inducing long-term antibacterial immunity, which are not harmful during vaccination and are convenient for mass application.
The N.F. Gamaleya NRCEM has developed a live recombinant vaccine for intranasal administration for pertussis prophylaxis, called GamLPV. To date, the GamLPV vaccine has undergone preclinical studies and phase II clinical trials on adult healthy volunteers, which proved its safety, immunogenicity and protective activity, and the method and scheme of drug administration have been worked out [6–10]. A similar recombinant live pertussis vaccine (BPZE1) developed in France is at the stage of clinical trials [11–13].
The aim of this study is to confirm the immunogenicity and protective activity of the GamLPV vaccine when administered twice in adult volunteers aged 18–65 years in a multicenter clinical trial.
Materials and methods
Study design
A randomized placebo-controlled, blind, multicenter clinical trial was conducted to investigate the immunogenicity and safety of the GamLPV live vaccine according to:
- Тhe Protocol of the clinical trial No. 03-GamLPV-2021, version 3 dated 28.01.2021, permission of the Ministry of Health of Russia No. 277 dated 08.06.2021.
- The Protocol of Clinical Trial No. 03-GamLPV-2021 was approved by the Ethics Council under the Ministry of Health of the Russian Federation (extract No. 277 dated 08.06.2021), as well as by local ethics committees:
- Ethical Committee of the Infectious Disease Clinical Hospital No. 1 of the Moscow Health Department (meeting protocol No. 5 of 08.12.2021);
- Ethics Committee of the Eco-safety research center LLC (extract No. 5 from meeting protocol No. 221 dated 20.01.2022; extract No. 2 from meeting protocol No. 222 dated 27.01.2022).
The inclusion and non-inclusion criteria relevant for assessing the immunogenicity of the vaccine used and the bacterial load in nasopharyngeal aspirates are summarized below. The full list of inclusion criteria, non-inclusion criteria, early exclusion of the volunteer from the study, composition and route of administration of the preparation, criteria for assessing the immunogenicity and efficacy of the preparation according to the Protocol are presented in the Appendix to the article on the journal's website.
When the protocol was being prepared, the level of IgG antibodies to Bordetella pertussis at the time of inclusion was used as one of the inclusion criteria in the study. The range of values of IgG antibody level was determined on the basis of data obtained using the Ridascreen test system. According to this system, volunteers with IgG antibody levels < 14 U/mL were considered seronegative (SN), antibody levels were considered indeterminate (gray zone, GZ) between 14 and < 18 U/mL, and volunteers with IgG antibody levels ≥ 18 U/mL were considered seropositive (SP). According to the protocol, both SN and mild to moderate SP volunteers with IgG antibody levels of 45 U/mL or less could be included in the study. By the time of inclusion of volunteers in the study, the Ridascreen test system was replaced by the ESR120G test system (Virion/Serion GmbH) due to organizational difficulties associated with the unavailability of the Ridascreen test system in Russia. According to the instructions for the ESR120G kit (Virion/Serion GmbH), samples with antibody levels < 40 IU/mL are considered to be SN, 40-50 IU/mL is considered to be the gray zone, and antibody levels of 50 IU/mL or higher are considered to be SP. An IgG antibody level of 45 IU/mL when determined using the Ridascreen kit is equivalent to an IgG antibody level of 126 IU/mL when determined using the ESR120G kit (Virion/Serion GmbH).
Inclusion criteria: men and women aged 18–65 years; medically stable condition, absence of pertussis-specific IgM antibodies, pertussis-specific IgG antibodies < 126 IU/mL (SERION ELISA classic ESR120G), absence of B. pertussis DNA in nasopharyngeal aspirates confirmed by polymerase chain reaction (PCR).
Exclusion criteria: history of pertussis; vaccination against pertussis within the last 10 years and any vaccination within the last year; any disease that, in the opinion of the investigator, may affect the results of the study or may lead to deterioration of health status during the study; reported severe post-vaccination complications in the history; taking medications for prophylactic or therapeutic purposes within 1 month before screening; participation in other clinical trials, presence of specific IgM antibodies to pertussis pathogen, specific IgG antibody level > 126 IU/mL (SERION ELISA classic ESR120G), presence of B. pertussis DNA in nasopharyngeal/ oropharyngeal aspirates.
Survey of volunteers and randomization
All volunteers participating in the study signed a voluntary informed consent form, after which they were assigned a number and screened. Compliance with all inclusion/non-inclusion criteria was confirmed before inclusion of volunteers in the study. The study population consisted of 260 male and female volunteers aged 18-65 years (inclusive), selected according to inclusion criteria and without non-inclusion criteria. Volunteers were allocated into 2 groups: those who received GamLPV (vaccinated group; n = 210) and those who received placebo (placebo group; n = 50) according to the protocol's randomization procedure. The vaccinated group was divided into 3 subgroups according to the content of specific pertussis IgG antibodies before the 1st vaccine administration: 1st subgroup, SN (n = 168); 2nd subgroup, GZ (n = 7); 3rd subgroup, SP (n = 35).
The clinical study included the 1st administration of the drug, observation period of 60 ± 5 days, and the 2nd administration of the drug and observation period of 60 ± 5 days. Determination of the level of specific IgG and IgA antibodies (enzyme-linked immunosorbent assay (ELISA), in blood serum, oro- and nasopharyngeal secretions) and titers of agglutination of bacterial suspension of pertussis pathogen (agglutination reaction, AR) by serum of volunteers at each drug administration were performed before administration (day 1), after administration on days 8, 15, 29 and 60. Determination of bacterial load in oro- and nasopharyngeal secretions by PCR at each drug administration was performed before drug administration, after the 1st administration after 1 h (day 1), on days 4, 8, 15, 29 and 60. Randomization and visit procedures are presented in the Appendix of the article on the journal website.
Study drug, doses and route of administration
GamLPV, a live vaccine for intranasal administration for the prevention of pertussis based on attenuated B. pertussis 4MKS bacteria [14], in the form of a lyophilizate for the preparation of a suspension for intranasal administration was produced by the Medgamal branch of the N.F. Gamaleya NRCEM. The vaccine series used in the clinical study passed all the necessary control stages.
The drug was administered at a dose of 5 × 109 CFU in the form of a suspension intranasally by injecting 0.25 mL into each nasal passage through a syringe without a needle, twice, with an interval of 2 months (60 ± 5 days). Sterile solution of stabilizer lyophilizate was used as placebo. Both preparations were reconstituted in 1 mL of 0.9% NaCl for injection.
