Protective potential of structural proteins of the SARS-CoV-2 virus in protecting against COVID-19
- Authors: Dolzhikova I.V.1, Grousova D.M.1, Zorkov I.D.1, Ilyukhina A.A.1, Kovyrshina A.V.1, Zubkova O.V.1, Popova O.D.1, Ozharovskaya T.A.1, Zrelkin D.I.1, Savina D.M.1, Samokhvalova E.G.1, Tukhvatulin A.I.1, Shcheblyakov D.V.1, Logunov D.Y.1, Gintsburg A.L.1,2
-
Affiliations:
- National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
- First Moscow State Medical University named after I.M. Sechenov (Sechenov University)
- Issue: Vol 101, No 6 (2024)
- Pages: 769-778
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18637
- DOI: https://doi.org/10.36233/0372-9311-577
- EDN: https://elibrary.ru/wrxlsr
- ID: 18637
Cite item
Abstract
Introduction. Many different vaccines for the prevention of COVID-19 have received emergency use approval in the shortest possible time. Due to the high rate of variability of the pathogen, in this study we analyzed the variability of the structural proteins of the SARS-CoV-2 virus and compared their protective potential in protecting animals from COVID-19.
The aim of the study was to compare the protective potential of the SARS-CoV-2 structural proteins in protecting animals from COVID-19.
Materials and methods. The SARS-CoV-2 virus was used in the study. Transgenic mice B6.Cg-Tg(K18-ACE2)2Prlmn/J (F1) were used as model animals. Recombinant adenoviral vectors rAd5-S, rAd5-N, rAd5-M were used for immunization of animals. Various genetic, virological and immunological methods, as well as methods of working with animals, were used in the study.
Results. The largest number of amino acid substitutions in the structural proteins of different SARS-CoV-2 variants was detected in glycoprotein S, the smallest — in nucleoprotein N. In the COVID-19 animal model, it was shown that only the use of glycoprotein S as a vaccine antigen allows to form protective immunity that protects 100% of animals from a lethal infection caused by the SARS-CoV-2 virus, while the use of protein N protects 50% of animals from a lethal infection, and protein M does not have a protective potential.
Conclusion. The data obtained, as well as the analysis of the epidemiological efficacy of various mRNA and vector vaccines, demonstrate that the use of the SARS-CoV-2 glycoprotein S as an antigen allows to form the highest level of protection. Due to the constant change in circulating variants of the SARS-CoV-2 virus, the decrease in the effectiveness of the vaccines with the original antigen composition against new variants of the virus and the continuing high incidence of COVID-19, it is necessary to continuously monitor the effectiveness of vaccines against new variants of the virus and promptly update the antigen composition of vaccines when a decrease in effectiveness is detected.
Keywords
Full Text
Introduction
Since the identification of the causative agent of COVID-19, the SARS-CoV-2 virus, vaccine development has proceeded at an unprecedented and extraordinary pace thanks to the collaborative efforts of researchers, manufacturers and governments around the world. This has enabled the vaccines to be quickly approved for emergency use and introduced into civilian circulation to vaccinate populations around the world.
SARS-CoV-2 virus belongs to single-stranded (+)RNA-containing viruses of the Betacoronavirus genus of the Coronaviridae family, the genome size varies from 29.8 to 29.9 thousand nucleotides, virions have a spherical or ellipsoidal shape with an average diameter of 108 ± 8 nm and contain 4 major structural proteins. The outer surface of the virion is covered with a surface protein, glycoprotein S, while the virus outer membrane contains membrane protein M and envelope protein E. Inside the virion is a ribonucleoprotein complex consisting of nucleocapsid protein (N) and virus RNA [1]. The surface glycoprotein S of SARS-CoV-2 virus is a trimer with a molecular mass of about 600 kDa. Located on the virion envelope, it plays a key role in viral infection through receptor recognition and subsequent fusion of the virus and host cell membranes. Protein S has been shown to elicit a robust immune response, making it a dominant target for the development of vaccines to prevent COVID-19 [2-5]. The major protein component within the virion is the nucleocapsid protein N, which is essential for binding and packaging genomic RNA into a ribonucleoprotein complex (RNP complex) within the virion [6]. Coronavirus membrane protein (M) is the most abundant viral structural protein and plays a central role in virus assembly and morphogenesis [7]. The M and N proteins could potentially serve as targets for inclusion in candidate vaccine for COVID-19 prevention [8]. In addition to these structural proteins, SARS-CoV-2 encodes 16 non-structural proteins (nsp1-16) and 9 accessory proteins.
