Prevalence, spectrum, and the relations between short-term and long-term post-acute sequelae of COVID-19 in children
- Authors: Meskina E.R.1, Khadisova M.K.1, Ushakova A.Y.1, Tselipanova E.E.1, Galkina L.A.1
-
Affiliations:
- Moscow Regional Research and Clinical Institute
- Issue: Vol 102, No 1 (2025)
- Pages: 43-61
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18705
- DOI: https://doi.org/10.36233/0372-9311-617
- EDN: https://elibrary.ru/ppuklt
- ID: 18705
Cite item
Abstract
Background. Data on the prevalence of post-COVID-19 condition (PCC) in children differ due to the lack of specific diagnostic criteria, reliable biomarkers, and limitations of many studies. It is important to study the association between PCC, previous functional disorders, and any events in the post-acute period of COVID-19 to estimate the true burden of PCC in children.
The aim. To determine of the prevalence of PCC in relation to initial functional disorders in children during the year after a mild form of COVID-19 and acute respiratory tract infections (ARTI) of other etiologies.
Materials and methods. The prospective study using a continuous sampling method included children under 18 years of age hospitalized with suspected COVID-19, in whom COVID-19 was confirmed by RT-PCR (n = 121) or excluded by molecular and serological methods (ARTI group, n = 105). Information on patients was collected from September 2020 to December 2021 by questionnaires at index points: hospital discharge and after 1, 3, 6 and 12 months. Severe COVID-19 and patients with chronic diseases were excluded. Functional disorders in the anamnesis and any events associated with health disorders during the year of observation, the severity of symptoms were taken into account. The analysis was performed in groups of children < 6 years and ≥ 6 years of age. Symptoms were grouped into clusters.
Results. Any symptoms were identified with a frequency of 32–39% (in children < 6 years and ≥6 years, respectively). The most common were psycho-emotional, respiratory and autonomic dysfunction (AD) cluster. Symptoms in most cases were mild with a duration of 3–6 months. After adjustment for age, AD history and recurrent ARTI episodes, no difference was found in the symptom clusters prevalence between confirmed and excluded COVID-19, except for hyposmia, the incidence of which in children ≥6 years decreased from 14.3% at the first index point to 2.4% after one year. Among patients without an AD history and without recurrent ARTI during the year, de novo PCC was a rare phenomenon (2.7–8.0%) without differences between COVID-19 and controls. The risks of PCC were significantly increased in patients with an AD history (adjusted OR at 1 month 3.19 (95% CI 1.89–5.38), at 6 months 3.33 (95% CI 1.81–6.15)). Multiple (5–25) and persistent (at all index points) symptoms rated by patients as significant occurred de novo rarely (1.7% (95% CI 0.4–4.9)), but significantly more often in children with an AD history — 25%) 95% CI 13.6–39.6%) (difference 23.3% (10.9–35.7%), OR 14.8 (4.4–50.6), p < 0.001). Recurrent episodes of ARTI were an important risk factor for an increase in cognitive cluster complaints and vagotonic AD after 3–6 months of observation.
Conclusion. The results obtained indirectly support the concept that PCC is a somatoform functional disorder, probably of sociogenic nature, in patients who are anxious at baseline. Some patients with the COVID-19 and ARTI consequences really need medical and psychosocial rehabilitation. The study of PCC provides new insights into the consequences of widespread respiratory viral infections.
Full Text
Introduction
Among the many global challenges of the COVID-19 pandemic, health impairments that persist, progress or emerge after the acute period of COVID-19 have a significant impact on daily human activities and quality of life.
Several terms are used in the scientific literature to define the long-term effects of COVID-19. They were proposed later than the identified problem and refer to the timing of onset and duration of persistent symptoms without specifying their epidemiological and clinical and characteristics. According to the World Health Organization (WHO) definition, Post-COVID-19 condition (PCC) occurs in individuals with a history of confirmed or probable SARS-CoV-2 infection when symptoms initially occurring within 3 months after the acute period last for at least 2 months and typically affect daily activities. This definition can be used in patients of all ages1 and is adopted in this publication. The term Post-acute sequelae of SARS-CoV-2 (PASC) proposed by the National Institutes of Health is essentially the same as the WHO definition [1]. According to the clarification of another group of experts, the term Long COVID takes into account at least one physical symptom under other similar conditions [2]. The absence of an alternative cause of impairment is mentioned as one of the key definitions of PCC. Currently, PCC has a special code of nosological unit of clinical diagnosis.
The prevalence, spectrum, hierarchy and duration of PCC symptoms are characterized by significant heterogeneity (0.8–74.5%) [3, 4]. More than 200 symptoms have been described in children and adolescents [5]. Weakness, fatigue, fever, dyspnea, cough, headache, dizziness, exercise intolerance, emotional disturbances, sleep disturbances, decreased concentration, learning difficulties, hyposmia, dysgeusia and abdominal pain are listed with variable ranking (< 10–20% in most studies) [5–7]. Late large pediatric controlled studies and meta-analyses with hundreds of thousands of participants have identified a 25–30% PCC symptom rate after SARS-CoV-2 infection of any severity in outpatients and hospitalized patients [6–8], as well as in asymptomatic carriers [9]. High quality studies are associated with lower confirmation of PCC [10].
Pooled assessments of SARS-CoV-2-infected and uninfected children (by polymerase chain reaction testing) identified the same consistent symptoms with little or no difference. Risks of individual symptoms (hyposmia, dysgeusia, exercise intolerance, dyspnea, cognitive deficits, hair loss, palpitations and fatigue) were slightly higher in SARS-CoV-2-positive patients, by 2–8% [3, 11], increasing to 15–30% among adolescents older than 11 years [12]. Risk factors for pediatric PCC, in addition to adolescence, may include female sex (although there is no gender difference in the acute COVID-19 period), European race, severe illness, NICU stay, length of hospitalization, combined comorbidity, and baseline mental health disorders [3, 7, 13]. Other studies have found no association of PCC with demographics (age, gender, and race) [8].
Symptoms may first appear in the early COVID-19 recollection period, persist with varying duration, fluctuate or recur. In children, the trajectory of major PCC symptoms decreases over time from 20–35% to 3–15% with no statistical difference from controls [7, 14–16]. The prevalence of some symptoms may increase after 3–6 months (up to 50–60%) and then be maintained at a high level, while others may decrease at different rates or persist with an initial low frequency [16]. In some children, symptoms occurred de novo after 6–12 months of follow-up [17]. However, 6 months after testing, both SARS-CoV-2-positive (24%) and SARS CoV-2-negative (17%) patients had symptoms that met the definition of PCC [18].
In general, compared with adults, the burden of pediatric PCC was moderate to low, symptoms were milder and reported significantly less frequently [3, 11, 16]. Nevertheless, PCC significantly reduced children's quality of life [19] and daily activities, interfered with schooling, and required more frequent visits to medical care [20].
PCC has a number of obvious problems [10, 21]. Limitations include the lack of standardized clinical patterns and biomarkers to describe and diagnose it, which leads to certain difficulties in diagnosis and planning clinical trials. Only 35% of published reports used 1 of 3 definitions of PCC (PASC) [22]. Out of the 110 children who sought medical care with suspected PCC, the diagnosis was confirmed in 29%, alternative somatic/psychiatric conditions were established in 47%, and the cause of complaints remained unexplained in 23% [23]. Cancellation, wrong selection, misclassification, non-response and/or loss of follow-up, as well as the substantial heterogeneity of the results obtained and the limitations of meta-analyses due to insufficient data may be objective shortcomings of many publications. Thereby, the quality of evidence and methodological rigor of a number of scientific papers may have been suboptimal [4, 6, 10].
On the other hand, the similarity of symptoms in SARS-CoV-2-positive and SARS-CoV-2-negative patients suggests the influence of additional factors that have not yet been clearly defined. Most studies have identified chronic diseases routinely recorded in medical databases and electronic records as predictors of PCC risk, but have not investigated the role of baseline functional impairment and possible new diseases in the context of the heterogeneous labile PCC symptom complex and new infection pandemic settings.
This prospective study was planned and performed early in the SARS-CoV-2 pandemic, but despite this, its results may be useful as it is the first attempt to consider baseline functional impairment in children as a predictor of PCC risk, as well as the possibility of common sequelae of COVID-19 and other respiratory infections, which seems to help achieve a better understanding of PCC.
The aim of the study was to determine the prevalence of PCC in relation to baseline functional impairment in children one year after non-severe COVID-19 and acute respiratory tract infection (ARTI) of other etiologies.
Materials and methods
Study design
We conducted an observational longitudinal study using a continuous sampling method of short- and long-term effects of non-severe COVID-19 compared with acute respiratory infection of other etiologies in children of any age hospitalized in an infectious disease hospital. During the acute period of illness and for a year afterward, children without baseline health problems or with a history of only functional impairment were observed.
Participants
Children with upper and lower respiratory tract infections were included in the study, regardless of its timing. In order to avoid bias associated with risk factors, the study excluded children with severe and complicated forms of the disease, with established chronic diseases and signs of primary autonomic dysfunction (AD) including organic lesions and traumas of the central and peripheral nervous system, excessive overstrain of the nervous system (clearly defined physical or emotional stress, sports in professional sections), diabetes mellitus, obesity, other endocrine disorders, chromosomal disorders and other chronic diseases.