Quantification of B. pertussis DNA
For real-time PCR (qPCR), we used DNA isolated from oropharyngeal and nasopharyngeal probes (hereinafter referred to as aspirates) placed in a single tube. After centrifugation, the samples were treated with guanidinedithiocyanate solution followed by DNA sorption on a sorbent [15]. To determine the amount of genome equivalent (GE) of B. pertussis DNA, the qPCR test system developed and validated by us was used [16]. qPCR was performed on the CFX-96 Touch thermocycler (Bio-Rad).
The elimination time of attenuated bacteria from the nasopharynx and oropharynx was estimated as a time interval in days from the moment of administration (1 h) until the established level was reached. The average B. pertussis DNA level of 0.7 GE/mL in both groups at the screening stage was taken as the level at which the elimination of bacteria was determined to be complete. If the indicated level was not reached, the elimination time was taken as 100 days. To compare the groups of volunteers after the 2nd bacterial administration and the 1st vaccination, we used the Kaplan–Meier method (Survival Analysis — Comparison of Two Samples module of the Statistica v. 10.0 program), which allows the use of censored data. Cases that did not reach the set level of 0.7 GE/mL were classified as censored data. Such cases were reported after the 1st vaccination, representing 6.2% (13 of 210), and 1 case less than 1.0% after the 2nd vaccination (1 of 202). The appropriateness of this method is because, as with the survival analysis, not all individuals had completed excretion by the time the study was completed (censored data), but they were not excluded but were used in the analysis. Differences were assessed using the log-rank test.
Methods for assessing the immunogenicity of GamLPV
Immunogenicity of GamLPV was evaluated by induction of specific IgG and IgA antibodies in the blood serum of volunteers detected by ELISA and AR. Local immune response was evaluated by the dynamics of specific secretory IgA antibodies to B. pertussis in nasopharyngeal aspirates.
Blood samples were collected in vacuum tubes with blood clotting activator (Vacuette). Serum was collected after centrifugation of samples for 20 min at 300g to thicken the clot. The level of specific IgG and IgA antibodies to pertussis pathogen in serum was determined by ELISA according to the instructions of the manufacturer of the ESR120G, ESR120A and ESR120M test systems (Virion/Serion GmbH). IgA antibodies in aspirates, as well as in blood serum, was determined using the ESR120A test system (Virion/Serion GmbH), but the samples were diluted 2-fold instead of 100 (according to the manufacturer's instructions). To obtain absolute values of antibody concentration in aspirates, the values calculated from the calibration curve were divided by 50.
AR was performed on kits produced by Research and Production Association Ecolab. The titer was considered to be the highest serum dilution at which an AR of at least “+++” (three crosses) was obtained.
Statistical processing of data
Mathematical and statistical analysis of the results was performed using the Statistica v. 10.0, R 4.2.1 and Microsoft Office Excel 2013 software packages to generate graphs and tables.
The obtained quantitative data were checked for compliance with the law of normal distribution. To determine normality, the Kolmogorov–Smirnov criterion (for a sample size of more than 50 volunteers) or the W Shapiro–Wilk criterion (for a sample size of less than 50 volunteers) were used. If the statistic was significant, the hypothesis of normal distribution of the variable values was rejected.
Immunogenicity in the studied groups represented by qualitative features (fractions) was assessed using the χ2 test criterion when the expected frequency of occurrence of a feature was more than 5 or the two-sided Fisher's exact test when the condition for the χ2 criterion was not met.
Immunogenicity points for qualitative criteria are presented as absolute frequencies (trait occurrence), relative frequencies (%), and 95% confidence interval of the proportion (Clopper-Pearson).
Quantitative criteria of immunogenicity were evaluated using nonparametric methods (Mann–Whitney test) or Student's t-test for independent samples according to the nature of distribution. The data were also presented taking into account the nature of distribution.
To compare several independent samples (more than 2) represented by quantitative variables, we used the Kraskell–Wallis rank analysis of variances. If statistically significant differences between all groups were established, the “a posteriori” method “Comparison of mean ranks for all groups” (nonparametric Dunn's test), which takes into account the problem of multiple comparisons, was used to identify differences between individual groups.
In addition to the descriptive statistics given for safety, geometric mean values with 95% confidence intervals were used to represent agglutination titers.
Log-rank test was used to compare groups of volunteers after the 1st and 2nd bacterial administration.
Two-sided criteria were used for immunogenicity points.
The magnitude of the error to confirm the null hypothesis should be greater than 0.05 (at p ≤ 0.05 the null hypothesis is rejected, at p > 0.05 — accepted).
Results
Dynamics of specific anti-pertussis IgG and IgA antibodies in serum and IgA antibodies in nasopharyngeal and oropharyngeal aspirates.
The results of IgG and IgA antibody measurements by subgroups are presented in Figs. 1, 2. The significance of statistical differences between the total vaccinated group and placebo was assessed using the Mann–Whitney test. Statistically significant differences between subgroups were assessed using nonparametric analysis of variance — Kraskell–Wallis rank analysis of variances and Dunn's posterior nonparametric test (tables not shown).
Fig. 1. B. pertussis-spesific IgG antibody dynamics in blood serum of vaccinated volunteers and additional groups SN, GZ and SP.
Here and on the Figs. 2–4: days after administration of GamLPV to volunteers from additional groups are plot on X-axis: 0_1 — the 1st day of administration of GamLPV (before administration), 8_1, 15_1, 29_1, 60_1 – 8, 15, 29 and 60 days after the 1st administration of GamLPV respectively; 8_2, 15_2, 29_2, 60_2 — 8, 15, 29, 60 days after the 2nd administration of GamLPV respectively. IgG antibody concentration (IU/mL) in the blood serum of volunteers is plot on Y-axis.
Fig. 2. B. pertussis-spesific IgA antibody dynamics in blood serum of vaccinated volunteers and additional groups SN, GZ and SP.
IgA antibody concentration (IU/mL) in the blood serum of volunteers is plot on Y-axis.
There were no significant differences in IgG antibodies between the total vaccinated group and the placebo group at the time of administration of GamLPV. On the 8th day after the 1st administration, IgG antibody levels in the total vaccinated group increased significantly compared to the placebo group (p = 0.012); starting on the 15th day after the 1st vaccination, differences between groups became highly statistically significant (p < 0.0001). Compared to baseline (19 IU/mL), median IgG antibody levels in the vaccinated group after the 1st administration of the drug on days 8, 15, 29, and 60 increased 1.1, 2.3, 3.6, and 3.8-fold, respectively. After the 2nd administration of GamLPV, the multiplicity of increase in IgG levels on days 8, 15, 29, and 60 was 3.7, 4.2, 3.9 and 3.8-fold, respectively (70.5–79.5 IU/mL). In the placebo group, median IgG antibody levels did not change significantly and ranged from 16.8–21.0 IU/mL during the course of the study.