To date, about 50 vaccines for COVID-19 prevention have been registered1. The most widely used vaccines are those developed on 4 technological platforms: vector vaccines, mRNA vaccines, inactivated vaccines and subunit vaccines. Vector and mRNA vaccines are the most effective in protecting against COVID-19 [9]. Most vaccines contain the key protective antigen – surface glycoprotein S of the SARS-CoV-2 virus. Immunization with such vaccines allows the formation of a full immune response to the protein [10]: both humoral immune response (preventing the virus from entering the cell) and cellular cytotoxic immune response (necessary for the elimination of infected cells) are formed [11–15]. Immunization with inactivated vaccines induces a weaker humoral immune response and almost no T-cell immune response. In this case, antibodies of IgG, IgM and IgA classes are formed to various SARS-CoV-2 proteins, not only to S [16, 17]. The issue of immune correlates of protection against COVID-19 is still being studied, and a direct correlation between the level of neutralizing antibodies and protection against the disease has been shown [18]. The majority of epitopes for neutralizing antibodies are located in the region of the receptor-binding domain and the N-terminal domain of glycoprotein S [19].
SARS-CoV-2 virus has been actively evolving since its entry into the human population [20]. During the 4 years of the COVID-19 pandemic, there was a constant change of circulating variants of the pathogen, which has been accompanied by an increase in disease incidence. The most significant wave of desease was registered in early 2022 and was associated with the spread of the first sublineages (BA.1 and BA.2) of the Omicron variant of the SARS-CoV-2 virus2. The emergence and spread of new Omicron variant sublineages (BA.1, BA.5, XBB, BA.2.86, etc.) were accompanied by an increase in disease incidence around the world, including among the vaccinated population, therefore, it is critical to conduct continuous monitoring of the efficacy of vaccines against circulating variants for rapidly change of antigenic composition of vaccines3.
In the Russian-language literature, there are no data on comparative analysis of the protective efficacy of different SARS-CoV-2 virus antigens. These data will allow adequate assessment of the need to include different antigens in the composition of vaccines for COVID-19 prevention for the greatest efficacy against different variants of SARS-CoV-2 virus.
Given the high level of variability of the virus, the aim of this study was to compare the protective potential of structural proteins of SARS-CoV-2 virus in the protection of animals against COVID-19. To achieve this goal, the following objectives were set: to analyze the variability of structural proteins of SARS-CoV-2 virus, to study the protective efficacy of different structural proteins of SARS-CoV-2 virus on the COVID-19 laboratory animals model.
Materials and methods
Virus
SARS-CoV-2 virus was obtained from the State Virus Collection of N.F. Gamaleya NRCEM: Wuhan B.1.1.1 hCoV-19/Russia/Moscow_PMVL-1/2020. All studies with viable SARS-CoV-2 virus were conducted in BSL-3 laboratory according to SanPiN 3.3686-21 “Sanitary and Epidemiologic Requirements for the Prevention of Infectious Diseases”. SARS-CoV-2 virus production was carried out in Vero E6 cell culture. The infectious virus titer was determined on Vero E6 cell culture by 50% tissue culture infectious dose 50 (TCID50). The TCID50 titer was calculated using the Spearman–Kerber method.
Mammallian cell lines
Vero E6 cell culture (African green monkey kidney epithelial cells) was obtained from the Laboratory of Cellular Microbiology of the N.F. Gamaleya NRCEM.
Animal models
The study used F1 transgenic mice obtained from crossing transgenic males B6.Cg-Tg(K18-ACE2)2Prlmn/J (Jackson Laboratory, https://www.jax.org/strain/034860; health status SOPF) and nontransgenic females C57BL/6 Gamrc (N.F. Gamaleya NRCEM; health status SPF), hereinafter referred to as hACE2-transgenic mice. Female hACE2-transgenic mice weighing 18–20 g were used in the study. All animal studies were approved by the Biomedical Ethics Committee of the N.F. Gamaleya NRCEM (protocol No. 24 dated 21/04/2022). Expression of the hACE2 gene in F1-generation C57BL/6 Tg(K18-ACE2)2Prlmn mice was confirmed by real-time polymerase chain reaction according to the Jackson Laboratory protocol for this line of mice [21].
Work with animal models
Animals were housed and handled in accordance with laboratory animal husbandry requirements4. Laboratory animals (n = 40) were housed in conventional cages for immunization; for experiments involving SARS-CoV-2 virus, animals were housed in the IsoCage N system (Tecniplast). The animals had free access to food and water.