Patients were recruited from September 2020 to December 2021, i.e., after the first wave of the Ukhan strain of SARS-CoV-2 and before the beginning of the spread of genetic variants of SARS-CoV-2 Omicron in Russia. 250 children under 18 years of age were included in the study. COVID-19 was confirmed by routine methods on the basis of positive SARS-CoV-2 RNA test in nasal and oropharyngeal swabs taken at the time of hospitalization. The comparison group (ARTI group) were patients in whom COVID-19 was excluded by negative nasal and oropharyngeal swabs in reverse transcription polymerase chain reaction and serologic tests with detection of anti-SARS-CoV-2 IgM and IgG in enzyme-linked immunosorbent assay before hospital discharge on days 7–10 of the illness. Testing for other respiratory viruses was not performed. Recruitment of patients in each group (COVID-19 and acute respiratory viruses) was stopped at the 125th included patient. A total of 9.6% of patients were excluded from the study (COVID-19 — 3.2%, ARTI — 16%) due to the development of severe pneumonia or loss of contact during the study period. Additionally, patients with diagnostic titers of specific anti-SARS-CoV-2 IgM and IgG were excluded from the ARTI group. Comparative data analysis was performed in groups of 121 (COVID-19) and 105 (ARTI) patients.
Treatment of COVID-19 in hospital was performed according to the Temporary Methodological Recommendations of the Ministry of Health of the Russian Federation “Prevention, Diagnosis and Treatment of New Coronavirus Infection (COVID-19)”, versions 1–7, as well as the Methodological Recommendations of the Ministry of Health of the Russian Federation “Features of Clinical Manifestations and Treatment of Disease Caused by New Coronavirus Infection (COVID-19) in Children”, versions 1 (03.04.2020) and 2 (03.07.2020), relevant at that time.
Patient information was collected by questionnaires administered to mothers and older children at the time of hospitalization and during the following year by telephone contact at index (contact) points at 1, 3, 6 and 12 months (± 5 days) after hospitalization.
The physical, psycho-emotional and cognitive development of children varies with age. Assessment of the health status of young children depends more on information provided by the mother. School-aged and adolescent children are able to present active complaints. Therefore, age-adapted questionnaires were developed for children under and over 6 years of age. The questionnaires were tailored to the literature and possible symptoms after COVID-19. Both questionnaires contained two blocks of questions. The first block (anamnestic) contained 15 questions to assess functional disorders (including gastrointestinal, autonomic nervous system (ANS) dysfunction, psycho-emotional state, and morbidity) in the child's history. The second block questionnaire (observational) for children < 6 years of age included 53 questions and for children ≥ 6 years of age 76 questions. Both questionnaires contained questions about active complaints, general condition of the patient, intensity of disorders, if any, signs of psycho-emotional, cognitive, functional (including gastrointestinal) disorders and AD, subjective sensations of pain, frequency of visits to a pediatrician and specialists, any illnesses, including those that occurred for the first time, facts of hospitalizations, and pharmacological treatment. The answers to the following questions were also evaluated: “Are you feeling worse than before the disease or the same?”, ‘Do you assess your own symptoms as mild, moderate or serious?’, ‘Were the symptoms you have now observed before the disease?’. Furthermore, it was offered to evaluate the state of general health on a 100-point scale. In case of recurrent acute respiratory infections, information about SARS-CoV-2 testing was collected from the words of their mothers.
During the follow-up period, a total of 277 recurrent episodes of acute respiratory infections were recorded, of which 85.9% of children were routinely tested. COVID-19 during the one-year follow-up period was confirmed in 3 cases (these patients were not excluded from the study). The study did not include clinical and instrumental diagnosis of AD, myalgic encephalomyelitis/chronic fatigue syndrome, or other investigations evaluating the function of various organs and systems. The analysis was performed in groups of children stratified by age.
Ethical expertise
The patients' legal representatives signed informed consent to participate in the study, agreed to fill out the developed questionnaires and answer questions during telephone contacts within a year after discharge from the hospital. The study protocol and informed consent to participate in the study were approved by the Independent Ethical Committee of the Moscow Regional Research and Clinical Institute (Protocol No. 10 of 01.09.2020).
Terms and definitions used
This publication uses the term Post-COVID-19 condition (PCC) as defined by WHO, taking into account all symptoms (at least one) [2] that persisted or occurred during the year. Groups of symptoms were grouped into clusters for ease of their statistical analysis: emotional, cognitive, fatigue, gastrointestinal, respiratory, and AD with subclusters of cardiovascular autonomic disorders, sympticotonic or vagotonic types. Definitions of all terms used are detailed in the Supplementary Material on the journal website. It should be emphasized that the symptoms grouped into clusters logically overlap. The individual most frequently occurring symptoms are also presented. The frequency of new symptoms was defined as the proportion of participants with a symptom on the date of telephone contact but who did not have that symptom at all previous index points.
Statistical analysis
Statistical analysis of the study results was carried out using the Microsoft Excel 2011, Statistica v. 7.0 (StatSoft Inc. 7.0) program packages. Quantitative and qualitative data were described with calculation of arithmetic mean (M), standard deviation (SD), median (Me), lower and upper quartiles [Q1; Q3]. Discrete features are presented as a fraction (%) of cases from the total number of observations. When comparing several dynamic measurements of dichotomous variables, the Cochran's Q-criterion was used, and the rank analysis of variance was used for dependent qualitative indicators. When comparing frequencies of events in independent groups, Pearson's χ2 test for quadratic and randomized tables was used; the two-sided Mann-Whitney test was used for qualitative variables; the McNemar test with Yates correction was used for pairwise comparisons of dependent variables. When the number of variables in 1 cell of the quadratic table was less than 5, the two-sided Fisher's criterion was used.
The following criteria were used to assess the magnitude of risks: frequency of the event in the compared groups, relative risk (RR) of the event in the compared groups, odds ratio (OR) of the event to no event in the compared groups with calculation of 95% confidence intervals (CI). OR < 1 indicates decreased risk, OR = 1 indicates no effect, and OR > 1 indicates increased risk. Predictors of long-term symptoms were assessed using univariate and multivariate logistic regression models and are represented by adjusted OR and 95% CI. Differences were considered statistically significant at p < 0.05.
Results
Children < 6 years of age
At baseline in the hospital, the COVID-19 group differed from the acute respiratory tract infection group in the absence of croup (vs. 8.6%) and bronchitis (vs. 31%, respectively) but a longer course of antibacterial and antiviral therapy. The difference was formal because of the lack of recommendations to distinguish the respective clinical forms of COVID-19. The incidence of out-of-hospital pneumonia was comparable in the compared groups. The history of patients < 6 years of age with COVID-19 was more frequently recorded as symptoms of AD, functional, including gastrointestinal disorders (24.3% vs. 5.2%; p = 0.006) and atopic dermatitis (18.9% vs. 3.4%; p = 0.026). A more detailed characterization of the compared groups is presented in Table S1 and Table S2 in the Supplementary Material on the journal website.
At the time of hospital discharge, complaints/signs were identified in 32 and 38% of patients < 6 years of age, with no statistical difference between the COVID-19 and ARTI groups (Table 1). The children's impairments were mostly mild and the number of symptoms/signs per child was small (usually 1–2, no more than 3–5). During the year, the frequency of active complaints was stable between 5–20%. The peak of symptoms/signs detected by questionnaires was registered by 3–6 months of follow-up with maximum frequency in the acute respiratory tract infection group. The maternal score (integral) of children's health status was associated with the questionnaires and showed consistent improvement after COVID-19 and no significant change after acute respiratory viral infections with worse scores compared to COVID-19 starting at 3 months of follow-up (Table 1).
