HIV-1 drug resistance among naïve patients in Armenia in 2017–2021
- Authors: Osadchaya O.A.1, Kireev D.E.1, Saleeva D.V.1, Kirichenko A.A.1, Lapovok I.A.1, Lopatukhin A.E.1, Shlykova A.V.1, Makhmudova L.F.1, Ladnaya N.N.1, Hovakimyan E.M.2, Martoyan S.V.2, Kazaryan H.K.2, Hovsepyan T.V.2, Sargsiants N.K.2, Pokrovsky V.V.1
-
Affiliations:
- Central Research Institute of Epidemiology
- National Center for Infectious Diseases
- Issue: Vol 101, No 2 (2024)
- Pages: 184-192
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18581
- DOI: https://doi.org/10.36233/0372-9311-398
- EDN: https://elibrary.ru/mnxbqo
- ID: 18581
Cite item
Abstract
Background. The increased antiretroviral therapy (ART) coverage of patients in the absence of routine drug resistance (DR) tests highlight the importance of HIV-1 drug resistance surveillance in Armenia.
Aim. The aim of this study was a determination of the prevalence of HIV-1 DR on a large-scale cohort of HIV-infected citizens of the Republic of Armenia who had no experience of taking antiretroviral drugs.
Materials and methods. The study was carried out on a cohort of more than 20% of PLHIV in the Republic of Armenia. The resulting 982 nucleotide sequences of the HIV-1 pol gene fragment, encoding the protease and reverse transcriptase region, as well as 367 sequences of the integrase gene, were analyzed using the Stanford University database and the CPR tool for the presence of drug resistance mutations and determination of the resistance level to ARV drugs. The HIV-1 subtype was determined using the Stanford University database and confirmed by phylogenetic analysis.
Results. The overall prevalence of HIV DR to ARV drugs in naïve patients was 13.8%. Resistance to non-nucleoside reverse transcriptase inhibitors was 11.2%, nucleotide reverse transcriptase inhibitors — 1.4%, protease inhibitors — 2.0% and integrase inhibitors — 0.5%. The predominant genetic variant among viruses containing DR mutations was subtype B. Resistance was most often recorded among men who have sex with men living in Yerevan.
Conclusion. In our study, prevalence of DR was high only for the NNRTI drugs. The results show that the first-line ARV drugs recommended in current national guidelines are highly likely to be effective. The analysis was carried out on a significant proportion of HIV-infected citizens of the Republic of Armenia, which increases the reliability and accuracy of the data obtained.
Keywords
Full Text
Introduction
The Republic of Armenia is a country in Eastern Europe and Central Asia (EECA) that has been actively engaged in the fight against HIV/AIDS over the past decades. According to the United Nations Global Statistics on HIV/AIDS (UNAIDS), there were 1.8 million people living with HIV in EECA as of 2021, and the number of new infections continues to increase1.
After the collapse of the Soviet Union, a period of economic collapse severely weakened the health infrastructure of the newly independent republics. At that time, little attention was paid to the HIV-1 epidemic. Much of the former Soviet Union was experiencing one of the fastest growing HIV-1 epidemics in the world, with the number of infected individuals doubling annually in certain regions [1]. Open border policies in the regions did not prevent migration, which in turn facilitated the spread of infectious diseases2. Research results show that among Armenian citizens the highest HIV-1 prevalence was recorded among migrant workers [2, 3].
One of the main reasons for the increase in HIV-1 infections in Armenia was the low level of access to antiretroviral therapy (ART). However, in low- and middle-income countries, ART was introduced only in the mid-2000s [4]. Thanks to the efforts of the World Health Organization (WHO) in the early twenty first century, the inequality between rich and poor countries in terms of treatment options decreased. Developing countries were able to purchase drugs at affordable prices, which in turn reduced mortality. During this period, the first guidelines for HIV-1 treatment in resource-limited settings were published, which contained simplified treatment regimens [5].