Immunization and virus challenge of animals
The study used recombinant viral vectors based on human adenovirus type 5 (rAd5) carrying genes of structural proteins of SARS-CoV-2 virus of Wuhan B.1 variant: rAd5-S (carries the gene of glycoprotein S), rAd5-N (carries the gene of nucleoprotein N), rAd5-M (carries the gene of membrane protein M) were used for immunization of animals. Animals of the groups (n = 10 per group) that received the vaccine were injected with rAd5 vaccines at a dose of 109 v.p./animal intramuscularly twice with an interval of 21 days. Animals in the control group (n = 10) were injected with an equivalent volume of sterile buffer solution. One week after the 2nd immunization, animals were infected intranasally with SARS-CoV-2 virus at a dose of 105 TCID50 and weight dynamics and survival rate were evaluated daily for 14 days after infection.
Organ sampling and viral load determination
Animals of all study groups (n = 4 per group) were euthanized on the 4th day after infection with an increased dose of inhalation anesthetic followed by cervical dislocation. Postmortem examination of the animals was performed, and lungs were sampled for macroscopic and viral load analysis. The selmpled organs were washed with saline and 10% homogenate was prepared using the MPbio FastPrep-24 device. The homogenates were centrifuged at 12,000g for 10 min and the supernatant was used for further analysis. The infectious titer of the virus was determined on Vero E6 cells according to the method described above.
Statistical and bioinformatics methods
Statistical processing of the research results was performed using the GraphPad Prism 10.2.3 computer program. The Student's t-test was used to analyze the data [22]. The covSPECTRUM online database was used to analyze the amino acid sequences of structural proteins of SARS-CoV-2 virus5. On the cov-spectrum.org portal in the Compare variants mode we selected the sequence of the desired protein comparison of new variants of SARS-CoV-2 virus6 in the Amino acid changes section and performed a pairwise with the original virus variant7. To calculate the variability of amino acid composition, the number of detectable amino acid substitutions was divided by the total number of amino acid substitutions and demonstrated as a percentage.
Results
Variability of structural antigens
We conducted a bioinformatics analysis of amino acid sequences of structural proteins of SARS-CoV-2 virus of different variants that circulated in Russia from March 2020 to July 2024 (Table). Among the 4 analyzed proteins, the largest number of amino acid substitutions was detected in glycoprotein S, the smallest – in nucleoprotein N. Thus, in variant KS.1, which has been circulating in Russia since spring 2024, 64 amino acid substitutions were detected in glycoprotein S and 8 in nucleoprotein N.
Variability of amino acid composition of structural proteins of SARS-CoV-2 virus circulating in Russia in 2020–2024
SARS-CoV-2 variant | Variability of amino acid composition, % relative to the original virus variant | |||
S | N | M | E | |
Delta | 0,79 | 0,59 | 0,45 | 0,00 |
Omicron BA.1 | 2,83 | 0,88 | 1,35 | 1,33 |
Omicron BA.5 | 2,67 | 1,03 | 1,35 | 1,33 |
Omicron XBB | 3,22 | 1,03 | 0,90 | 2,67 |
Omicron BA.2.86 | 4,63 | 1,17 | 2,25 | 1,33 |
Omicron KS.1 | 5,03 | 1,17 | 2,25 | 1,33 |
Source: covSPECTRUM online database.
Protective efficacy of SARS-CoV-2 virus structural proteins in a lethal infection model in hACE2-transgenic mice
In order to compare the protective efficacy of structural proteins of SARS-CoV-2 virus, we obtained recombinant viral vectors based on human adenovirus serotype 5 carrying genes of glycoprotein S, nucleoprotein N or membrane protein M. The study was performed on hACE2-transgenic mice. Animals were immunized twice at 21-day intervals and 7 days after the 2nd immunization, animals were infected intranasally with SARS-CoV-2 (Wuhan-like) virus at a dose of 105 TCID50 per animal. The effectiveness of antigens in protection against infection was analyzed by several parameters: lethality, severity of the course of infection (weight loss) and reduction of viral load in the lungs of vaccinated animals compared to controls.
Survival analysis showed that only the use of glycoprotein S as antigen can protect 100% of animals from lethal infection caused by SARS-CoV-2 virus (Fig. 1, a). Analysis of the severity of the course of infection also demonstrated that only the use of glycoprotein S can protect 100% of animals from infection (Fig. 1, b).
Fig. 1. Survival (a) and weight dynamics (b) of hACE2-transgenic mice from immunized and control groups (n = 10, days 0–4; n = 6, days 5–14) after infection with the SARS-CoV-2 virus.
The mean and standard deviation for each time point are shown in the figure (b).