Table 1. Frequency of registration of symptoms and signs in children under 6 years in post-acute of COVID-19 (n = 37) and ARTI of other etiology (n = 58)
Сomplaints, symptoms, signs | Discharge from the hospital | After 1 month | After 3 months | After 6 months | After 12 months | р2 | |
Well-being is impaired (self-assessment), n (%) | COVID-19 ARTI | 6 (16.2) 8 (13.8) | 4 (10.8) 7 (12.1) | 7 (18.9) 12 (20.7) | 8 (21.6) 8 (13.8) | 6 (16.2) 3 (5.2) | 0.699 0.185 |
including mild cases | COVID-19 ARTI | 5 (13.5) 5 (8.6) | 4 (10.8) 4 (6.9) | 6 (16.2) 11 (19.0) | 7 (18.9) 6 (10.3) | 6 (16.2) 3 (5.2) | 0.914 0.066 |
Complaints/symptoms, total (any, at least one sign identified by the questionnaire), n (%) | COVID-19 ARTI | 12 (32.4) 22 (37.9) | 8 (21.6) 25 (43.1) р1 = 0.033 | 10 (27.0) 35 (60.3) р1 = 0.002 | 17 (45.9) 26 (44.8) | 10 (27.0) 25 (43.1) | 0.061 0.035 |
The average quantity of signs, M ± SD | COVID-19 ARTI | 1.3 ± 0.1 1.8 ± 0.8 | 1.5 ± 0.8 2.4 ± 1.2 | 1.4 ± 0.8 1.8 ± 0.9 | 2.4 ± 1.6 1.8 ± 1.2 | 1.9 ± 1.1 1.8 ± 1.0 | 0.670 0.734 |
The average quantity of signs, M ± SD / Me [Q1; Q3] | COVID-19 ARTI | 87.8 ± 17.3 / 90 [80; 100] 91.3 ± 12.2 / 100 [80; 100] р1 = 0.329 | 95.4 ± 7.3 / 100 [90; 100] 90.7 ± 11.1 / 100 [80; 100] р1 = 0.076 | 97.3 ± 6.5 / 100 [100; 100] 92.2 ± 10.9 / 100 [80; 100] р1 = 0.021 | 97.3 ± 8.7 / 100 [100; 100] 92.4 ± 11.7 / 100 [80; 100] р1 = 0.018 | 98.9 ± 5.2 / 100 [100; 100] 94.7 ± 9.2 / 100 [90; 100] р1 = 0.008 | < 0.001 0.314 |
Weakness, fatigue, locomotor activity decreased, n (%) | COVID-19 ARTI | 1 (2.7) 1 (1.7) | 1 (2.7) 2 (3.4) | 1 (2.7) 1 (1.7) | 2 (5.4) – | 1 (2.7) – | 0.406 0.147 |
Skin rash, n (%) | COVID-19 ARTI | – 3 (5.2) | – – | 2 (5.4) 5 (8.6) | 1 (2.7) 2 (3.4) | – 3 (5.2) | – – |
Hair loss, n (%) | COVID-19 ARTI | – – | – 2 (3.4) | 1 (2.7) – | – – | – – | – – |
dyspnea without catarrhal symptoms, n (%) | COVID-19 ARTI | – 1 (1.7) | 1 (2.7) – | – – | – – | – – | – – |
Psychoemotional disorders, n (%) | COVID-19 ARTI | 2 (5.4) 3 (5.2) | 6 (16.2) 10 (17.2) | 6 (16.2) 12 (20.7) | 5 (13.5) 8 (13.8) | 2 (5.4) 7 (12.1) | 0.234 0.002 |
Psychoemotional disorders de novo in relation to the previous time point, n (%) | COVID-19 ARTI | 1 (2.7) – | 2 (5.4) 6 (10.3) | 4 (10.9) 3 (5.2) | 1 (2.7) – | – – | – – |
Sleep disorders, restless sleep, n (%) | COVID-19 ARTI | 6 (16.2) 1 (1.7) р1 = 0.013 | 2 (5.4) 2 (3.4) | 5 (13.5) 2 (3.4) | 3 (8.1) 2 (3.4) | 1 (2.7) 3 (5.2) | 0.060 0.644 |
Sleep disorders, restless sleep de novo in relation to the previous time point, n (%) | COVID-19 ARTI | 5 (13.5) 1 (1.7) р1 = 0.032 | 1 (2.7) 1 (1.7) | 2 (5.4) 1 (1.7) | – 1 (1.7) | – – | – – |
Cognitive dysfunction, n (%) | COVID-19 ARTI | 1 (2.7) – | 2 (5.4) – | 1 (2.7) – | 3 (8.1) – | 2 (5.4) – | 0.406 0.406 |
Аutonomic dysfunction (any signs), n (%) | COVID-19 ARTI | 10 (27.0) 11 (19.0) | 7 (18.9) 16 (27.6) | 7 (18.9) 13 (22.4) | 7 (18.9) 10 (17.2) | 7 (18.9) 12 (20.7) | 0.670 0.111 |
AD de novo in relation to the previous time point, n (%) | COVID-19 ARTI | 5 (13.5) 6 (10.3) | 3 (8.1) 8 (13.8) | – 1 (1.7) | – 2 (3.4) | 2 (5.4) 2 (3.4) | – – |
Gastrointestinal disorders (total), n (%) including: | COVID-19 ARTI | 1 (8.1) 5 (8.6) | 2 (5.4) 13 (22.4) р1 = 0.041 | 1 (2.7) 5 (8.6) | 12 (32.4) 5 (8.6) р1 = 0.004 | 3 (8.1) 3 (3.4) | < 0.001 0.016 |
abdominal pain | COVID-19 ARTI | – 2 (3.4) | 1 (2.7) 2 (3.4) | – 1 (1.7) | – 1 (1.7) | 2 (5.4) 1 (1.7) | |
constipation | COVID-19 ARTI | 1 (2.7) 1 (1.7) | 1 (2.7) – | 1 (2.7) 2 (3.4) | 1 (2.7) 1 (1.7) | – – | |
diarrhea | COVID-19 ARTI | – 2 (3.4) | – 12 (20.7) | – 2 (3.4) | 11 (29.7) 3 (5.2) | 1 (2.7) 1 (1.7) | |
Gastrointestinal disorders de novo in relation to the previous time point, n (%) | COVID-19 ARTI | – 3 (5.0) | 1 (2.7) 11 (18.3) р1 = 0.026 | – 3 (5.2) | 3 (8.1) 1 (1.7) | 1 (2.7) – | – – |
Catarrhal symptoms at the telephone contact, n (%) | COVID-19 ARTI | 2 (5.4) 15 (25.9) р1 = 0.013 | 1 (2.7) 14 (24.1) р1 = 0.003 | 2 (5.4) 21 (36.2) р1 < 0.001 | 4 (10.8) 18 (31.0) р1 = 0.023 | 4 (10.8) 21 (36.2) р1 = 0.004 | 0.435 0.433 |
Catarrhal symptoms only, n (%) | COVID-19 ARTI | 2 (5.4) 11 (19.0) 0.061 | 2 (5.4) 3 (5.2) | 1 (2.7) 16 (27.6) р1 = 0.002 | 2 (5.4) 10 (17.2) р1 = 0.091 | 2 (5.4) 10 (17.2) р1 = 0.091 | 0.910 0.006 |
Recurrent episodes of ARTI episodes between the points of telephone contact, n (%) | COVID-19 ARTI | – – | 6 (16.2) 16 (27.6) | 5 (13.5) 27 (46.6) р1 < 0.001 | 11 (29.7) 29 (50.0) р1 = 0.052 | 18 (48.6) 25 (43.1) | < 0.001 0.045 |
Frequency of complicated course of ARTI (croup, pneumonia, febrile convulsions, lymphadenitis), n (%) | COVID-19 ARTI | – – | – 3 (5.1) | – 2 (3.4) | 1 (2.7) 2(3.4) | 1 (2.7) 1 (1.7) | – – |
ABT frequency, n (%) | COVID-19 ARTI | – – | – 3 (5.1) | – 7 (12.1) | 2 (5.4) 9 (15.5) | – 7 (12.1) | – 0.089 |
The children number with doctor’s appointment between telephone contact points, n (%) | COVID-19 ARTI | – – | 6 (16.2) 17 (29.3) | 7 (18.9) 27 (46.6) р1 = 0.007 | 13 (35.1) 26 (44.8) | 12 (32.4) 22 (37.9) | 0.056 0.155 |
Аverage number of medical appointments, M ± SD | COVID-19 ARTI | 1.2 ± 0.4 1.1 ± 0.7 | 1.9 ± 0.9 1.2 ± 0.5 | 1.5 ± 0.9 1.2 ± 0.6 | 1.3 ± 0.5 1.2 ± 0.5 | 0.428 0.391 | |
The hospitalizations number between telephone contact points, n (%) | COVID-19 ARTI | – – | – 3 (5.1) | 1 (2.7) – | 1 (2.7) 3 (5.1) | 2 (5.4) – | – – |
Note. Here and in the Table 2: *At the time of discharge from the hospital, signs de novo in relation to the period before the disease. AD — autonomic dysfunction, ABT — antibacterial therapy. The Cochran’s Q test was used to compare the dynamics of the frequency of signs in groups (р1), rank analysis of variance was used to compare qualitative signs, the McNemar's test was used to compare paired dependent characteristics (р2), χ2 for four-field tables with the Yeats correction or two-tailed Fisher's test were used to compare paired independent features.
Typical symptoms (weakness, fatigue, respiratory distress) were very rare (1–2%) at all contact points. AD symptoms, psycho-emotional disturbances, sleep problems and catarrhal symptoms were more common than others regardless of the comparison group. Respondents in the COVID-19 and ARTI groups noted de novo AD symptoms at the time of hospital discharge relative to the pre-disease period in 13.5% and 10.3% of cases, respectively (p > 0.05; see Table 1). Further, relative to the prior point of contact, new AD symptoms occurred in the range of 2.0–13.8% in both groups. After COVID-19, the prevalence of emotional disturbances was relatively stable, but in the ARTI group had the character of a parabolic curve with a peak (20.7%) at 3 months after hospital discharge. Overall, during the year, de novo signs of AD appeared in 27.0% of respondents in the COVID-19 group and 34.5% in the ARTI group (p > 0.05), and new cases of psycho-emotional disorders in 21.6 and 15.5%, respectively (p > 0.05). All patients with a history of AD occasionally indicated its symptoms at one or another point of telephone contact. Symptoms of AD throughout the year were absent in 54% of children in the COVID-19 group and 65.5% of children in the ARTI group, psycho-emotional disorders in 73 and 82.8%, and sleep disorders in 75.7 and 93.1%, respectively (for all indicators, p > 0.05).
The maximum number of complaints 3-6 months after hospital discharge was associated with new episodes of acute respiratory and intestinal infections, which were the main reason for seeking medical help. Catarrhal symptoms without other signs during this time frame were frequent and significantly more frequent after acute respiratory infections than after COVID-19 (Table 1). Twenty-seven and 55.2% (p = 0.008) of children in the compared groups became repeatedly ill with acute respiratory infections within 3 months after hospital discharge, and 40.5 and 89.9% within 6 months, respectively (p < 0.001). Confirmed cases of acute gastroenteritis occurred (at different time points) in 13.5% of children in the COVID-19 group and 6.9% of children in the ARTI group.
Additional diagnoses after COVID-19 were hepatitis of undetermined etiology of low activity of 3 months' duration (2.7%; 95% CI 0.1–15.8), cervical lymphadenitis, and urinary tract infection; in the acute respiratory tract infection group, varicella (3 cases), community-acquired pneumonia, otitis media, COVID-19 (1 case), and urinary tract infection. 24.3 children in the COVID-19 group and 6.9% of children in the acute respiratory tract infection group had no illnesses during the year (p = 0.033).
At all of the time points, 3 (8.1% 95% CI 1.7–21.9) children in the COVID-19 group and 1 (1.7% 95% CI 0.04–9.20%) in the acute respiratory tract infection group (p = 0.050) had any persistent disorders significantly affecting their general health (as assessed by mothers). However, similar symptoms were recorded in the history of these patients before inclusion in the study. In the remaining children, the complaints/symptoms were transient in nature. There was no effect of age and gender on the studied parameters in children younger than 6 years of age.