At present, the incidence of HIV infection in Armenia is low. The total number of infected cases does not exceed 4,600 people, which is only 0.12% of the country's population3. Much like in other countries, programs in Armenia are aimed at achieving the goals of UNAIDS. As a result of the "90-90-90" strategy, a good result was achieved in the region — in 2020, 81% of people living with HIV knew their status and 86% of people who were prescribed ART achieved an undetectable viral load4. As of 2018, the preferred treatment regimen includes the integrase inhibitor (INI) dolutegravir in combination with two nucleoside reverse transcriptase inhibitors (NRTIs) — tenofovir, lamivudine or emtricitabine. An alternative regimen is two NRTIs (tenofovir and lamivudine) and one non-nucleoside reverse transcriptase inhibitor (NNRTI) drug, efavirenz5.
Due to the increasing number of patients on ART, the development of viral resistance is a significant challenge in combating the HIV epidemic. WHO recommends drug resistance testing prior to treatment initiation and when ART is ineffective. Unfortunately, this approach is not available to all countries. In most developing countries, only certain groups of patients are tested and only in cases of virological failure of ART6.
HIV resistance testing is also recommended for epidemiologic surveillance of drug resistance (DR) [6]. According to WHO reports, the prevalence of transmitted DR for 2017 and 2020 has exceeded the 5% threshold in most countries, with 67% of countries exceeding 10%. The presence of HIV-1 DR in naive patients reduces the selection of effective ART and may increase the risk of virologic treatment failure [7].
Previously published studies have investigated the prevalence of HIV-1 DR in Armenia in untreated patients and reported a significant increase in resistance. While in 2015 the resistance rate was only 1.5%, in the 2020 and 2021 studies, the prevalence of DR increased to 5.5% and 9.2%, respectively, and approached the WHO 10% threshold, upon reaching which clinical treatment guidelines should be revised [8–10]. However, the 2022 publication indicated a decrease in DR in naive patients to 7.5% in 2020–2021 [11]. Previous studies of HIV-1 DR were characterized by small sample sizes and therefore had limited reliability of results.
The aim of the scientific research is to assess the prevalence and pattern of HIV-1 drug resistance in the Republic of Armenia by analyzing a large cohort of patients with no history of taking antiretroviral drugs.
Materials and methods
The collection of biological material and related patient information took place in 2018–2022. A total of 982 patients who had not previously received ART participated in the study. Patients were included consecutively during routine visits to the Republican AIDS Prevention Center of the Ministry of Health of the Republic of Armenia. Demographic and clinical data collected included age, sex, date of first positive immune blot, presumed HIV-1 transmission route, HIV-1 RNA viral load and CD4+ cell count.
Samples were analyzed by mass parallel sequencing using a laboratory technique developed at the Central Research Institute of Epidemiology (n = 367) or by classical Sanger sequencing using the AmpliSense HIV-Resist-Seq reagent kit (Central Research Institute of Epidemiology) (n = 615). If mass parallel sequencing was used, three viral genes were analyzed: protease, reverse transcriptase and integrase. If Sanger sequencing was used, the nucleotide sequences of the protease gene and a fragment of the reverse transcriptase gene, in which resistance mutations may appear, were obtained. Sequencing was performed using MiSeq (Illumina) and Applied Biosystems 3500 Genetic Analyzer (Life Technologies) instruments.
Preliminary HIV-1 subtyping was performed using the online tools REGA v. 3.07 and Stanford University database8. The results of preliminary subtyping were verified by phylogenetic analysis using the MEGA v. 6.0 software with reference sequences of HIV-1 subtypes and recombinant forms downloaded from the Los-Alamos database9. Nucleotide sequence alignment and further editing were performed using BioEdit v. 7.210.
The quality of nucleotide sequences was assessed using the WHO HIV DR v. 2.3011 and Calibrated Population Resistance Tool12.