Before the 1st administration of GamLPV, the median IgG level in each subgroup (SN, GZ, SP) was significantly different from the median of the total vaccinated group. Significant differences were also observed between the subgroups of SN and SP, SN and GZ. Within the observation period, the median IgG antibody level in the SP subgroup was significantly different from the median for the total vaccinated group on day 15 after the 1st administration (p = 0.001), and on days 29 (p = 0.023) and 60 (p = 0.001) after the 2nd administration of GamLPV. Significant differences between the SN and SP groups were observed on days 15 (p = 0.001) and 29 (p = 0.023) after the 1st administration and on days 29 (p = 0.003) and 60 (p = 0.001) after the 2nd administration of GamLPV. IgG antibody levels in the GZ subgroup were not significantly different from values for the other subgroups or the total vaccinated group at any of the post-vaccination controls.
When assessing the level of specific IgA antibodies in the blood, significant differences were found between the vaccinated and placebo groups — 7.0 and 4.8 IU/mL, respectively (p = 0.014). Both values were significantly less than the seronegativity cut-off limit (< 25 IU/mL). After the 1st vaccination from day 8 onwards, the significance of differences between groups became highly significant (p < 0.0001) and remained at this level until the end of the study. In the vaccinated group after the 1st administration, the median values on days 8, 15, 29 and 60 increased 1.3, 3.6, 4.9 and 5.4 times, respectively, compared to baseline. After the 2nd vaccination, a booster effect was observed on day 29 — IgA antibody levels increased 6.2-fold compared to baseline. By day 60, the IgA level returned to the level detected before the 2nd administration. In the placebo group no significant changes were found during the observation period — the median values ranged from 4.0–6.0 IU/mL. Subgroup analysis showed that at the time before the 1st administration of GamLPV the level of IgA antibodies to B. pertussis significantly differed between the SP and SN subgroups (p = 0.001), while in the SP subgroup the antibody level also significantly differed from the value characteristic of the general group of vaccinated persons (p = 0.001). At subsequent follow-up checkpoints, up to day 8 after the 2nd administration of GamLPV, there were no significant differences between the subgroups. On day 15 after the 2nd administration, significant differences were again found between the SP and SN subgroups (p = 0.001), as well as between the SP subgroup and the value for the total vaccinated group (p = 0.001). On days 29 and 60 after the 2nd administration, significant differences in IgA antibody levels to B. pertussis persisted only between the SP and SN groups (p = 0.049). For the SP subgroup, no significant differences in the median IgA antibody level with the other subgroups were found at any of the study controls.
Determination of specific secretory anti-pertussis IgA antibodies in nasopharyngeal and oropharyngeal flushes from volunteers revealed statistically highly significant (p < 0.0001) differences between the vaccinated and placebo groups at all controls (Fig. 3). In the vaccinated group, compared to baseline (0.20 IU/mL), the median levels of secretory IgA antibodies on days 15, 29 and 60 after the 1st vaccination increased 2.5, 4.5 and 3.2-fold, respectively. This increased continuously after the 2nd vaccination and on days 8, 15 and 29, the multiplicity of increase was 4.4, 5.2 and 5.8, respectively. By day 60, the median level of secretory IgA antibodies returned to the values detected on day 60 after the 1st administration of GamLPV. Subgroup analysis showed significant differences in secretory IgA antibody levels between the SN and SP subgroups on day 29 after the 2nd vaccine administration (p = 0.031).
Fig. 3. B. pertussis-specific secretory IgA antibody (median values) dynamics in nasopharyngeal/oropharyngeal aspirates of vaccinated volunteers and additional groups SN, GZ and SP.
Secretory IgA antibody concentration (IU/ml) in nasopharyngeal/oropharyngeal aspirates is plot on Y-axis.
Dynamics of change in the proportion of volunteers with more than twofold increase in the level of specific anti-pertussis IgA and IgG antibodies in serum and nasopharyngeal aspirates. The multiplicity of increase in the amount of specific anti-pertussis IgA and IgG antibodies in aspirates and in serum at each visit was analyzed in comparison with the data before drug administration (Fig. 4).
Fig. 4. The dynamics of the proportion of vaccinated volunteers with more than a twofold increase of B. pertussis-specific IgG antibodies in blood serum.
The proportion of volunteers (%) with more than a twofold increase of B. pertussis-specific IgG antibodies in blood serum is plot on Y-axis.
The proportion of volunteers with at least a twofold increase in the level of IgG antibodies in the blood in the vaccinated group was significantly different (χ2 test, p < 0.0001) from the placebo group as early as day 15 after the 1st injection, and the differences remained significant until the end of the study. On days 15, 29, and 60 after the 1st administration and on days 8, 15, 29 and 60 after the 2nd administration, the proportion of volunteers who achieved this rate was 46.4, 69.3, 72.8, 73.1, 75.0, 77.9, and 77.0%, respectively. Subgroup analysis showed that in the SN subgroup, at day 29 after the 1st administration, the proportion was 84.3%. This value increased at the following time points, reaching a maximum of 92.4% on day 29 after the 2nd administration. In the SP and GZ subgroups, these values ranged from 11.4–17.1 and 14.3–57.1% during the same period, respectively.
When assessing IgA antibody levels, it was found that from day 15 after the 1st injection until the end of the analysis, the proportion of patients with at least a twofold increase in serum IgA antibody levels in the vaccinated group differed from the placebo group with high reliability (χ2 test, p < 0.0001). On days 15, 29 and 60 after the 1st administration, the percentage of such volunteers in the vaccinated group was 71.8, 85.1 and 86.1%, respectively. After the 2nd vaccination on days 8, 15, 29 and 60, this proportion in the vaccinated group was 88.1, 88.1, 90.0, and 89.0%, respectively. Subanalysis of subgroups showed that in the SN subgroup, a twofold increase in the level of IgA antibodies in blood was observed in more than 90.0% of volunteers from day 29 of the study to the end of the study (91.6–94.9%). In the SP subgroup these values ranged from 60.0–74.3%, in the SP subgroup — 57.1–85.7%.