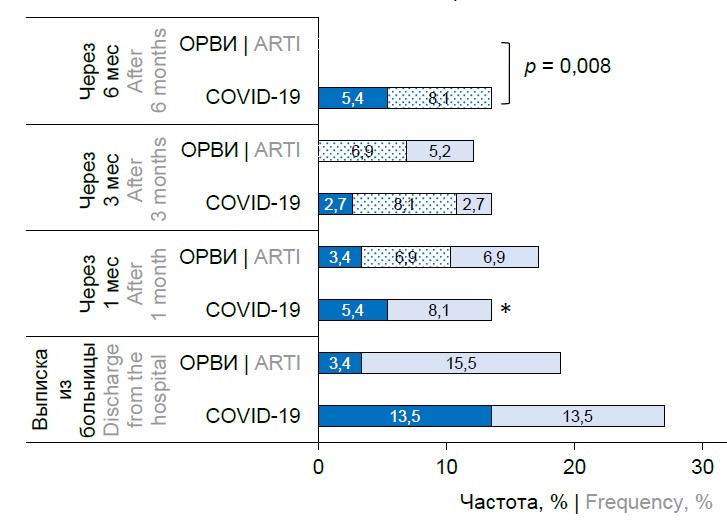
The overall incidence of cases meeting the definition of PCC in children younger than 6 years at 1, 3, 6 and 12 months after hospital discharge was relatively stable and was 19, 24, 30 and 16% after COVID-19 and 19, 24, 24 and 15.5% after acute respiratory infection (at all points p > 0.05). But after excluding patients who had acute infections or other established diseases, it appeared that PCC was rarely reported de novo, 2.7–8.1% at 1–3 months after hospital discharge and was absent at 6 months (Figure, a).
Incidence of PCC (as defined by WHO) in non-sick children in COVID-19 and ARTI groups over time, adjusted for AD history and de novo symptoms.
a — children under 6 years old; b — children aged 6–18 years.
*p = 0.027 between children < 6 years and ≥ 6 years; the "were before" series presented symptoms recorded in relation to the previous time point; PCC — post-COVID-19 condition; AD — autonomic dysfunction.
The adjusted odds of having symptoms of AD (adjusted OR = 2.192; 95% CI 1.246–3.865; p = 0.007) or emotional distress (adjusted OR = 2.081; 95% CI 1.035–4.187; p = 0.039) were increased by 6 months after hospital discharge in both SARS-CoV-2-positive and SARS-CoV-2-negative patients if they had a recurrent acute respiratory illness.
Children ≥ 6 years of age
The COVID-19 group differed from the acute respiratory tract infection group by a shift in age structure toward a predominance of adolescents 10–17 years of age (70.2% vs 51.1%; p = 0.024), the acute respiratory tract infection group by a higher incidence of bronchitis and a shorter duration of antibiotic therapy and antiviral therapy (similar to younger children). Patient history ≥ 6 years did not differ, except for a more frequent history of out-of-hospital pneumonia among those hospitalized with acute respiratory infections (Table S3 and Table S4 in the Supplementary Material on the journal website).
Children ≥ 6 years of age rated their health status worse than patients < 6 years of age (Table 2; Table S5 in the Supplementary Material on the journal website). At the time of hospital discharge, the incidence of complaints and symptoms was higher (36.9%) in the COVID-19 group than in the ARTI group (8.5%; Table 2). At one year, self-assessment of health impairment after COVID-19 indicated moderate improvement according to the mean integral score. In the ARTI group, the dynamics of changes had an opposite direction (Table 2). Patients ≥ 6 years of age presented a wider range of complaints and their mean per 1 child was higher than that of children < 6 years of age at all telephone contact points. The proportion of children with more complaints/signs (6–25) ranged from 2–13% with no differences between groups or time points. In almost all cases (87–88%), multiple complaints were made by children with a history of underlying functional health disorders.
Table 2. Frequency of registration of symptoms and signs in children from 6 to 18 years in post-acute of COVID-19 (n = 84) and ARTI of other etiology (n = 47)
Сomplaints, symptoms, signs | Discharge from the hospital | After 1 month | After 3 months | After 6 months | After 12 months | р2 | |
Well-being is impaired (self-assessment), n (%) | COVID-19 ARTI | 31 (36.9) 4 (8.5) р1 < 0.001 | 30 (35.7) 11 (23.4) | 23 (27.4) 11 (23.4) | 25 (29.8) 10 (21.3) | 19 (22.6) 11 (23.4) | < 0.009 0.149 |
including to a mild | COVID-19 ARTI | 20 (23.8) 2 (4.3) р1 = 0.001 | 20 (23.8) 9 (19.1) | 16 (19.0) 9 (19.1) | 15 (17.9) 8 (17.0) | 15 (17.9) 8 (17.0) | < 0.009 0.267 |
Complaints/symptoms, total (any, at least one sign identified by the questionnaire), n (%) | COVID-19 ARTI | 33 (39.3) 10 (21.3) р1 = 0.036 | 38 (45.2) 22 (46.8) | 36 (42.9) 24 (51.1) | 38 (45.2) 23 (48.9) | 33 (39.3) 24(51.7) | 0.617 0.002 |
The average quantity of signs, M ± SD / Me [Q1; Q3] | COVID-19 ARTI | 4.5 ± 5.0 / 3 [2–5] 2.0 ± 1.9 / 1 [1–2] р1 = 0.019 | 5.0 ± 4.9 / 3 [2–5] 3.0 ± 2.4 / 2 [1–4] р1 = 0.081 | 4.8 ± 5.0 / 3 [2.0–5.5] 3.6 ± 02.5 / 3 [1.5–5.5] р1 = 0.718 | 4.3 ± 3.9 / 3 [2–5] 3.3 ± 2.5 / 2 [1.0–5.5] р1 = 0.344 | 4.3 ± 3.6 / 3 [2–6] 2.9 ± 1.9 / 2 [2.0–4.5] р1 = 0.209 | 0.464 0.346 |
The average quantity of signs, M ± SD / Me [Q1; Q3] | COVID-19 ARTI | 86.5 ± 16.6 95 [70; 100] 95.1 ± 10.0 / 100 [100; 100] р1 = 0.001 | 89.3 ± 15.6 100 [80; 100] 89.8 ± 15.4 / 100 [80; 100] р1 = 0.803 | 91.4 ± 14.4 100 [80; 100] 89.8 ± 15.4 / 100 [80; 100] р1 = 0.576 | 92.4 ± 14.7 100 [85; 100] 90.4 ± 14.1 / 100 [80; 100] р1 = 0.354 | 94.9 ± 11.7 100 [80; 100] 89.4 ± 15.7 / 100 [70; 100] р1 = 0.040 | < 0.001 0.049 |
Weakness, fatigue, locomotor activity decreased, n (%) | COVID-19 ARTI | 20 (23.8) 5 (10.6) | 23 (27.4) 11 (23.4) | 18 (21.4) 11 (23.4) | 17 (20.2) 10 (21.3) | 11 (13.1) 6 (12.8) | 0.018 0.044 |
Weakness, fatigue, locomotor activity decreased de novo in relation to the previous time point, n (%) | COVID-19 ARTI | 12 (14.3) 3 (6.4) | 5 (6.0) 5 (10.6) | 1 (1.2) – | 5 (6.0) 2 (4.3) | – 1 (2.1) | – |
Subfebrile conditions (more than 2 weeks), n (%) | COVID-19 ARTI | 9 (10.7) 1 (2.1) р1 = 0.076 | 12 (14.3) 1 (2.1) р1 = 0.031 | 10 (11.9) 1 (2.1) р1 = 0.053 | 7 (8.3) 1 (2.1) | 5 (6.0) – | 0.080 0.809 |
Headache, n (%) | COVID-19 ARTI | 15 (17.9) 3 (6.4) р1 = 0.068 | 16 (19.0) 6 (12.8) | 14 (16.7) 6 (12.8) | 15 (17.9) 6 (12.8) | 15 (17.9) 3 (6.4) р1 = 0.068 | 0.982 0.224 |
Headache de novo in relation to the previous time point, n (%) | COVID-19 ARTI | 7 (8.3) 1 (2.1) | 4 (4.8) 2 (4.3) | – – | 1 (1.2) 2 (4.3) | 3 (3.6) – | – – |
Muscle pain, muscle spasms, n (%) | COVID-19 ARTI | 2 (2.4) – | 2 (2.4) 1 (2.1) | 3 (3.6) – | 3 (3.6) – | 3 (3.6) – | – – |
Hyposmia, n (%) | COVID-19 ARTI | 12 (14.3) – | 6 (7.1) 1 (2.1) | 3 (3.6) 1 (2.1) | 3 (3.6) – | 2 (2.4) – | < 0.001 – |
Skin rash, n (%) | COVID-19 ARTI | – – | 1 (1.2) 1 (2.1) | 2 (2.4) 1 (2.1) | 1 (1.2) – | 1 (1.2) 1 (2.1) | – – |
Hair loss, n (%) | COVID-19 ARTI | 1 (1.2) – | 3 (3.6) 1 (2.1) | 3 (3.6) 1 (2.1) | 2 (2.4) 1 (2.1) | 3 (3.6) – | – – |
Аrthritis, joint pain, n (%) | COVID-19 ARTI | – – | 1 (1.2) – | 2 (2.4) – | 2 (2.4) – | 2 (2.4) – | – – |
Dyspnea without catarrhal symptoms, n (%) | |||||||
at rest | COVID-19 ARTI | 1 (1.2) – | 1 (1.2) – | 1 (1.2) – | 1 (1.2) – | 1 (1.2) – | – |