DR to antiretroviral drugs were identified using the Stanford University database. Surveillance drug resistance mutations (SDRM) were identified using WHO Surveillance Drug Resistance Mutation Worksheet 2014.
The study was conducted with the informed consent of the patients. The study was approved by the local ethical committee of the Central Research Institute of Epidemiology of Rospotrebnadzor (Moscow, Russia) on May 21, 2019 (protocol No. 92).
RESULTS
Characteristics of the study group
The mean age of participants at the time of inclusion in the study was 41 (19-75) years, with the largest number of people with HIV infection being aged 30-40 years; 68.9% of patients were male. Heterosexual transmission was predominant (83.2%) as the most likely route of transmission in the study group.
Subtyping results showed a high degree of genetic diversity among the variants circulating in the study region. HIV-1 sub-subtype A6 was predominant with a prevalence of 87.0%, with subtype B being the next most frequent (5.9%). Subtypes A1, C and G were also detected in isolated cases. In addition, 6 different recombinant forms were detected –CRF02_AG, CRF03_A6B, CRF06_cpx, CRF20_BG, CRF24_BG and CRF63_02A6, which are frequently found in EECA countries.
The epidemiologic data of the study participants are presented in Table 1. A 95% confidence interval (CI) was calculated for each epidemiologic group.
Table 1. Epidemiological characteristics of patients included in the study, n (%; 95% CI)
Characteristics | Men | Women | All |
Number of patients | 677 (68.9; 66.0–71.8) | 305 (31.1; 28.2–34.0) | 982 |
Transmission risk group of infection | |||
Heterosexual | 514 (52.3; 49.2–55.5) | 303 (30.9; 28.0–33.8) | 817 (83.2; 80.7–85.4) |
Men who have sex with men | 98 (10.0; 8.3–12.0) | – | 98 (10.0; 8.3–12.0) |
Injecting drug users | 65 (6.6; 5.2–8.4) | 2 (0.2; < 0.01–0.8) | 67 (6.8; 5.4–8.6) |
HIV-1 genetic variants | |||
A1 | 1 (0.1; < 0.01–0.6) | 0 | 1 (0.1; < 0.01–0.6) |
A6 | 567 (57.7; 54.6–60.8) | 287 (29.2;26.5–32.2) | 854 (87.0; 84.7–88.9) |
B | 56 (5.7; 4.4–7.3) | 2 (0.2; < 0.01–0.8) | 58 (5.9; 4.6–7.6) |
C | 1 (0.1; < 0.01–0.6) | 1 (0.1; < 0.01–0.6) | 2 (0.2; < 0.01–0.8) |
G | 1 (0.1; < 0.01–0.6) | 0 | 1 (0.1; < 0.01–0.6) |
CRF02_AG | 16 (1.6; 1.0–2.7) | 6 (0.6; 0.3–1.4) | 22 (2.2; 1.5–3.4) |
CRF03_A6B | 5 (0.5; 0.2–1.2) | 2 (0.2; < 0.01–0.8) | 7 (0.7; 0.3–1.5) |
CRF06_cpx | 2 (0.2; < 0.01–0.8) | 1 (0.1; < 0.01–0.6) | 3 (0.3; 0.1–0.9) |
CRF20_BG | 1 (0.1; < 0.01–0.6) | 0 | 1 (0.1; < 0.01–0.6) |
CRF24_BG | 7 (0.7; 0.3–1.5) | 0 | 7 (0.7; 0.3–1.5) |
CRF63_02A6 | 20 (2.0; 1.3–3.1) | 6 (0.6; 0.3–1.4) | 26 (2.7; 1.8–3.9) |
Assessment of prevalence of DR to antiretroviral drugs and resistance mutations
We analyzed 982 nucleotide sequences for HIV-1 DR to NNRTI, NRTI, and PI class antiretroviral drugs and 367 nucleotide sequences for HIV-1 DR to PI class drugs.