The dynamics of the level of secretory IgA antibodies was similar to that of IgA antibodies in serum. Highly significant differences (p < 0.0001) between the vaccinated and placebo groups were detected from day 15 after the 1st vaccine injection. The proportion of volunteers with a twofold increase in IgA antibody levels on days 15, 29 and 60 after the 1st administration and on days 8, 15, 29 and 60 after the 2nd vaccine administration was 58.4, 71.6, 70.8, 80.2, 82.6, 84.0 and 73.5%, respectively. There were no significant differences between the SN, GZ and SP subgroups, except for one point, 29 days after the 2nd vaccine administration, at which the SN and SP subgroups differed significantly (p = 0.031).
Timing of reaching maximum values of IgA and IgG antibodies in serum after the 2nd vaccination compared to the 1st vaccination. In order to analyze the time to reach the maximum concentrations, the data of only those volunteers of the vaccinated group were analyzed, in which the antibody level was evaluated at least in 3 points, except for the time of drug administration.
After the 2nd vaccination, a significant reduction in the time to reach maximum antibody levels was observed: more than two-fold for serum IgG and 4-fold for serum IgA; two-fold for secretory IgA (Table).
Comparative evaluation of B. pertussis-specific IgA and IgG antibody median values in blood serum and secretory IgA antibodies in nasopharyngeal/oropharyngeal aspirates of volunteers with time to Сmax in group of vaccinated volunteers after the 1st and the 2nd vaccination
Class of antibodies | Vaccination number | n | Median value of specific antibody content, IU/mL | р1 | Median value of bacterial elimination time, day | р2 |
Serum IgA | 1 | 207 | 44,0 | 0,006 | 56,0 | < 0,0001 |
2 | 200 | 55,5 | 14,0 | |||
Serum IgG | 1 | 208 | 77,5 | < 0,0001 | 59,0 | < 0,0001 |
2 | 200 | 90,0 | 28,0 | |||
Secretory IgA | 1 | 208 | 1,98 | < 0,0001 | 28,0 | < 0,0001 |
2 | 199 | 6,28 | 14,0 |
Note. Reliability of differences was calculated using the Wilcoxon test.
Evaluation of the immune response to vaccination of GamLPV volunteers using AR. The immune response, which characterizes the level of agglutinating antibodies in the serum of vaccinated volunteers, was assessed by the AR method. Antibody titers were presented as geometric mean values with 95% confidence intervals and descriptive statistics for asymmetrically distributed data (tables not presented).
Statistically significant differences between the groups of vaccinated volunteers and those who received placebo were established at all points analyzed. Starting on day 15 after the 1st vaccination, these differences became statistically highly significant (p < 0.0001). The antibody titer in the placebo group did not change significantly throughout the entire examination period.
In the vaccinated group, the geometric mean agglutination titer on day 8 after the 1st vaccination (45.9) was generally consistent with the baseline (42.4). An increase in titer was observed from day 15 after the 1st vaccine administration. The multiplicity of increase in the geometric mean agglutination titer on days 15, 29 and 60 was 1.7, 2.3, and 2.0, respectively. After the 2nd vaccination on days 8, 15 and 29, the multiples were 2.0, 2.6 and 2.7, respectively. By day 60 after the 2nd vaccination, the titer decreased slightly, but the multiplicity increase of 2.6 exceeded that on day 60 after the 1st vaccination.
The proportion of volunteers with an agglutination titer of 1 : 80 or higher on days 8, 15, 29, and 60 after the 1st vaccination was 38.1, 56.0, 73.6, and 65.8%, and 66.8, 77.6, 77.6 and 80.0% after the 2nd vaccination. After the 1st vaccination, the proportion of volunteers with an agglutination titer of 1 : 80 in the total vaccinated group was statistically significantly different from the distinguished subgroups. After the 2nd vaccination, no statistically significant differences were found between subgroups. The highest percentage of individuals who met the specified criterion was found in the group of SP volunteers (from 80.0% on day 15 after the 1st administration to 94.3% on days 15 and 60 after the 2nd vaccine administration). In the SN subgroup, for the same time interval, this indicator ranged from 51.5–75.9%, in the SP subgroup — 42.9–100.0%.
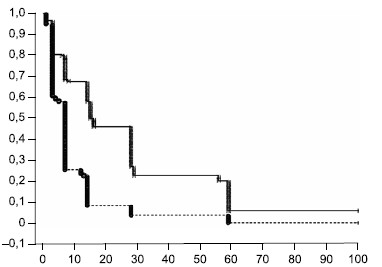
Elimination time of attenuated bacteria from the nasopharynx after the 2nd administration of bacteria compared with the 1st vaccination. Fig. 5 shows the dynamics of elimination of attenuated bacteria from the nasopharynx as a decrease in the cumulative proportion of persons with non-eliminated B. pertussis bacteria in separate time intervals. From the presented data it is evident that elimination of vaccine B. pertussis from the nasopharynx after the 2nd administration of bacteria is faster than after the 1st vaccination of volunteers. At the same time, after the 2nd vaccine administration, there is a greater proportion of volunteers whose elimination process was completed early — within the first 7 days after vaccination.
Fig. 5. The dynamics of cumulative fraction of volunteers who did not eliminate B. pertussis bacteria from the nasopharynx after second administration of bacteria compared with the first vaccination.
Time duration of B. pertussis bacteria elimination starting from the first hour after GamLPV administration are plot on X-axis; the cumulative fraction of volunteers in whose aspirates the DNA of B. pertussis bacteria was detected by PCR in an amount more than 0,7 GE/mL, is plot on Y-axis. The dashed line indicates the second vaccination schedule of volunteers.
Comparative evaluation of the elimination time of attenuated bacteria from the nasopharynx of vaccinated volunteers of the general group after the 1st and 2nd vaccinations using the log-rank criterion showed a statistically highly significant (p < 0.0001) decrease in the elimination duration after the 2nd vaccination from 16 to 7 days according to the median value. The SN subgroup showed the greatest difference in the duration of bacterial elimination period after the 1st and 2nd vaccinations, 28 and 7 days, respectively. In the SP subgroup, the differences were also significant (p = 0.013) — the median duration of bacterial elimination was 14 and 7 days, respectively.
In addition to the elimination time analysis, the total amount of B. pertussis DNA in the nasopharynx of volunteers, during follow-up after the 1st and 2nd vaccinations, was estimated using the linear trapezoidal method. The area under the curve after the 1st vaccination was 6714 (1539) GE day/mL and after the 2nd vaccination was 443 (44) GE day/mL at the median when calculated between days 3 and 60.