at load | COVID-19 ARTI | 2 (2.4) – | 4 (4.8) – | 4 (4.8) – | 3 (3.6) – | 3 (3.6) – | – |
Psychoemotional disorders, n (%) | COVID-19 ARTI | 10 (11.9) 2 (4.3) | 12 (14.3) 4 (8.5) | 14 (16.7) 4 (8.5) | 12 (14.3) 5 (10.6) | 13 (15.5) 5 (10.6) | 0.354 0.329 |
including: anxiety and depression | COVID-19 ARTI | 3 (3.6) 1 (2.1) | 3 (3.6) 1 (2.1) | 4 (4.8) 1 (2.1) | 4 (4.8) 1 (2.1) | 3 (3.6) 1 (2.1) | 0.531 0.406 |
panic attacks | COVID-19 ARTI | 1 (1.2) – | – – | 1 (1.2) – | 1 (1.2) – | – – | – – |
Psychoemotional disorders de novo in relation to the previous time point, n (%) | COVID-19 ARTI | 5 (6.0) 1 (2.1) | 3 (3.6) 2 (4.2) | 1 (1.2) – | – 1 (2.1) | 2 (2.4) – | – – |
Intolerance to physical activity, n (%) | COVID-19 ARTI | 6 (7.1) – | 5 (6.0) 3 (6.4) | 9 (10.7) 3 (6.4) | 7 (8.7) 3 (6.4) | 5 (6.0) 1 (2.1) | 0.405 0.092 |
Intolerance to emotional stress, n (%) | COVID-19 ARTI | 5 (6.0) – | 5 (6.0) 2 (4.3) | 4 (4.8) 2 (4.3) | 3 (3.6) 1 (2.1) | 4 (4.8) 3 (6.4) | 0.800 0.316 |
Sleep disorders, restless sleep, n (%) | COVID-19 ARTI | 2 (2.4) 1 (2.1) | 5 (6.0) 2 (4.3) | 4 (4.8) 2 (4.3) | 5 (6.0) 1 (2.1) | 5 (6.0) 2 (4.3) | 0.437 0.558 |
Sleep disorders, restless sleep de novo in relation to the previous time point, n (%) | COVID-19 ARTI | 1 (1.2) 1 (2.1) | 2 (2.4) 1 (2.1) | 1 (1.2) – | 1 (1.2) – | 2 (2.4) – | – – |
Cognitive dysfunction, n (%) | COVID-19 ARTI | 8 (9.5) 2 (4.3) | 13 (15.5) 5 (10.6) | 11 (13.1) 5 (10.6) | 12 (14.3) 8 (17.0) | 9 (10.7) 1 (2.1) | 0.220 0.009 |
including memory impairment | COVID-19 ARTI | 3 (3.6) – | 5 (6.0) – | 5 (6.0) – | 6 (7.1) 1 (2.1) | 7 (8.3) 1 (2.1) | 0.149 0.406 |
Autonomic dysfunction (any signs), n (%) | COVID-19 ARTI | 34 (40.5) 10 (21.3) р1 = 0.026 | 34 (40.5) 15 (31.9) | 30 (35.7) 16 (34.0) | 31 (36.9) 15 (31.9) | 26 (31.0) 9 (19.1) | 0.436 0.002 |
AD de novo in relation to the previous time point, n (%) | COVID-19 ARTI | 16 (19.0) 3 (6.4) р1 = 0.086 | 4 (4.8) 5 (10.6) | – – | 6 (7.1) 3 (6.4) | 2 (2.4) – | – – |
Cardiovascular symptoms, n (%) | COVID-19 ARTI | 13 (15.5) 1 (2.1) р1 = 0.018 | 18 (21.4) 4 (8.5) р1 = 0.058 | 6 (7.1) 2 (4.3) | 9 (10.7) 2 (4.3) | 11 (13.1) 1 (2.1) р1 = 0.054 | 0.011 0.406 |
Adrenergic autonomic dysfunction, n (%) | COVID-19 ARTI | 13 (15.5) 2 (4.3) р1 = 0.054 | 19 (22.6) 10 (21.3) | 17 (20.2) 5 (10.6) | 15 (17.9) 6 (12.8) | 11 (13.1) 4 (14.9) | 0.243 0.033 |
Vagotonic autonomic dysfunction, n (%) | COVID-19 ARTI | 14 (16.7) 5 (10.6) | 10 (11.9) 4 (8.5) | 5 (6.0) 8 (17.0) р1 = 0.043 | 8 (9.5) 7 (14.9) | 8 (9.5) 1 (2.1) | 0.032 0.046 |
Mixed-type autonomic dysfunction, n (%) | COVID-19 ARTI | 5 (6.0) – | 5 (6.0) 1 (2.1) | 8 (9.5) 3 (6.4) | 8 (9.5) 2 (4.2) | 7 (8.3) 1 (2.1) | 0.771 0.363 |
Gastrointestinal disorders, n (%) | COVID-19 ARTI | 5 (6.0) — | 10 (11.9) 6 (12.8) | 11 (13.1) 6 (12.8) | 11 (13.1) 3 (6.4) | 10 (11.9) 2 (4.3) | 0.135 0.005 |
including: abdominal pain | COVID-19 ARTI | 4 (4.8) – | 9 (10.7) 5 (10.6) | 9 (10.7) 5 (10.6) | 9 (10.7) 3 (6.4) | 9 (10.7) 2 (4.2) | |
constipation | COVID-19 ARTI | 2 (2.4) – | 2 (2.4) – | 2 (2.4) – | – – | – – | |
diarrhea | COVID-19 ARTI | 1 (1.2) – | 1 (1.2) 2 (4.3) | 2 (2.4) – | 1 (1.2) – | 1 (1.2) – | |
Gastrointestinal disorders de novo in relation to the previous time point, n (%) | COVID-19 ARTI | 5 (6.0) – | 1 (1.2) – | 1 (1.2) – | 2 (2.4) 1 (4.3) | 1 (1.2) – | – – |
Catarrhal symptoms at the telephone contact, n (%) | COVID-19 ARTI | 5 (6.0) 1 (2.1) | 5 (6.0) 7 (14.9) р1 = 0.089 | 5 (6.0) 14 (29.8) р1 < 0.001 | 8 (9.5) 15 (31.9) р1 = 0.067 | 5 (6.0) 16 (34.0) р1 < 0.001 | 0.725 < 0.001 |
Catarrhal symptoms only, n (%) | COVID-19 ARTI | – – | 1 (1.2) 3 (6.4) р1 = 0.092 | 1 (1.2) 5 (10.6) р1 = 0.022 | 5 (6.0) 7 (14.9) р1 = 0.017 | – 9 (19.1) р1 < 0.001 | 0.287 0.009 |
Recurrent episodes of ARTI episodes between the points of telephone contact, n (%) | COVID-19 ARTI | – – | 7 (8.3) 19 (40.4) р1 < 0.001 | 14 (16.7) 19 (40.4) р1 = 0.003 | 26 (31.0) 20 (42.6) | 17 (20.2) 18 (28.3) | 0.002 0.976 |
Total diseases, n (%) | COVID-19 ARTI | 10 (11.9) 21 (44.7) р1 < 0.001 | 17 (20.2) 21 (44.7) р1 = 0.004 | 34 (30.5) 21 (44.7) | 19 (22.6) 18 (38.3) р1 = 0.056 | < 0.001 0.872 | |
ABT frequency, n (%) | COVID-19 ARTI | – – | 6 (7.1) 5 (10.6) | 1 (1.2) 5 (10.6) р1 = 0.022 | 8 (9.5) 3 (6.4) | 7 (8.3) 3 (6.4) | 0.079 0.082 |
The children number with doctor’s appointment between telephone contact points, n (%) | COVID-19 ARTI | – – | 19 (22.6) 21 (44.7) р1 = 0.009 | 25 (29.8) 22 (46.8) р1 =0.052 | 36 (42.9) 19 (40.4) | 28 (33.3) 16 (34.0) | 0.014 0.506 |
Average number of medical appointments, M ± SD | COVID-19 ARTI | – | 2.3 ± 1.4 2.9 ± 2.1 | 1.8 ± 2.1 1.7 ± 1.2 | 1.3 ± 0.7 1.5 ± 0.6 | 1.4 ± 0.9 1.7 ± 0.9 | 0.029 1.0 |
The hospitalizations number between telephone contact points, n (%) | COVID-19 ARTI | – – | – 1 (2.1) | – 1 (2.1) | 3 (3.6) 1 (2.1) | 1 (1.2) 1 (2.1) | – – |
Children ≥ 6 years of age complained more frequently of weakness/fatigue, headache, and emotional disturbances (2.4–23.8%) and rarely (1–7%) of physical and mental intolerance, cognitive dysfunction, breathing difficulties, sleep disturbances, hair loss, and gastrointestinal disturbances. In a “crude” assessment in the early period of recongestion, the prevalence of signs of AD (difference 19.2% [3.5–34.9%]; OR = 0.9 [0.04–2.50], cardiovascular symptoms (difference 13.3% [4.6–22.1%]; OR = 6.27 [0.02–53.10]), subfebrile state (difference 12.2% [3.6–20.7%]; OR = 5.7 [0.10–49.2]), and hyposmia (difference 14.3%) were higher after COVID-19 than after acute respiratory infections (Table 2). However, the increased risks associated with COVID-19 were not significant.
During the year after COVID-19, the prevalence of dominant features, including the vagotonic-type AD symptom cluster, consistently decreased (Table 2). Other clusters and symptoms were recorded at a stable level. Hyposmia gradually resolved but subjectively persisted after 12 months in 2.4% (95% CI 0.6–9.1) of cases. The number of new illnesses (mainly recurrent acute respiratory infections) increased by 6 months and declined thereafter. Within 6 months after COVID-19, 2 cases of arthritis (2.4%; 95% CI 0.4–9.1%), hemorrhagic vasculitis (1.2%; 95% CI 0–7.4%), out-of-hospital pneumonia, and purulent inflammatory diseases (2 cases) were diagnosed.