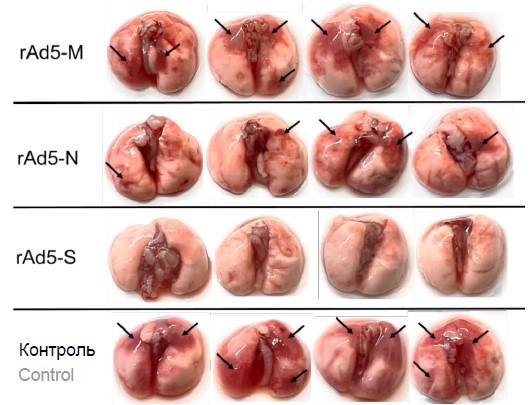
On the 4th day after infection, some animals were euthanized for macroscopic analysis of lung condition and viral load analysis. Analysis of the lung condition on the 4th day after infection showed that the most pronounced damage was found in the group of control animals and the group of animals that received the vaccine based on protein M (leukocytic infiltration, atelectasis and changes in membrane structures of the alveolar wall were observed). Less pronounced damage was detected in animals in the group that received the vaccine based on nucleoprotein N. Animals that received the vaccine based on glycoprotein S had no lung damage (Fig. 2).
Fig. 2. Photographs of the lungs of hACE2-transgenic mice from the immunized and control groups (n = 4) on day 4 after infection with the SARS-CoV-2 virus. Arrows indicate areas of lung tissue damage.
When analyzing the viral load in the lungs of infected animals in the control group and in the groups that received vaccines based on nucleoprotein N and membrane protein M, viable virus was detected. The animals that received the vaccine based on nucleoprotein N showed a significant decrease in viral load by 1.3 log10 TCID50. In the group that received a vaccine based on glycoprotein S no viable SARS-CoV-2 virus was detected and the viral load decreased by 5 lg TCID50 (Fig. 3).
Fig. 3. Viral load in the lungs of hACE2-transgenic mice from the immunized and control groups (n = 4) on day 4 after infection with the SARS-CoV-2 virus.
The figure shows individual data for each animal, the arithmetic mean and standard deviation, as well as the significance level p (Student's t-test).
Discussion
When selecting an antigen for inclusion in candidate vaccines, it is important to have an understanding of the immunologic features of the response to natural COVID-19 infection. The interaction of immune cells with the major structural proteins of the virus induces the formation of an antiviral immune response. For SARS-CoV-2 virus, these structural proteins are S, M, N and E. Despite the immunogenicity of glycoprotein S, nucleoprotein N and membrane protein M also contribute significantly to the development of a specific immune response. In patients with COVID-19, specific antibodies to protein N are detected early, whereas antibodies to glycoprotein S are detected 4-8 days after the onset of disease symptoms, which is probably due to the highest representation of nucleoprotein N in the virion [23, 24]. The cellular immune response also plays an important role in protection against COVID-19. A number of studies have shown that active proliferation of CD4+ and CD8+ T cells correlates with a less severe course of the disease and a high degree of virus elimination8. The cellular immune response is also highly specific to SARS-CoV-2 structural proteins. Determination of the SARS-CoV-2 T-cell epitopes, including 21 studies, showed that of the total number of CD4+ epitopes analyzed, 33% belonged to protein S, 11% to protein N, and 10% to protein M; of the total number of CD8+ epitopes analyzed, 26% belonged to protein S, 7% to protein N, and 6% to protein M [25]. A polyfunctional N-specific CD8+ T-cell response is associated with milder COVID-19 disease severity [26]. Because N is conserved between different SARS-CoV-2 variants, N-specific CD4+ T cells could potentially provide protection against different genetic variants of SARS-CoV-2 [27].
Nowadays, most vaccines for COVID-19 prevention used in clinical practice are based on glycoprotein S of the SARS-CoV-2 virus. However, there is still debate about which antigen should be included in COVID-19 vaccines. This is due to the high variability of glycoprotein S and the decreased efficacy of existing vaccines based on it against new SARS-CoV-2 virus variants.
When analyzing the amino acid sequences of structural proteins of SARS-CoV-2 virus — variants from Delta to current KS.1 — we demonstrated that the largest number of substitutions was found in glycoprotein S, the smallest — in nucleoprotein N, which makes protein N the most conservative among structural proteins of SARS-CoV-2 virus.