Discussion
The results of studying the immunogenicity of GamLPV and virulent B. pertussis bacteria on the experimental model of nonhuman monkeys showed that the first contact with infection induced a slow and unexpressed growth of specific IgG antibodies, lasting, as a rule, until the 46th–60th day of observation. Repeated administration of bacteria resulted in a rapid and pronounced increase in IgG antibodies starting on day 3–7 with a maximum on day 14–28 after the 2nd infection [6, 7]. The results of the phase I clinical trial GamLPV confirmed the slow increase of IgG antibody values after the 1st vaccination [8]. At that time, it was also shown that, at least among volunteers in Moscow and the Moscow region when using the Ridacreen kit, less than 50% of adults aged 40–60 years were SN [8–10].
The tested vaccine is intended for revaccination of adults regardless of their health status and level of specific IgG antibodies. Therefore, it is of practical interest to study the tolerability, immunogenicity and protective activity of GamLPV for volunteers 18–65 years of age who have no obvious health disorder, no symptoms of pertussis and serum IgG antibody level lower than the threshold defined in the country for laboratory diagnosis of pertussis. The lack of studies in Russia determining the serologic status of the population (IgG, IgM and IgA antibody levels) and the absence of in-house ELISA test systems does not allow quantitative assessment of the level of protective antibodies after vaccination and/or disease. Therefore, based on the results of our previous studies and some data from the national literature, an IgG antibody value of 45 U/mL [9, 10], measured using the Ridacreen kit, was accepted as the threshold for inclusion in the study, which allowed more than 80% of screened volunteers to be included in the study. The impossibility of using Ridacreen test systems in the study presented in this article led to the necessity to determine the conversion factor for converting the value of the IgG inclusion criterion limit point < 45 U/mL into international units (IU/mL) measured using the available and registered in Russia ESR120G test system (Institut Virion/Serion GmbH). As a result of semi-empirical calculations, a value equal to 2.8 was accepted as such a coefficient. Thus, the level of 126 IU/mL determined by the ESR120G test system (Virion/Serion GmbH) was taken as the upper limit of the IgG antibody level at which a volunteer could be included in the study. The calculated coefficient was used only to determine the boundary in the inclusion criteria. All other values of IgG and IgA antibody parameters of blood serum of volunteers and aspirates were determined using the ESR120A and ESR120G test systems (Institut Virion/Serion GmbH) in the units used by them (IU/mL).
The choice of parameters and criteria (control points) for assessing the immunogenicity of GamLPV intranasal application by the main indicators is traditional for multicenter studies and is justified in protocols, brochures and reports on the conducted clinical trials. Control points characterizing the level of immunogenicity of the drug are given in the Appendix to the article on the journal's website.
The conducted study demonstrated a significant seroconversion of IgG and IgA antibodies and an increase in the level of secretory IgA antibodies in nasopharyngeal aspirates of volunteers. The values obtained exceeded the baseline antibody levels in volunteers included in the vaccinated group and the antibody levels in volunteers in the placebo group. Significant differences between the levels of IgG and IgA antibodies in the vaccinated and placebo groups were observed already on day 8. Thus, the humoral immune response to intranasal vaccination of volunteers with GamLPV begins to manifest itself one week after the drug administration. The maximum increase of median values of antibody levels to B. pertussis in the serum of volunteers was 6.2 times for IgG and 4.2 times for IgA. The level of IgA antibodies in aspirates maximally increased 5.8 times after the 2nd vaccination. Similar dynamics was registered when assessing seroconversion by AR indicator.
The time to reach maximum levels of IgG and IgA antibodies in serum and secretory IgA antibodies in nasal aspirates after the 1st and 2nd vaccination of volunteers is indicative. In all cases, antibody levels, regardless of isotype, not only exceeded those after the 2nd administration but also peaked at shorter times after the 1st administration.
An important indicator is the proportion of volunteers who reached the target value of exceeding the initial antibody level. The control point chosen by us and included in the protocol assumes achievement of at least a twofold increase in IgG and IgA antibody levels in at least 80% of vaccinated volunteers. When calculated for the total group, this reference point was achieved and exceeded for IgA antibody levels in serum and in aspirates. For IgG antibodies, a twofold increase in antibody levels was observed in 77.9% of vaccinated volunteers.
Analysis of the dynamics of changes in the level of IgG and IgA antibodies in serum by subgroups confirmed the general dynamics of increasing levels of IgA and IgG antibodies. Quite expectedly, the multiplicity of increase in the total vaccinated group and the SN subgroup were close to each other and significantly exceeded the multiplicity of increase in the SP and GZ subgroups (Fig. 4), which is due to the effect of the base (initial antibody level against which the multiplicity was calculated). The insignificant lag in the proportion of volunteers who achieved a twofold increase in the level of IgG antibodies from 80% in the total group of the vaccinated is fully explained by the presence in it of volunteers of the SP and GZ subgroups with initially high IgG antibody values and, accordingly, the impossibility of achieving a high multiplicity of antibody increase by them. Thus, on the 29th day after the 2nd vaccination, the proportion of volunteers with a twofold or more increase in the level of IgG antibodies in serum among all vaccinated persons was 77.9%, whereas in the SN subgroup this level exceeded 92%, in the SP subgroup it was only 17.1%, and in the GZ subgroup — 57.1%. The SP and GZ subgroups together account for about 20% of the total vaccinated group, which affected the final value and determined the minimum value (2.1%) of this indicator, which was insufficient to achieve the stated primary endpoint of 80.0% of patients with a twofold increase in IgG antibody levels.
It should be noted that our chosen level of 80% and the achievement of this level with double seroconversion were determined by the results of the previous stage of the study, which included only SN by IgG antibody volunteers. As can be seen from the data obtained, the reported rate was achieved in the SN group [8–10]. Moreover, in similar experiments conducted during clinical trials of the BPZE1 vaccine, the authors calculated the proportion of patients with a 1.5-fold increase in antibody levels and reached the control point only at this criterion [13]. Recalculation of our values for the 1.5-fold increase showed achievement of this parameter in our study in more than 80% of volunteers starting from day 29 after the 1st administration of GamLPV.