After acute respiratory viral infection, the trajectory of the prevalence of the main symptoms was different. The sum of symptoms of AD due to weakness/fatigue, headaches and in general AD of vagotonic type increased by 3-6 months, then decreased by the end of the year. The registration of other nonrespiratory symptoms did not differ from COVID-19. New episodes of acute respiratory infections and associated catarrhal symptoms accounted for a substantial proportion of the causes of impairment. After the initial acute respiratory infection, cystitis, bacterial sinusitis, and 2 episodes of COVID-19, including one with hyposmia, were diagnosed in isolated cases (Table 2).
Overall, the risks of new episodes of respiratory infections were lower after COVID-19 (as among children < 6 years of age) compared with the ARTI group after 3 months (by 31.3% [95% CI 16.9–47.3]; OR = 0.79 [0.55–0.91]) and after 6 months (by 23.7% [7.6–39.9]; OR = 0.59 [0.26–0.77]). There was a correspondingly lower (22.1% [5.3–38.9]; OR = 0.49 [0.16–0.70]) referral to a pediatrician. During the year, among children ≥ 6 years of age in the compared groups, no symptoms were present in 47.6% of cases in the COVID-19 group and in 61.7% of cases in the ARTI group (p = 0.122).
The incidence of PCC in the compared groups was stable at 6 months and moderately decreased after one year (COVID-19 group: 44.0; 41.7; 42.9 and 33.3%; ARTI group: 40.4; 40.4; 36.2 and 17.0% at index points, respectively; p > 0.05). PCC was more common in children ≥ 6 years than in children <6 years, 1 month after hospital discharge: COVID-19 group: age difference 22.7% [95% CI 6.3–39.3]; OR = 1.20 [0.08–3.50]; p = 0.009), ARTI group: difference 21.5% [4.2–38.7]; OR = 1.13 [0.13–3.03], OR = 2.9 [1.20–6.98]; p = 0.018). No age differences were found at later ages.
The risk of symptom-complex PCC in patients ≥ 6 years without acute infections or other established diseases was higher after COVID-19 than after acute respiratory infections at 1 month, and the risk of de novo PCC was higher only at the time of hospital discharge (p = 0.039). Thereafter, no statistical difference was obtained for this indicator. After 6 months of follow-up, there were practically no cases of de novo PCC in children ≥ 6 years of age (Figure, b).
Multiple and persistent (throughout the year) disorders significantly affecting well-being were significantly more common in children with a history of AD than de novo: COVID-19 group: 26.5% vs. 1.1%, respectively; p < 0.001; ARTI group: 21.4 and 2.2%, respectively; p = 0.016. Table 3 presents the risk scores of the main symptom clusters adjusted for age, history of AD and repeated episodes of acute respiratory infections.
Table 3. History of autonomic dysfunction and incidence of acute respiratory infections adjusted odds ratio in children ≥ 6 years
Sign | Аdjusted OR | 95% CI | р |
Odds are increased after COVID-19 | |||
Well-being is impaired (self-assessment) | 2.038 | 1.141–3.644 | 0.016 |
including children ≥ 12 years | 1.634 | 1.065–2.507 | 0.037 |
Autonomic dysfunction (any signs), discharge from the hospital | 4.509 | 2.377–8.559 | < 0.001 |
Odds are increased after ARTI | |||
Well-being is impaired (self-assessment) after 6 months | 2.484 | 1.365–4.518 | 0.003 |
Odds are increased in children with a history of autonomic dysfunction regardless of COVID-19/ARTI group | |||
After 1 months | 3.187 | 1.889–5.381 | < 0.001 |
After 6 months | 3.333 | 1.806–6.147 | < 0.001 |
Well-being is impaired (self-assessment) | |||
after 1 months | 1.756 | 2.112–1.068 | 0.015 |
after 6 months | 1.824 | 1.157–2.872 | 0.009 |
Headache after 1 months | |||
after 1 months | 2.809 | 1.614–4.889 | < 0.001 |
after 6 months | 3.274 | 1.831–5.795 | < 0.001 |
Weakness, fatigue, locomotor activity | |||
after 1 months | 2.440 | 1.551–3.838 | < 0.001 |
after 6 months | 2.472 | 1.527–4.007 | < 0.001 |
Psychoemotional disorders | |||
after 1 months | 3.447 | 1.774–7.007 | < 0.001 |
after 6 months | 2.918 | 1.596–5.333 | < 0.001 |
Autonomic dysfunction (any signs) after 1 month | 3.387 | 2.076–5.474 | < 0.001 |
ncluding children ≥ 12 years | 1.718 | 1.070–2.759 | 0.024 |
Autonomic dysfunction (any signs) after 6 months | 3.187 | 1.917–5.296 | < 0.001 |
Odds are increased in children with repeated ARTI regardless of the COVID-19/ARTI group | |||
Cognitive dysfunction after 6 months | 1.745 | 1.023–2.977 | 0.041 |
Vagotonic autonomic dysfunction after 6 months | 1.788 | 1.007–3.171 | 0.047 |
Autonomic dysfunction (any signs) after 6 months | 1.768 | 1.181–2.649 | 0.006 |
Note: *Аt all time points of contact, odds were increased for these signs only points of 1 and 6 months are given for convenience.
Table 3 shows that the odds of complaints after COVID-19 were elevated compared to ARTI only at the time of hospital discharge, especially in children 12 years and older, but after 6 months the odds were higher in the ARTI group. It appeared that it was not the etiology of the initial respiratory infection but rather a history of ARTI that significantly increased the risks of predominant symptoms at all telephone contact points. Adolescents ≥ 12 years of age had higher such risks than children <12 years of age if AD was reported before the initial illness (adjusted OR = 3.2; 95% CI 2.0–5.2; p < 0.001). Interestingly, repeated respiratory episodes increased the odds of the most significant clusters (AD, including vagotonic type and cognitive impairment) by 6 months of follow-up regardless of the etiology of the initial infection.
Discussion
According to recent data, PCC in children is characterized by several groups of signs/conditions [1, 24]. After severe COVID-19 and in comorbid patients, PCC is diagnosed more frequently and has a more significant impact on pediatric health [1, 3, 25]. The present work is an attempt to explain the heterogeneity of clinical manifestations and temporal dynamics of PCC.
In general, we obtained information on the prevalence, spectrum and dynamics of symptoms that coincides with most published systematic reviews and meta-analyses [1, 4, 7, 8]. Symptoms identified in the questionnaires occurred with a frequency of 32–39%. The most common were the psycho-emotional cluster and the AD cluster, although older children were more detailed in labeling perceived symptoms, which is natural. The respiratory cluster was a significant proportion, often the only one.
In general, patients in the COVID-19 and ARTI groups experienced similar symptoms. Crude (unadjusted for possible confounding factors) assessment of differences between the COVID-19 group and the SARS-CoV-2-negative group found higher risks of well-being disorders (additionally, sleep problems in children < 6 years of age and hyposmia and AD cluster in children ≥ 6 years of age) only in the early recollection period. There was no difference in most specific features, which is probably due to the exclusion of comorbid patients. We confirmed the increased prevalence of some symptoms among older children (especially ≥ 12 years) described previously [12].
In the present study, different dynamics of individual symptoms (heterogeneous timing of onset and duration) were observed, as noted by other authors [14–16]. After COVID-19, the total number of complaints consistently decreased in parallel with an increase in the mean personal health assessment score. In children < 6 years of age, there was no statistical dynamics of individual clusters, and in children ≥ 6 years of age, the frequency of hyposmia, fatigue clusters, and AD (cardiovascular autonomic symptoms and vagotonic-type AD) decreased significantly over the course of the year. In the ARTI group, the sum of negative subjective feelings increased by 3–6 months in association with a significant frequency of repeated episodes of respiratory infections: in children < 6 years of age due to the psycho-emotional cluster, and in patients ≥ 6 years of age due to the cognitive, fatigue and vagotonic AD clusters. The mean estimated health status score in the acute respiratory trauma group did not change much. Throughout the year, most patients in both groups experienced mild health impairments and their duration did not exceed 3-6 months.
Even in the early stages of the pandemic, researchers noted significant similarities between PCC and clinical manifestations of AD [6, 26]. Prior to the spread of COVID-19, it was shown that postural orthostatic tachycardia syndrome, whose main clinical features are dizziness, tachycardia, headache, and difficulty concentrating, most commonly occurs at a young age with a median age of 14 years and is diagnosed with a significant delay in the majority of patients [27]. Given these data, we focused on determining the difference in prevalence of PCC symptoms between children and adolescents with and without these conditions before COVID-19, which we believe is a strength of the study.
In children < 6 years of age, a history of AD was rare, so there were limited opportunities for in-depth analysis for this characteristic. In the older age group, calculation of age-adjusted OR and past history of AD significantly narrowed the range and size of COVID-19 effects. The COVID-19 group differed from SARS-CoV-2-negative controls only at the time of hospital discharge by increased risk of malaise complaints. At other time points, symptom frequency was associated not with the etiology of the initial respiratory infection (COVID-19 or acute respiratory infection) but specifically with a history of ANS dysfunction before the pandemic. Among patients without a history of similar symptoms and who had not been sick for a year, de novo PCC was rare (2.7–8.0%) with no statistical differences between the COVID-19 and acute respiratory infection groups. Cumulatively (regardless of initial infection), multiple (5–25) and persistent (at all index points) symptoms rated by patients as significant occurred de novo even less frequently, in 1.7% of cases (95% CI 0.4–4.9), but significantly more frequently among children with a history of AD, 25% of cases (95% CI 13.6–39.6%; difference 23.3% (10.9–35.7%), OR = 14.8 (4.4–50.6); p < 0.001). It seems that the risks of PCC in most patients were due to their baseline physical and mental health status, and personal perception of the stressful situation in the current pandemic played a significant role as a supportive trigger of symptoms. At the same time, some patients did feel sick, as evidenced by frequent visits to the doctor.