In order to directly compare the protective potential of SARS-CoV-2 virus structural proteins most represented in the virion, we obtained candidate vaccines based on recombinant human adenoviruses of serotype 5 carrying SARS-CoV-2 virus structural proteins: rAd5-M, rAd5-N and rAd5-S. These vaccines were used for immunization of hACE2-transgenic mice, after which the animals were infected with SARS-CoV-2 B.1.1.1 virus to evaluate the protective efficacy. Membrane protein M showed no protective efficacy — all immunized animals died after infection, while viable virus was detected in the lungs on the 4th day after infection at a titer similar to that of control animals. Similar data were obtained by J. Chen et al. when studying the effectiveness of plasmid DNA-based vaccines [28]. The use of nucleoprotein N as an antigen reduces the viral load in the lungs of immunized animals, but the reduction does not reach the required 2 log10; at the same time, pathological lesions were detected in the lungs of vaccinated animals, and protection against lethal infection caused by SARS-CoV-2 virus was only 50%, which correlates with the studies of other authors [29–32]. The use of glycoprotein S as an antigen made it possible to immunize animal models (no pathologic lesions and viable virus in the lungs) and protect all of them from lethal infection caused by SARS-CoV-2 virus, which is confirmed by the studies of other authors [33–36]. J. Chen et al. demonstrated that joint immunization with two DNA-based vaccines carrying S and N protein genes induced a more pronounced cellular and humoral immune response and had a greater protective efficacy against SARS-CoV-2 virus in a mouse model of infection [32]. R.L. Hajnik et al. showed on the COVID-19 hamster model that immunization with mRNA-S+N combined preparation induces a more pronounced protective response against SARS-CoV-2 virus veriants Delta and Omicron compared to single-component preparations [37]. Based on the above, the possibility of combining antigens in vaccine formulations for COVID-19 prevention should be considered in future studies. However, it should be taken into account that the inclusion of several antigens in vaccines significantly increases the cost of the production process and, consequently, the cost of the vaccine.
Analysis of the efficacy data of different vaccines for COVID-19 prevention in controlled clinical trials worldwide showed that mRNA and vector vaccines carrying the glycoprotein S gene of SARS-CoV-2 virus allow to provide the highest level of population protection in terms of morbidity, hospitalization and COVID-19-associated deaths [9, 38]. Given the constant change of circulating SARS-CoV-2 virus variants, the decreasing efficacy of the vaccines used against new virus variants [39] and the continuing high incidence of COVID-19, it is necessary to continuously monitor the efficacy of vaccines against new virus variants. If a decrease in efficacy is detected, the antigenic composition of vaccines should be updated. These studies are harmonized with the WHO studies, based on the results of which, starting from 2022, WHO issues recommendations on changing the antigenic composition of vaccines9. In 2023, based on the results of efficacy monitoring, the antigenic composition of Russian vaccines of Gam-COVID-Vac line (vector vaccines carrying the gene of glycoprotein S of SARS-CoV-2 virus) was updated for XBB sublineage. Clinical trials of vaccines with updated composition showed a favorable safety profile, formation of neutralizing antibodies against Omicron sublineages circulating in 2023 and circulating in the first half of 2024, and today the vaccines have been introduced into civil circulation to protect the population against current circulating variants of SARS-CoV-2.
Conclusion
SARS-CoV-2 virus actively mutates, which leads to the emergence of new virus variants. Among the structural proteins, the one subject to the greatest variability is surface glycoprotein S, which plays an important role in the virus life cycle — internalization, and is also a key target for neutralizing antibodies.
Comparative analysis of the protective potential of different structural proteins of SARS-CoV-2 virus on the animal model of lethal infection showed that only the use of glycoprotein S allows to form a protective immune response that protects 100% of animals from lethal infection caused by SARS-CoV-2 virus, while the reduction of viral load in the lungs of animals on the 4th day after infection amounted to 5.0 log10 TCID50 (100 000 times). At the same time, the use of nucleoprotein N resulted in a decrease in viral load of 1.3 log10 TCID50 (20-fold), and the level of protection against lethal infection was at 50%.
1 COVID-19 Vaccine tracker. Approved vaccines.
URL: https://covid19.trackvaccines.org/vaccines/approved/
2 NextStrain. Genomic epidemiology of SARS-CoV-2 with subsampling focused globally since pandemic start.
URL: https://nextstrain.org/ncov/gisaid/global/all-time
WHO. WHO COVID-19 dashboard. URL: https://data.who.int/dashboards/covid19/cases?m49 = 001
3 OurWorldInData. United States: COVID-19 weekly death rate by vaccination status, All ages.
URL: https://ourworldindata.org/grapher/united-states-rates-of-covid-19-deaths-by-vaccination-status
WHO. Statement on the antigen composition of COVID-19 vaccines. 18.05.2023.
URL: https://www.who.int/news/item/18-05-2023-statement-on-the-antigen-composition-of-covid-19-vaccines
WHO. Statement on the antigen composition of COVID-19 vaccines. 26.04.2024.