Assessment of immunogenicity of GamLPV using AR did not reveal significant differences with the results obtained by ELISA method, except for a more pronounced increase in the proportion of volunteers with agglutination titer 1 : 80 and higher in the SP subgroup compared to SN, while the dynamics of IgA and IgG antibody levels in these groups is of the opposite nature. Reliability and biological meaning of the revealed differences require further investigation. Generalization of the results of the analysis of blood serum of volunteers in two clinical trials of the candidate GamLPV vaccine, as well as the data obtained in the study of monkeys immunized with GamLPV and/or experimentally infected with virulent bacteria of the pertussis pathogen, showed that ELISA and AR in general reveal the general picture of immunological reactions of the organism to immunization and experimental infection. The use of AR in most cases did not reveal significant differences from the results obtained by ELISA, and the differences in AR results obtained using different series of the preparation, with good reproducibility of ELISA results, indicate low standardization of the kits used for staging AR. The obtained results make it inexpedient to use AR in further clinical studies of GamLPV. The non-alternativity of ELISA to determine the serologic status of patients and vaccinated people once again emphasizes the necessity to develop and produce a domestic ELISA test system for quantitation of antibodies to the main antigens of the pertussis pathogen.
There were no significant differences in the level of secretory IgA antibodies in aspirates, whereas the levels of IgA and IgG antibodies in blood serum differed significantly between subgroups, including before the beginning of the experiment, which is especially pronounced when comparing the subgroups of SN and SP. The observed differences in median values of IgA- antibody levels in serum before vaccination are apparently due to the correlation between IgG and IgA antibody levels in volunteers and reflect the effects of prior exposure to B. pertussis antigens.
The dynamics of the proportion of volunteers with more than twofold increase of IgA antibodies in aspirates did not differ significantly in the subgroups and reached the control values of 80% on day 15–29 after the 2nd vaccination. The noted feature may indicate the relative independence of local immunity formation from the initial IgG antibody level. The second vaccination enhances the local response irrespective of the serologic status of the volunteer, which once again indicates the extremely important role of local immune defense in the formation of antibacterial immunity in pertussis. This is also indicated by the results of determining the bacterial load in the naso- and oropharynx of vaccinated volunteers in dynamics. For each of the subgroups, with the exception of GZ, a sharp decrease in the time of bacterial elimination after the 2nd vaccination and the absence of a pronounced difference in the value of parameters for different subgroups of volunteers were noted.
The number of bacteria registered in volunteers during the observation period after the 1st and 2nd immunizations can be considered as an additional value to the bacterial elimination time in relation to the assessment of the antibacterial activity of GamLPV. This value was estimated by calculating the area under the bacterial elimination curve after each immunization. Analysis of the presented data indicates that the elimination efficiency for the period from day 3 to day 60 after the 2nd vaccination was 33 times higher compared to the 1st vaccine administration. Since it takes time to develop an immune response, it is most appropriate to determine the area under the curve not from day 1, when the body has not yet had time to react to the introduction of bacteria, but from day 3 to day 60. In addition, the results of measuring IgG and IgA antibody values indicate that the immune response of the organism after the 1st and 2nd vaccinations is manifested closer to the 8th day after the introduction of bacteria.
The above results of the analysis of the elimination efficiency of attenuated bacteria after the 2nd vaccine administration suggest that the level of specific IgG and IgA antibodies in serum does not fully reflect the level of antibacterial protective activity. This is consistent with the statement that there are no correlations between serum IgG antibody levels and the protective activity of pertussis vaccines in practice [5]. This statement, at first glance, limits the use of serum IgG and IgA antibody levels in pertussis vaccine immunogenicity studies. Seroconversion is undoubtedly an important characteristic of the humoral immune response in pertussis or vaccination, and the antibodies produced participate in the formation of immune protection aimed at neutralizing the toxic activity of the pathogen and preventing clinical manifestations of the disease, but it does not indicate the formation of sterile antibacterial immunity, as after reconvalescence or vaccination with live pertussis vaccine. It is now generally accepted that the cellular component of immunity is responsible for the formation of antimicrobial defense in pertussis disease. Direct experiments on monkeys demonstrate that the presence of all isotypes of specific antibodies to pertussis toxin, filamentous hemagglutinin, pertactin and fimbriae after their immunization with cell-free pertussis vaccine does not ensure prevention or at least inhibition of virulent bacteria multiplication in the nasopharynx after experimental infection of animals [5]. The demonstrated absence of dependence of bacterial elimination time on the initial level of IgG and IgA antibodies in blood serum before vaccination can be considered as an additional argument in favor of the made assumption. To assess the induced antibacterial response, most likely, data on the level of IgA antibodies in oro- and nasopharyngeal washes and cellular response parameters, primarily characterizing the Th17 response, should be used.
The recently published results of the BPZE1 vaccine clinical trial are generally consistent with those presented in the present study [13]. Furthermore, they contain a comparison of BPZE1-induced and TDaP-induced immune response rates. According to the authors, the level of seroconversion after intranasal vaccination of BPZE1 volunteers is slightly lower, but comparable to the values of IgG and IgA antibodies in the serum of volunteers after TDaP vaccination. At the same time, injectable TDaP vaccination, unlike intranasal BPZE1, is not able to induce specific secretory antibodies in the nasopharynx of volunteers. The high level of tolerability of the BPZE1 vaccine and the safety of its intranasal administration in adult volunteers have been demonstrated.
Conclusion
The results of this study showed immunologic efficacy (immunogenicity) of the GamLPV live pertussis vaccine when administered twice and once intranasally in comparison with placebo in adult volunteers. The basis was the achievement of immunogenicity control points of the vaccine:
- The proportion of volunteers with more than two-fold increase of IgG and IgA antibody levels in serum after vaccination was more than 80%;
- The proportion of volunteers with an agglutination titer of 1 : 80 in AR after vaccination was more than 80%;
- The proportion of volunteers with more than two-fold increase of IgA antibody levels in naso- and oropharyngeal aspirates after vaccination was more than 80%.
A statistically significant decrease in the time to reach maximum values of IgA antibodies in blood serum and naso- and oropharyngeal aspirates after the 2nd vaccination in comparison with the 1st and a reduction in the elimination time of attenuated bacteria from the nasopharynx after the 2nd administration of bacteria in comparison with the 1st vaccination of volunteers, assessed by the number of GE (using qPCR) were shown.
The difference in the proportion of volunteers with a more than twofold increase in IgG antibody levels in SN volunteers (more than 90%) and in SP volunteers (less than 78%) is due to the initially high antibody levels of the latter and, consequently, the lack of doubling after the next exposure to infection. The increase in the proportion of volunteers with more than doubling of IgA and IgG antibody levels is mainly due to SN volunteers with lower initial IgA antibody levels.