Although COVID-19-associated pediatric AD is understudied, compelling biomarkers of ANS imbalance both during and after the acute period of infection have been presented [28–30]. R. Buchhorn showed that heart rate variability data after COVID-19 are not significantly different from those of adolescents with autonomic dysfunction due to psychosomatic diseases before the COVID-19 pandemic [31]. A study by B.H. Shaw et al. conducted shortly before the COVID-19 pandemic demonstrated different triggers of dysautonomia with a significant predominance of infection (41%) and psychological problems (28%). Pubertal period of child development was also noted as a significant predictor of AD. Interestingly, in this study, 40% of patients reported the onset of symptoms 3 months after the initiating event [27]. ANS imbalance has also been described in other respiratory viral infections, such as influenza [32] or respiratory syncytial infection [33].
Indirect confirmation of these data was also obtained in the present study, as the frequency of vagotonic AD and cognitive cluster complaints increased after 3–6 months in SARS-CoV-2-negative patients who were more likely to have repeated acute respiratory infections. And adjustment for distorting factors revealed a significant role of recurrent respiratory infections in increasing the risks of these complaints. In-depth studies have demonstrated autonomic disorders with predominant parasympathetic tone in COVID-19 [34], although multifactorial generation of symptoms and individual patient characteristics mediate complex mechanisms of ANS imbalance [30].
Stressful events are a critical trigger of AD, especially in predisposed patients. The SARS-CoV-2 pandemic globally has caused a significant deterioration in mental and cognitive health not only in adults but also in children, which has been named coronavirus anxiety [35–37]. Worry about their own and their loved ones' health, fear, negative media coverage, social isolation and physical inactivity increased the stressful pressure of the pandemic. It was not only adolescents, who respond more consciously to negative information flow, who suffered from sustained experiences. Young children also experienced subjective anxiety in response to maternal anxiety [38, 39]. Patients with special psychological needs or anxious at baseline were hypersensitive to the challenges of the epidemiologic situation associated with the spread of SARS-CoV-2 [37, 40]. Excessive health concern may persist for many months after COVID-19 [40]. In adults, anticipation of symptoms, baseline symptom burden and history of coronavirus disease, but not serologic confirmation, were predictors of worsening somatic symptoms [41]. The use of scales to assess neuropsychiatric symptoms and somatization confirmed subjective stress intolerance and subjective cognitive deficits in PCC patients [42]. In our data, repeated respiratory episodes in an ongoing pandemic per se or as a nocebo effect appeared to maintain/deepen feelings of fear, anxiety and depression.
The results of our study support a model of PCC in which the symptom cluster represents genuinely experienced persistent somatic symptoms that are usually functional in nature and therefore potentially reversible. PCC is likely to be a disorder with somatic symptoms and predisposing, triggering and perpetuating factors [10].
Thus, it appears that PCC is not a unique event peculiar to COVID-19 and likely cannot be a nosologic unit of clinical diagnosis. In our data, the only clinical characteristic of SARS-CoV-2 infection was chemosensory disorders, which are common in older children and adolescents (younger children are unable to formulate their sensations as a complaint) and persist for a long time [43]. Interestingly, the initial severity of hyposmia, emotional stress and depression may be risk factors for its persistence for 2 years [44]. We have not identified other specific symptoms for COVID-19 and PCC compared to SARS-CoV-2-negative patients.
The limitations of this study can be considered to be the collection of information by questionnaire without instrumental investigations that are accepted for the diagnosis of AD and related cardiovascular disorders. It is logical to assume that mothers of younger children and older children, being aware of their disease and under the stress of the pandemic, tend to unconsciously distort the true picture of their own health, which was probably further stimulated by leading questions in the surveys. We evaluated the information about repeated episodes of acute respiratory viral infections and repeated testing for SARC-CoV-2 from patients' words, and it is impossible to exclude subjective data errors, although in Russia during this period testing of all patients with acute respiratory viral infections was strictly regulated. The age of patients in the COVID-19 group was slightly skewed toward children older than 12 years of age who had more frequent complaints. We should then assume the possibility of lower risks of COVID-19 than established in this paper. However, the prospective type of our study, the exclusion of factors (severity of disease and comorbidity) that could knowingly cause bias in the data, consideration of the severity of symptoms and their dynamic variability, low dropout rate and consideration of new events (diseases) during the follow-up period allow us to believe that the data obtained are scientifically and practically useful.
Conclusion
Data on the prevalence of PCC in children vary due to the lack of specific diagnostic criteria, reliable biomarkers, and significant limitations of many existing studies. The present study is an attempt to seek explanations for the structural and temporal variability of PCC per se and therefore focused on finding relationships of symptoms after COVID-19 with baseline functional impairment and any events during the year. Adjustment for age and past history of AD allowed for a rare prevalence of PCC (2.7–8.0%) and no differences between patients with SARS-CoV-2 infection confirmed or excluded by molecular and serologic methods. Apart from prolonged hyposmia, we found no specific effects of COVID-19 compared with acute respiratory infections. In most cases, the disorders were mild and persisted for 3–6 months. Persistent and multiple symptoms perceived as serious by patients were found in a quarter of patients with a history of AD and in only 1.7% of de novo patients. Our results suggest that recurrent respiratory infections in an ongoing pandemic may be a significant trigger of new symptoms, explaining the previously described temporal variability of PCC. Given our and recent evidence, we support the current concept that PCC is a functional somatic disorder, probably sociogenic in patients who have baseline anxiety.
The study of PCC presents new aspects of the consequences of widespread respiratory viral infections, as some patients do need both medical and psychosocial rehabilitation.
1 WHO. A clinical case definition for post COVID-19 condition in children and adolescents by expert consensus, 16 February 2023. Available at: https://who.int/publications/i/item/WHO-2019-nCoV-Post-COVID-19-condition-CA-Clinical-case-definition-2023-1
About the authors
Elena R. Meskina
Moscow Regional Research and Clinical Institute
Author for correspondence.
Email: meskinaelena@rambler.ru
ORCID iD: 0000-0002-1960-6868
Dr. Sci. (Med.), Head, Сhildren's infectious disease department, Professor, Chair of children's diseases, Postgraduate training faculty
Russian Federation, MoscowMarima K. Khadisova
Moscow Regional Research and Clinical Institute
Email: murzabekova.marina.1979@mail.ru
ORCID iD: 0000-0001-8293-6643
Cand. Sci. (Med.), senior researcher, Сhildren's infectious disease department, Therapy department
Russian Federation, MoscowAnna Y. Ushakova
Moscow Regional Research and Clinical Institute
Email: vaoe_08@mail.ru
ORCID iD: 0000-0001-8438-7609
Cand. Sci. (Med.), Associate Professor, Department of infectious diseases, Postgraduate training faculty
Russian Federation, MoscowElena E. Tselipanova
Moscow Regional Research and Clinical Institute
Email: elena-tselip@yandex.ru
ORCID iD: 0000-0002-0586-8402
Cand. Sci. (Med.), senior researcher, Department of children’s infections, Therapy department
Russian Federation, MoscowLidiya A. Galkina
Moscow Regional Research and Clinical Institute
Email: lidiya140855@mail.ru
ORCID iD: 0000-0002-0052-2867
Cand. Sci. (Med.), senior researcher, Department of children’s infections, Therapy department
Russian Federation, MoscowReferences
- Rao S., Gross R.S., Mohandas S., et al. Postacute sequelae of SARS-CoV-2 in children. Pediatrics. 2024;153(3):e2023062570. DOI: https://doi.org/10.1542/peds.2023-062570.