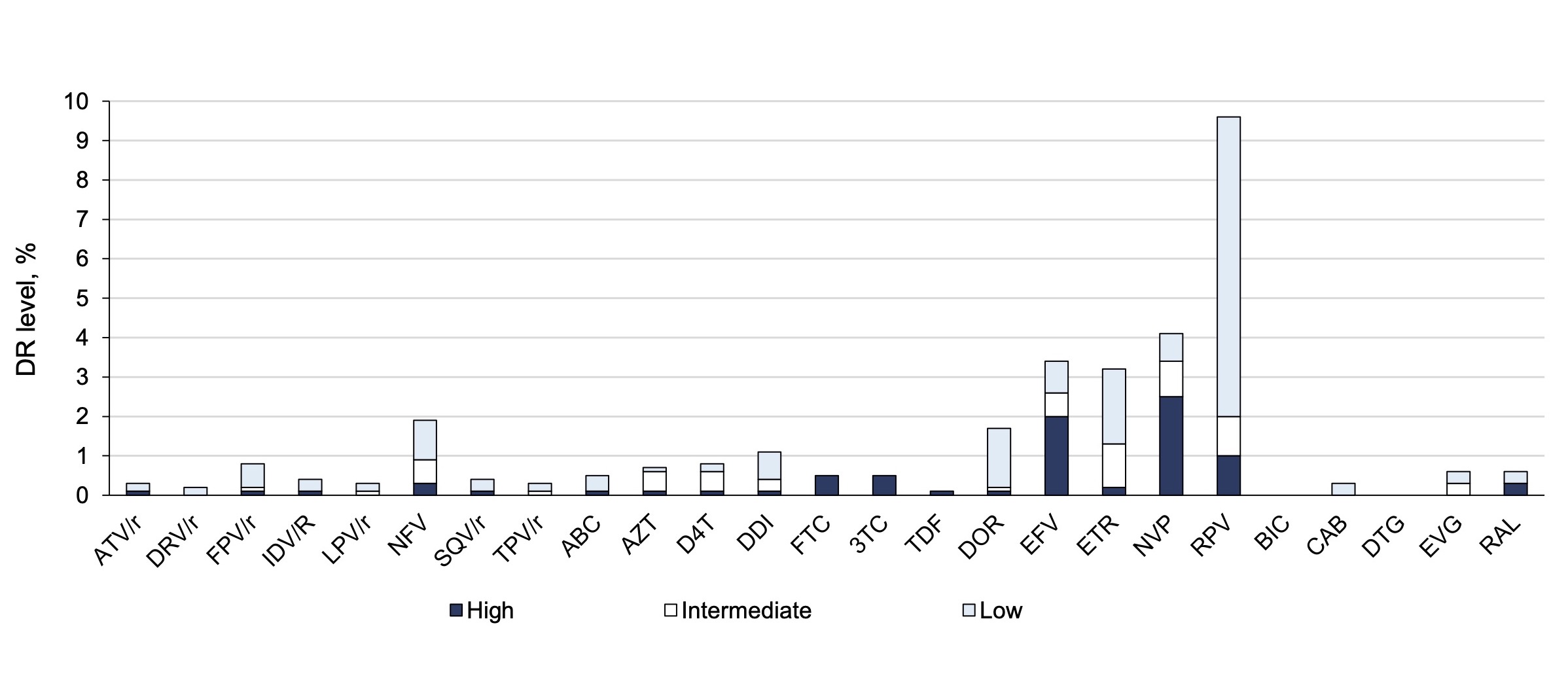
The overall prevalence of HIV DR to all drug classes was 13.8% (95% CI 11.8–16.2%). DR to individual antiretroviral drug classes occurred at a frequency of 2.0% (95% CI 1.3–3.1%) to PIs, 1.4% (95% CI 0.8–2.4%) to NRTIs, 11.2% (95% CI 9.4–13.3%) to NNRTIs, and 0.5% (95% CI 0.02–2.1%) to INIs. Details of the prevalence of DR to each drug separately are presented in Figure 1.