The contribution of SN and SP volunteers to the proportion of volunteers with more than a twofold increase in secretory IgA antibodies (> 80%) is approximately equal, with close initial median IgA antibody values in each group, but larger values achieved in SP-volunteers. The latter result may indicate a short period of secretory IgA antibody presence after infection/vaccination, but accelerated production and attainment of higher values in SP volunteers with previous contact with infection.
The reduction of bacterial elimination time after the 2nd vaccination compared to the 1st is equally recorded in SN and SP volunteers and is 2.0 and 2.3 times in each group, respectively.
Summarizing the presented results, it can be stated that the 1st intranasal vaccination of volunteers leads to the formation of specific humoral response of the organism, including the production of specific secretory IgA antibodies. Repeated administration of attenuated bacteria enhances the immune response and demonstrates the presence of antibacterial immunity formed as a result of the 1st intranasal vaccination of volunteers with the preparation GamLPV. A single intranasal administration of the preparation may be sufficient for revaccination of the adult population with GamLPV. The necessity for double vaccination of young children and adolescents should be determined by the results of clinical trials.
About the authors
Alevtina A. Lidzhieva
N.F. Gamaleya National Research Center for Epidemiology and Microbiology
Email: baburida@yandex.ru
ORCID iD: 0000-0002-1537-6444
junior researcher, Laboratory of bacterial genetics, Department of medical microbiology
Russian Federation, MoscowAlisa Yu. Medkova
N.F. Gamaleya National Research Center for Epidemiology and Microbiology
Author for correspondence.
Email: baburida@yandex.ru
ORCID iD: 0000-0002-1509-0622
Cand. Sci. (Med.), senior researcher, Laboratory of bacterial genetics, Department of medical microbiology
Russian Federation, MoscowSergey V. Kulikov
N.F. Gamaleya National Research Center for Epidemiology and Microbiology
Email: baburida@yandex.ru
ORCID iD: 0000-0001-7478-3624
junior researcher, Laboratory of bacterial genetics, Department of medical microbiology
Russian Federation, MoscowLudmila N. Sinyashina
N.F. Gamaleya National Research Center for Epidemiology and Microbiology
Email: baburida@yandex.ru
ORCID iD: 0000-0003-1708-5453
D. Sci. (Med.), leading researcher of laboratory of bacterial genetics of the department of medical microbiology
Russian Federation, MoscowRezida A. Sioundioukova
N.F. Gamaleya National Research Center for Epidemiology and Microbiology
Email: baburida@yandex.ru
ORCID iD: 0000-0001-5600-1967
Cand. Sci. (Biol.), senior researcher, Laboratory of bacterial genetics, Department of medical microbiology
Russian Federation, MoscowAndrey P. Markov
N.F. Gamaleya National Research Center for Epidemiology and Microbiology
Email: baburida@yandex.ru
ORCID iD: 0009-0000-1857-967X
Cand. Sci. (Biol.), senior researcher, Laboratory of bacterial genetics, Department of medical microbiology
Russian Federation, MoscowAleksey B. Verveda
Research Institute of Industrial and Maritime Medicine; Eco-Safety Research Center LLC
Email: baburida@yandex.ru
ORCID iD: 0000-0003-4029-3170
Cand. Sci. (Med.), leading researcher, senior researcher
Russian Federation, St. Petersburg; St. PetersburgNatalia E. Abaeva
I.I. Mechnikov Research Institute for Vaccines and Sera
Email: baburida@yandex.ru
ORCID iD: 0000-0003-3984-959X
researcher, Laboratory of biosynthesis of immunoglobulins
Russian Federation, MoscowIrina N. Chernyshova
I.I. Mechnikov Research Institute for Vaccines and Sera
Email: baburida@yandex.ru
ORCID iD: 0000-0001-5053-2433
Cand. Sci. (Med.), senior researcher, Laboratory of biosynthesis of immunoglobulins
MoscowMarina V. Gavrilova
N.F. Gamaleya National Research Center for Epidemiology and Microbiology; I.I. Mechnikov Research Institute for Vaccines and Sera
Email: baburida@yandex.ru
ORCID iD: 0000-0002-6936-2486
Cand. Sci. (Biol.), researcher, Laboratory of bacterial genetics, Department of medical microbiology, researcher, Laboratory of biosynthesis of immunoglobulins
Russian Federation, Moscow; MoscowKristina K. Bushkova
I.I. Mechnikov Research Institute for Vaccines and Sera
Email: baburida@yandex.ru
ORCID iD: 0000-0002-4757-0751
researcher. Laboratory of biosynthesis of immunoglobulins
Russian Federation, MoscowIlya N. Dyakov
N.F. Gamaleya National Research Center for Epidemiology and Microbiology; I.I. Mechnikov Research Institute for Vaccines and Sera
Email: baburida@yandex.ru
ORCID iD: 0000-0001-5384-9866
Cand. Sci. (Biol.), researcher, Laboratory of bacterial genetics, Department of medical microbiology, leading researcher, Head, Laboratory of biosynthesis of immunoglobulins
Russian Federation, Moscow; MoscowGennady I. Karataev
N.F. Gamaleya National Research Center for Epidemiology and Microbiology
Email: baburida@yandex.ru
ORCID iD: 0000-0001-8771-6092
D. Sci. (Biol.), leading researcher, head of the laboratory of bacterial genetics of the department of medical microbiology
Russian Federation, MoscowReferences
- Decker M.D., Edwards K.M. Pertussis (whooping cough). J. Infect. Dis. 2021;224(12 Suppl. 2):310–20. DOI: https://doi.org/10.1093/infdis/jiaa469
- Macina D., Evans K.E. Bordetella pertussis in school-age children, adolescents, and adults: a systematic review of epidemiology, burden, and mortality in the Middle East. Infect. Dis. Ther. 2021;10(2):719–38. DOI: https://doi.org/10.1007/s40121-021-00440-8
- Locht C., Carbonetti N.H., Cherry J.D., et al. Highlights of the 12th international Bordetella symposium. Clin. Infect. Dis. 2020;71(9):2521–6. DOI: https://doi.org/10.1093/cid/ciaa651