- Stephenson T., Allin B., Nugawela M.D., et al. Long COVID (post-COVID-19 condition) in children: a modified Delphi process. Arch. Dis. Child. 2022;107(7):674–80. DOI: https://doi.org/10.1136/archdischild-2021-323624
- Rao S., Lee G.M., Razzaghi H., et al. Clinical features and burden of post acute sequelae of SARS-CoV-2 infection in children and adolescents. JAMA Pediatr. 2022;176(10):1000–9. DOI: https://doi.org/10.1001/jamapediatrics.2022.2800
- Hua M.J., Butera G., Akinyemi O., Porterfield D. Biases and limitations in observational studies of Long COVID prevalence and risk factors: A rapid systematic umbrella review. PLoS One. 2024;19(5):e0302408. DOI: https://doi.org/10.1371/journal.pone.0302408
- Behnood S., Newlands F., O’Mahoney L., et al. Persistent symptoms are associated with long term effects of COVID-19 among children and young people: Results from a systematic review and meta-analysis of controlled studies. PLoS One. 2023;18(12):e0293600. DOI: https://doi.org/10.1371/journal.pone.0293600
- Lopez-Leon S., Wegman-Ostrosky T., Ayuzo Del Valle N.C., et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci. Rep. 2022;12(1):9950. DOI: https://doi.org/10.1038/s41598-022-13495-5
- Zheng Y.B., Zeng N., Yuan K., et al. Prevalence and risk factor for long COVID in children and adolescents: A meta-analysis and systematic review. J. Infect. Public Health. 2023;16(5): 660–72. DOI: https://doi.org/10.1016/j.jiph.2023.03.005
- Schiavo M., Di Filippo P., Porreca A., et al. Potential predictors of long COVID in Italian children: a cross-sectional survey. Children (Basel). 2024;11(2):221. DOI: https://doi.org/10.3390/children11020221
- Ma Y., Deng J., Liu Q., et al. Long-term consequences of asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2023;20(2):1613. DOI: https://doi.org/10.3390/ijerph20021613
- Joffe A.R., Elliott A. Long COVID as a functional somatic symptom disorder caused by abnormally precise prior expectations during Bayesian perceptual processing: а new hypothesis and implications for pandemic response. SAGE Open Med. 2023;11:20503121231194400. DOI: https://doi.org/10.1177/20503121231194400
- Xu Z., Wang W., Zhang D., et al. Excess risks of long COVID symptoms compared with identical symptoms in the general population: a systematic review and meta-analysis of studies with control groups. J. Glob. Health. 2024;14:05022. DOI: https://doi.org/10.7189/jogh.14.05022
- Stephenson T., Pinto Pereira S.M., Shafran R., et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc. Health. 2022;6(4):230–9. DOI: https://doi.org/10.1016/S2352-4642(22)00022-0
- Luo D., Mei B., Wang P., et al. Prevalence and risk factors for persistent symptoms after COVID-19: a systematic review and meta-analysis. Clin. Microbiol. Infect. 2024;30(3):328–35. DOI: https://doi.org/10.1016/j.cmi.2023.10.016
- Pazukhina E., Andreeva M., Spiridonova E., et al. Prevalence and risk factors of post-COVID-19 condition in adults and children at 6 and 12 months after hospital discharge: a prospective, cohort study in Moscow (StopCOVID). BMC Med. 2022;20(1):244. DOI: https://doi.org/10.1186/s12916-022-02448-4
- Чернявская А.С., Симонова О.И., Каширская Н.Ю. Особенности постковидного синдрома у детей, перенесших инфекцию в легкой форме. Медицинский совет. 2023;17(17):187–93. Chernyavskaya A.S., Simonova O.I., Kashirskaya N.Yu. Characteristics of post COVID-19 condition in children after mild COVID-19. Medical Council. 2023; 17(17):187–193. DOI: https://doi.org/10.21518/ms2023-386 EDN: https://elibrary.ru/wvozve
- Stephenson T., Pinto Pereira S.M., Nugawela M.D., et al. Long COVID-six months of prospective follow-up of changes in symptom profiles of non-hospitalised children and young people after SARS-CoV-2 testing: а national matched cohort study (The CLoCk) study. PLoS One. 2023;18(3):e0277704. DOI: https://doi.org/10.1371/journal.pone.0277704
- Pinto Pereira S.M., Shafran R., Nugawela M.D., et al. Natural course of health and well-being in non-hospitalised children and young people after testing for SARS-CoV-2: a prospective follow-up study over 12 months. Lancet Reg. Health Eur. 2023;25:100554. DOI: https://doi.org/10.1016/j.lanepe.2022.100554
- Pinto Pereira S.M., Nugawela M.D., Rojas N.K., et al. Post-COVID-19 condition at 6 months and COVID-19 vaccination in non-hospitalised children and young people. Arch. Dis. Child. 2023;108(4):289–95. DOI: https://doi.org/10.1136/archdischild-2022-324656
- Исаева Е.П., Зайцева О.В., Локшина Э.Э. и др. Качество жизни детей после перенесенной новой коронавирусной инфекции. Медицинский совет. 2023;17(1):198–204. Isaeva E.P., Zaytseva O.V., Lokshina E.E., et al. Quality of life in children after a new coronavirus infection. Medical Council. 2023;17(1):198–204. DOI: https://doi.org/10.21518/ms2022-013 EDN: https://elibrary.ru/pduzar
- Vu T.T., Nguyen K.C., Nguyen H.T., et al. Prevalence and symptom profile of long COVID among schoolchildren in Vietnam. Viruses. 2024;16(7):1021. DOI: https://doi.org/10.3390/v16071021
- Robinson J.L., Le Saux N. Children and long-COVID: Do they go together? J. Assoc. Med. Microbiol. Infect. Dis. Can. 2022;7(4):300–6. DOI: https://doi.org/10.3138/jammi-2022-09-26
- Chaichana U., Man K.K.C., Chen A., et al. Definition of post-COVID-19 condition among published research studies. JAMA Netw. Open. 2023;6(4):e235856. DOI: https://doi.org/10.1001/jamanetworkopen.2023.5856
- Goretzki S.C., Brasseler M., Dogan B., et al. High prevalence of alternative diagnoses in children and adolescents with suspected long COVID — a single center cohort study. Viruses. 2023;15(2):579. DOI: https://doi.org/10.3390/v15020579
- Parzen-Johnson S., Katz B.Z. Navigating the spectrum of two pediatric COVID-19 complications: multi-system inflammatory syndrome in children and post-acute sequelae of SARS-CoV-2 infection. J. Clin. Med. 2024;13(4):1147. DOI: https://doi.org/10.3390/jcm13041147
- Lap C.R., Brackel C.L.H., Winkel A.M.A.M., et al. Post-COVID-19 condition in children: epidemiological evidence stratified by acute disease severity. Pediatr. Res. 2024. DOI: https://doi.org/10.1038/s41390-024-03597-3
- Becker R.C. Autonomic dysfunction in SARS-CoV-2 infection acute and long-term implications COVID-19 editor’s page series. J. Thromb. Thrombolysis. 2021;52(3):692–707. DOI: https://doi.org/10.1007/s11239-021-02549-6
- Shaw B.H., Stiles L.E., Bourne K., et al. The face of postural tachycardia syndrome — insights from a large cross-sectional online community-based survey. J. Intern. Med. 2019;286(4):438–48. DOI: https://doi.org/10.1111/joim.12895
- Woo M.S., Shafiq M., Fitzek A., et al. Vagus nerve inflammation contributes to dysautonomia in COVID-19. Acta Neuropathol. 2023;146(3):387–94. DOI: https://doi.org/10.1007/s00401-023-02612-x.
- Haischer M.H., Opielinski L.E., Mirkes L.M., et al. Heart rate variability is reduced in COVID-19 survivors and associated with physical activity and fatigue. Physiol. Rep. 2024;12(2):e15912. DOI: https://doi.org/10.14814/phy2.15912
- Scala I., Rizzo P.A., Bellavia S., et al. Autonomic dysfunction during acute SARS-CoV-2 infection: a systematic review. J. Clin. Med. 2022;11(13):3883. DOI: https://doi.org/10.3390/jcm11133883
- Buchhorn R. Therapeutic approaches to dysautonomia in childhood, with a special focus on long COVID. Children (Basel). 2023;10(2):316. DOI: https://doi.org/10.3390/children10020316
- Mattei J., Teyssier G., Pichot V., et al. Autonomic dysfunction in 2009 pandemic influenza A (H1N1) virus-related infection: a pediatric comparative study. Auton. Neurosci. 2011;162(1-2):77–83. DOI: https://doi.org/10.1016/j.autneu.2011.03.003
- Stock C., Teyssier G., Pichot V., et al. Autonomic dysfunction with early respiratory syncytial virus-related infection. Auton. Neurosci. 2010;156(1-2):90–5. DOI: https://doi.org/10.1016/j.autneu.2010.03.012
- Delogu A.B., Aliberti C., Birritella L., et al. Autonomic cardiac function in children and adolescents with long COVID: a case-controlled study. Eur. J. Pediatr. 2024;183(5):2375–82. DOI: https://doi.org/10.1007/s00431-024-05503-9
- Racine N., McArthur B.A., Cooke J.E., et al. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: A meta-analysis. JAMA Pediatr. 2021;175(11):1142–50. DOI: https://doi.org/10.1001/jamapediatrics.2021.2482
- Fortuna L.R., Brown I.C., Lewis Woods G.G., Porche M.V. The impact of COVID-19 on anxiety disorders in youth: coping with stress, worry, and recovering from a pandemic. Child Adolesc. Psychiatr. Clin. N. Am. 2023;32(3):531–42. DOI: https://doi.org/10.1016/j.chc.2023.02.002
- Panchal U., Salazar de Pablo G., Franco M., et al. The impact of COVID-19 lockdown on child and adolescent mental health: systematic review. Eur. Child Adolesc. Psychiatry. 2023;32(7):1151–77. DOI: https://doi.org/10.1007/s00787-021-01856-w
- Terin H., Açıkel S.B., Yılmaz M.M., Şenel S. The effects of anxiety about their parents getting COVID-19 infection on children’s mental health. Eur. J. Pediatr. 2023;182(1):165–71. DOI: https://doi.org/10.1007/s00431-022-04660-z
- Hagan M.J., Roubinov D.R., Cordeiro A., et al. Young children’s traumatic stress reactions to the COVID-19 pandemic: the long reach of mothers’ adverse childhood experiences. J. Affect. Disord. 2022;318:130–8. DOI: https://doi.org/10.1016/j.jad.2022.08.061
- Horn M., Wathelet M., Amad A., et al. Persistent physical symptoms after COVID-19 infection and the risk of somatic symptom disorder. J. Psychosom. Res. 2023;166:111172. DOI: https://doi.org/10.1016/j.jpsychores.2023.111172
- Engelmann P., Löwe B., Brehm T.T., et al. Risk factors for worsening of somatic symptom burden in a prospective cohort during the COVID-19 pandemic. Front. Psychol. 2022;13:1022203. DOI: https://doi.org/10.3389/fpsyg.2022.1022203
- Tröscher A., Gebetsroither P., Rindler M., et al. High somatization rates, frequent spontaneous recovery, and a lack of organic biomarkers in post-COVID-19 condition. Brain Behav. 2024;14(10):e70087. DOI: https://doi.org/10.1002/brb3.70087
- Almoznino G., Gleicher D., Kharouba J., Blumer S. Olfactory and gustatory disorders associated with SARS-CoV-2 infection in children and adults: a topic review. Quintessence Int. 2023;54(10):852–66. DOI: https://doi.org/10.3290/j.qi.b4313291
- Liao B., Deng Y.K., Zeng M., Liu Z. Long-term consequences of COVID-19: chemosensory disorders. Curr. Allergy Asthma Rep. 2023;23(2):111–9. DOI: https://doi.org/10.1007/s11882-022-01062-x
Supplementary files