URL: https://www.who.int/news/item/26-04-2024-statement-on-the-antigen-composition-of-covid-19-vaccines
4 Руководство по лабораторным животным и альтернативным моделям в биомедицинских технологиях / под ред. Н.Н. Каркищенко, С.В. Грачева. М.; 2010. 354 с.
5 covSPECTRUM. Detect and analyze variants of SARS-CoV-2. URL: https://cov-spectrum.org/
6 covSPECTRUM. B.1.617.2 (Nextclade).
URL: https://cov-spectrum.org/explore/World/AllSamples/from%3D2020-01-01%26to%3D2024-10-11/variants?nextcladePangoLineage=B.1.617.2&
covSPECTRUM. BA.1 (Nextclade).
URL: https://cov-spectrum.org/explore/World/AllSamples/from%3D2020-01-01%26to%3D2024-10-11/variants?nextcladePangoLineage=ba.1&
covSPECTRUM. BA.5 (Nextclade).
URL: https://cov-spectrum.org/explore/World/AllSamples/from%3D2020-01-01%26to%3D2024-10-11/variants?nextcladePangoLineage=ba.5&
covSPECTRUM. XBB (Nextclade).
URL: https://cov-spectrum.org/explore/World/AllSamples/from%3D2020-01-01%26to%3D2024-10-11/variants?nextcladePangoLineage=xbb&
covSPECTRUM. BA.2.86 (Nextclade).
URL: https://cov-spectrum.org/explore/World/AllSamples/from%3D2020-01-01%26to%3D2024-10-11/variants?nextcladePangoLineage=ba.2.86&
covSPECTRUM. KS.1 (Nextclade).
URL: https://cov-spectrum.org/explore/World/AllSamples/from%3D2020-01-01%26to%3D2024-10-11/variants?nextcladePangoLineage=ks.1&
7 covSPECTRUM. B (Nextclade).
URL: https://cov-spectrum.org/explore/World/AllSamples/from%3D2020-01-01%26to%3D2024-10-11/variants?nextcladePangoLineage=B&nextcladePangoLineage1=B.1&
8 CDC. CDC Museum COVID-19 timeline. Centers for Disease Control and Preventio. URL: https://www.cdc.gov/museum/timeline/covid19.html
9 WHO. Technical Advisory Group on COVID-19 Vaccine Composition. URL: https://www.who.int/groups/technical-advisory-group-on-covid-19-vaccine-composition-(tag-co-vac)
About the authors
Inna V. Dolzhikova
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Author for correspondence.
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0003-2548-6142
Cand. Sci. (Biol.), Head, State virus collection laboratory
Russian Federation, MoscowDaria M. Grousova
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0002-3299-4818
junior researcher, State virus collection laboratory
Russian Federation, MoscowIlya D. Zorkov
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0001-8311-2283
junior researcher, State virus collection laboratory
Russian Federation, MoscowAnna A. Ilyukhina
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0003-0728-5478
junior researcher, State virus collection laboratory
Russian Federation, MoscowAnna V. Kovyrshina
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0002-8757-7026
researcher, Cellular microbiology laboratory
Russian Federation, MoscowOlga V. Zubkova
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0001-7893-8419
Cand. Sci. (Biol.), leading researcher, Immunobiotechnology laboratory
Russian Federation, MoscowOlga D. Popova
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0003-3248-1227
junior researcher, Immunobiotechnology laboratory
Russian Federation, MoscowTatiana A. Ozharovskaya
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0001-7147-1553
Cand. Sci. (Biol.), senior researcher, Immunobiotechnology laboratory
Russian Federation, MoscowDenis I. Zrelkin
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0003-0899-8357
junior researcher, Immunobiotechnology laboratory
Russian Federation, MoscowDaria M. Savina
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0002-2228-3406
researcher, Division for implementation of scientific developments into production
Russian Federation, MoscowEkaterina G. Samokhvalova
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0002-0127-173X
researcher, State virus collection laboratory
Russian Federation, MoscowAmir I. Tukhvatulin
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0001-8506-2339
Cand. Sci. (Biol.), Head, Mycoplasmas and L-forms of bacteria laboratory
Russian Federation, MoscowDmitry V. Shcheblyakov
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0002-1289-3411
Cand. Sci. (Biol.), Head, Immunobiotechnology laboratory
Russian Federation, MoscowDenis Yu. Logunov
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0003-4035-6581
D. Sci. (Biol.), Academician of the Russian Academy of Sciences, Head, Cellular microbiology laboratory
Russian Federation, MoscowAlexander L. Gintsburg
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya; First Moscow State Medical University named after I.M. Sechenov (Sechenov University)