- Locht C. Will we have new pertussis vaccines? Vaccine. 2018;36(36):5460–9. DOI: https://doi.org/10.1016/j.vaccine.2017.11.055
- Warfel J.M., Zimmerman L.I., Merkel T.J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl Acad. Sci. USA. 2014;111(2):787–92. DOI: https://doi.org/10.1073/pnas.1314688110
- Медкова А.Ю., Синяшина Л.Н., Амичба А.А. и др. Доклинические исследования безопасности, иммуногенности и защитной активности аттенуированных бактерий Bordetella pertussis на экспериментальной модели Macaca mulatta. Журнал микробиологии, эпидемиологии и иммунобиологии. 2020;97(4):312–23. Medkova A.Yu., Sinyashina L.N., Amichba A.A., et al. Preclinical studies of safety, immunogenicity and protective activity of attenuated Bordetella pertussis bacteria on the Macaca mulatta model. = Journal of Microbiology, Epidemiology and Immunobiology. 2020;97(4):312–23. DOI: https://doi.org/10.36233/0372-9311-2020-97-4-3 EDN: https://elibrary.ru/zcqxpb
- Джидарян А.А., Матуа А.З., Медкова А.Ю. и др. Безопасность и иммуногенность препарата живой коклюшной вакцины ГамЖВК интраназального применения на экспериментальной модели детёнышей обезьян вида павиан гамадрил. Журнал микробиологии, эпидемиологии и иммунобиологии. 2022;99(2):203–14. Djidaryan A.A., Matua A.Z., Medkova A.Yu., et al. Safety and immunogenicity of live intranasal pertussis vaccine GamLPV in the experimental infant hamadryas baboon model. Journal of Microbiology, Epidemiology and Immunobiology. 2022;99(2):203–14. DOI: https://doi.org/10.36233/0372-9311-190
- Медкова А.Ю., Лиджиева А.А., Сёмин Е.Г. и др. Иммуногенность препарата «Живая вакцина интраназального применения для профилактики коклюша» (ГамЖВК) при однократном применении у здоровых добровольцев. Журнал микробиологии, эпидемиологии и иммунобиологии. 2021;98(6):706–20. Medkova A.Yu., Lidzhiyeva A.A., Semin E.G., et al. Immunogenicity of the drug «Live intranasal vaccine for the prevention of pertussis» (GamLPV) with a single use in healthy volunteers. Journal of Microbiology, Epidemiology and Immunobiology. 2021;98(6):706–20. DOI: https://doi.org/10.36233/0372-9311-194 EDN: https://elibrary.ru/jkxdrn
- Медкова А.Ю., Лиджиева А.А., Сёмин Е.Г. и др. Клинические исследования безопасности и переносимости живой вакцины интраназального применения для профилактики коклюша. Разработка и регистрация лекарственных средств. 2021;10(1):114–9. Medkova A.Yu., Lidzhieva A.A., Semin E.G., et al. A clinical study of the safety and tolerability of live nasal vaccine for the prevention of pertussis. Drug Development & Registration. 2021;10(1):114–9. DOI: https://doi.org/10.33380/2305-2066-2021-10-1-114-11 EDN: https://elibrary.ru/ivumga
- Каратаев Г.И., Медкова А.Ю., Сёмин Е.Г. и др. Разработка способа и схемы применения живой рекомбинантной коклюшной вакцины «ГамЖВК». Безопасность и переносимость двукратной интраназальной вакцинации здоровыми взрослыми добровольцами. Разработка и регистрация лекарственных средств. 2022;11(3):202–8. Karataev G.I., Medkova A.Yu., Semin E.G., et al. Development of a method and a scheme for the use of a live recombinant vaccine «GamLPV». Safety and tolerability of double intranasal vaccination of healthy adult volunteers. Drug Development & Registration. 2022;11(3):202–8. DOI: https://doi.org/10.33380/2305-2066-2022-11-3-202-208 EDN: https://elibrary.ru/sytabk
- Li R., Lim A., Ow S.T., et al. Development of live attenuated Bordetella pertussis strains expressing the universal influenza vaccine candidate M2e. Vaccine. 2011;29(33):5502–11. DOI: https://doi.org/10.1016/j.vaccine.2011.05.052
- Jahnmatz M., Richert L., Al-Tawil N., et al. Safety and immunogenicity of the live attenuated intranasal pertussis vaccine BPZE1: a phase 1b, double-blind, randomised, placebo-controlled dose-escalation study. Lancet Infect Dis. 2020;20(11): 1290–301. DOI: https://doi.org/10.1016/S1473-3099(20)30274-7
- Keech C., Miller V.E., Rizzardi B., et al. Immunogenicity and safety of BPZE1, an intranasal live attenuated pertussis vaccine, versus tetanus-diphtheria-acellular pertussis vaccine: a randomised, double-blind, phase 2b trial. Lancet. 2023;401(10379):843–55. DOI: https://doi.org/10.1016/S0140-6736(22)02644-7
- Семин Е.Г., Синяшина Л.Н., Медкова А.Ю. и др. Конструирование рекомбинантных аттенуированных бактерий Bordetella pertussis генотипа ptxP3. Журнал микробиологии, эпидемиологии и микробиологии. 2018;95(4):33–41. Semin E.G., Sinyashina L.N., Medkova A.Y., et al. Construction of recombinant attenuated Bordetella pertussis bacteria of ptxP3 genotype. Journal of Microbiology, Epidemiology and Immunobiology. 2018;95(4):33–41. DOI: https://doi.org/10.36233/0372-9311-2018-4-33-41 EDN: https://elibrary.ru/npsjcf
- Медкова А.Ю., Синяшина Л.Н., Румянцева Ю.П. и др. Накопление авирулентных инсерционных bvg- мутантов Bordetella pertussis при экспериментальной инфекции лабораторных мышей. Молекулярная генетика, микробиология и вирусология. 2013;(4):22–6. EDN: https://elibrary.ru/rtwart Medkova A.Yu., Sinyashina L.N., Rumyantseva Yu.P., et al. Accumulation of avirulent Bordetella pertussis Bvg mutants in the course of experimental whooping cough in mice. Molecular Genetics, Microbiology and Virology. 2013;28(4):156–61. DOI: https://doi.org/10.3103/S0891416813040058 EDN: https://elibrary.ru/sljrat
- Нестерова Ю.В., Медкова А.Ю., Бабаченко И.В. и др. Клинико-диагностическое значение генетических маркеров Bordetella pertussis у контактных лиц в семейных очагах. Журнал инфектологии. 2019;11(1):17–24. Nesterova Yu.V., Medkova A.Yu., Babachenko I.V., et al. Clinical-diagnostic value of Bordetella pertussis genetic markers in contact persons in familial foci. Journal Infectology. 2019;11(1):17–24. DOI: https://doi.org/10.22625/2072-6732-2019-11-1-17-24 EDN: https://elibrary.ru/ubtkhk
Supplementary files