Email: dolzhikova@gamaleya.org
ORCID iD: 0000-0003-1769-5059
D. Sci. (Biol.), Professor, Academician of the Russian Academy of Sciences, Director, Head, Department of infectology and virology
Russian Federation, Moscow; MoscowReferences
- Ke Z., Oton J., Qu K., et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature. 2020;588(7838):498–502. DOI: https://doi.org/10.1038/s41586-020-2665-2
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–9. DOI: https://doi.org/10.1038/s41564-020-0688-y
- Du S., Cao Y., Zhu Q., et al. Structurally resolved SARS-CoV-2 antibody shows high efficacy in severely infected hamsters and provides a potent cocktail pairing strategy. Cell. 2020;183(4):1013–23.e13. DOI: https://doi.org/10.1016/j.cell.2020.09.035
- Zost S.J., Gilchuk P., Case J.B., et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584(7821):443–9. DOI: https://doi.org/10.1038/s41586-020-2548-6
- Robbiani D.F., Gaebler C., Muecksch F., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–42. DOI: https://doi.org/10.1038/s41586-020-2456-9
- Mu J., Xu J., Zhang L., et al. SARS-CoV-2-encoded nucleocapsid protein acts as a viral suppressor of RNA interference in cells. Sci. China Life Sci. 2020;63(9):1413–6. DOI: https://doi.org/10.1007/s11427-020-1692-1
- Zhang Z., Nomura N., Muramoto Y., et al. Structure of SARS-CoV-2 membrane protein essential for virus assembly. Nat. Commun. 2022;13(1):4399. DOI: https://doi.org/10.1038/s41467-022-32019-3
- Dai L., Gao G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021;21(2):73–82. DOI: https://doi.org/10.1038/s41577-020-00480-0
- Graña C., Ghosn L., Evrenoglou T., et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022;12(12):CD015477. DOI: https://doi.org/10.1002/14651858.CD015477
- Rijkers G.T., Weterings N., Obregon-Henao A., et al. Antigen presentation of mRNA-based and virus-vectored SARS-CoV-2 vaccines. Vaccines (Basel). 2021;9(8):848. DOI: https://doi.org/10.3390/vaccines9080848
- Sahin U., Muik A., Derhovanessian E., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–9. DOI: https://doi.org/10.1038/s41586-020-2814-7
- Mulligan M.J., Lyke K.E., Kitchin N., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–93. DOI: https://doi.org/10.1038/s41586-020-2639-4
- Logunov D.Y., Dolzhikova I.V., Zubkova O.V., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–97. DOI: https://doi.org/10.1016/S0140-6736(20)31866-3
- Tukhvatulin A.I., Dolzhikova I.V., Dzharullaeva A.S., et al. Safety and immunogenicity of rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine against SARS-CoV-2 in healthy adolescents: an open-label, non-randomized, multicenter, phase 1/2, dose-escalation study. Front. Immunol. 2023;14:1228461. DOI: https://doi.org/10.3389/fimmu.2023.1228461
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–81. DOI: https://doi.org/10.1016/S0140-6736(21)00234-8
- Al-Shudifat A.E., Al-Tamimi M., Dawoud R., et al. Anti-S and Anti-N antibody responses of COVID-19 vaccine recipients. Vaccines (Basel). 2023;11(9):1398. DOI: https://doi.org/10.3390/vaccines11091398
- Qaqish A., Abbas M.M., Al-Tamimi M., et al. SARS-CoV-2 antinucleocapsid antibody response of mRNA and inactivated virus vaccines compared to unvaccinated individuals. Vaccines (Basel). 2022;10(5):643. DOI: https://doi.org/10.3390/vaccines10050643
- Goldblatt D., Alter G., Crotty S., Plotkin S.A. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol. Rev. 2022;310(1):6–26. DOI: https://doi.org/10.1111/imr.13091
- Finkelstein M.T., Mermelstein A.G., Parker Miller E., et al. Structural analysis of neutralizing epitopes of the SARS-CoV-2 spike to guide therapy and vaccine design strategies. Viruses. 2021;13(1):134. DOI: https://doi.org/10.3390/v13010134
- Markov P.V., Ghafari M., Beer M., et al. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023;21(6):361–79. DOI: https://doi.org/10.1038/s41579-023-00878-2
- McCray PB Jr., Pewe L., Wohlford-Lenane C., et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007;81(2):813–21. DOI: https://doi.org/10.1128/JVI.02012-06
- Унгуряну Т.Н., Гржибовский А.М. Краткие рекомендации по описанию, статистическому анализу и представлению данных в научных публикациях. Экология человека. 2011; (5): 55–60. Unguryanu T.N., Grjibovski A.M. Brief recommendations on description, analysis and presentation of data in scientific papers. Human Ecology. 2011; (5): 55–60.
- Tan Y.J., Goh P.Y., Fielding B.C., et al. Profiles of antibody responses against severe acute respiratory syndrome coronavirus recombinant proteins and their potential use as diagnostic markers. Clin. Diagn. Lab. Immunol. 2004;11(2):362–71. DOI: https://doi.org/10.1128/cdli.11.2.362-371.2004
- Wu H.S., Hsieh Y.C., Su I.J., et al. Early detection of antibodies against various structural proteins of the SARS-associated coronavirus in SARS patients. J. Biomed. Sci. 2004;11(1):117–26. DOI: https://doi.org/10.1007/BF02256554
- Andersen K.G., Rambaut A., Lipkin W.I., et al. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–2. DOI: https://doi.org/10.1038/s41591-020-0820-9
- Peng Y., Mentzer A.J., Liu G., et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020;21(11):1336–45. DOI: https://doi.org/10.1038/s41590-020-0782-6
- Yang G., Wang J., Sun P., et al. SARS-CoV-2 epitope-specific T cells: Immunity response feature, TCR repertoire characteristics and cross-reactivity. Front. Immunol. 2023;14:1146196. DOI: https://doi.org/10.3389/fimmu.2023.1146196
- Chen J., Deng Y., Huang B., et al. DNA vaccines expressing the envelope and membrane proteins provide partial protection against SARS-CoV-2 in mice. Front. Immunol. 2022;13:827605. DOI: https://doi.org/10.3389/fimmu.2022.827605
- Matchett W.E., Joag V., Stolley J.M., et al. Cutting Edge: nucleocapsid vaccine elicits spike-independent SARS-CoV-2 protective immunity. J. Immunol. 2021;207(2):376–9. DOI: https://doi.org/10.4049/jimmunol.2100421
- Rabdano S.O., Ruzanova E.A., Pletyukhina I.V., et al. Immunogenicity and in vivo protective effects of recombinant nucleocapsid-based SARS-CoV-2 vaccine Convacell®. Vaccines (Basel). 2023;11(4):874. DOI: https://doi.org/10.3390/vaccines11040874
- Primard C., Monchâtre-Leroy E., Del Campo J., et al. OVX033, a nucleocapsid-based vaccine candidate, provides broad-spectrum protection against SARS-CoV-2 variants in a hamster challenge model. Front. Immunol. 2023;14:1188605. DOI: https://doi.org/10.3389/fimmu.2023.1188605
- Chen J., Huang B., Deng Y., et al. synergistic immunity and protection in mice by co-immunization with DNA vaccines encoding the spike protein and other structural proteins of SARS-CoV-2. Vaccines (Basel). 2023;11(2):243. DOI: https://doi.org/10.3390/vaccines11020243
- Wu Y., Huang X., Yuan L., et al. A recombinant spike protein subunit vaccine confers protective immunity against SARS-CoV-2 infection and transmission in hamsters. Sci. Transl. Med. 2021;13(606):eabg1143. DOI: https://doi.org/10.1126/scitranslmed.abg1143
- Brocato R.L., Kwilas S.A., Kim R.K., et al. Protective efficacy of a SARS-CoV-2 DNA vaccine in wild-type and immunosuppressed Syrian hamsters. NPJ Vaccines. 2021;6(1):16. DOI: https://doi.org/10.1038/s41541-020-00279-z
- DiPiazza A.T., Leist S.R., Abiona O.M., et al. COVID-19 vaccine mRNA-1273 elicits a protective immune profile in mice that is not associated with vaccine-enhanced disease upon SARS-CoV-2 challenge. Immunity. 2021;54(8):1869–82.e6. DOI: https://doi.org/10.1016/j.immuni.2021.06.018
- Darling T.L., Ying B., Whitener B., et al. mRNA-1273 and Ad26.COV2.S vaccines protect against the B.1.621 variant of SARS-CoV-2. Med. 2022;3(5):309–24.e6. DOI: https://doi.org/10.1016/j.medj.2022.03.009
- Hajnik R.L., Plante J.A., Liang Y., et al. Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models. Sci. Transl. Med. 2022;14(662):eabq1945. DOI: https://doi.org/10.1126/scitranslmed.abq1945
- Kyriakidis N.C., López-Cortés A., González E.V., et al. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6(1):28. DOI: https://doi.org/10.1038/s41541-021-00292-w
- Wu N., Joyal-Desmarais K., Ribeiro P.A.B., et al. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 2023;11(5):439–52. DOI: https://doi.org/10.1016/S2213-2600(23)00015-2
Supplementary files