Fig. 1. Frequency of occurrence and level of resistance to antiretroviral drugs.
HIV DR was most frequently registered for the drug rilpivirine of the NNRTI class — in 9.7% of cases, but in 7.6% of cases it was low-level resistance. To other drugs of this class, nevirapine and efavirenz, DR was detected in 4.2 and 3.5% of cases, respectively, and it was mostly high-level resistance. Resistance to antiretroviral drugs of NRTI, PI and INI classes individually did not exceed 2%.
HIV-1 surveillance drug resistance mutations (SDRM) detected in more than one patient are presented in Table 2.
Table 2. Surveillance HIV-1 drug resistance mutations HIV-1 identified more than in one patient
Surveillance DR mutations | Patients count | Prevalence mutations, % (95% CI) |
Mutations NNRTI | ||
K101E | 13 | 1.3 (0.8–2.3) |
K103N | 12 | 1.2 (0.7–2.2) |
Y181C | 7 | 0.7 (0.3–1.5) |
G190A | 8 | 0.8 (0.4–1.6) |
Mutations NRTI | ||
T69D | 3 | 0.3 (0.06–0.9) |
M184V | 4 | 0.4 (0.1–1.1) |
L210W | 5 | 0.5 (0.2–1.2) |
T215D | 4 | 0.4 (0.1–1.1) |
Mutations PI | ||
D30N | 2 | 0.2 (< 0.001–0.8) |
M46I | 6 | 0.6 (0.2–1.4) |
Surveillance mutations associated with DR to PI class antiretroviral drugs were detected in only two cases — in one patient E92G and in the second patient Y143H.
The overall prevalence of HIV-1 surveillance DR mutations was 1.4% (95% CI 0.8–2.4%) to PI class antiretroviral drugs, 1.5% (95% CI 0.9–2.5%) to NNRTI class, 3.1% (95% CI 2.1–4.3%) to NNRTI class, and 0.6% (95% CI 0.02–2.1%) to INI class.
Dependence of resistance prevalence on the analyzed indicators of the study sample
In this study, we researched the dynamics of the prevalence of DR to antiretroviral drugs of different classes depending on the year of the first positive immunoblot test. To obtain comparable cohort sizes, groups of patients with detected HIV infection were formed: up to and including 2017 (146 people), in 2018 (241 people), in 2019 (332 people), in 2020 (81 people) and in 2021 (182 people). The results of the analysis are presented in Figure 2. The dynamics of the overall prevalence of HIV-1 DR to antiretroviral drugs was largely driven by DR to NNRTI class antiretroviral drugs. During the study period, a slight upward trend of DR in the country was noted. It should also be noted that since 2020, a significant proportion of patients started to receive dolutegravir in the first-line therapy regimen of antiretroviral drugs from the INI group, and the share of such patients increased from 30% to 80% by the end of 2021 [8, 11]. This is possibly associated with the emergence of isolated cases of virus resistance to antiretroviral drugs of this class in 2019–2020.
The association between the prevalence of HIV-1 DR to antiretroviral drugs and genetic variants of the virus was analyzed. Among variants occurring in more than 1% of cases, subtype B viruses were the most frequently resistant, showing DR in 36.2% of cases (in 21 patients out of 58). DR prevalence above average was also reported in patients infected with CRF63_02A6, 15.4% (in 4 patients out of 26). DR detection rates below average were found in patients with sub-subtype A6, 11.5% (in 98 patients out of 854) and CRF02_AG, 9.1% (in 3 patients out of 22).
The prevalence of HIV-1 DR to antiretroviral drugs was recorded almost one and a half times more frequently in men than in women, 15.4% versus 10.5% (p = 0.041). Analysis of the prevalence of resistance according to the perceived risk group showed that resistant variants were found most frequently among men who have sex with men (27.6%, 27 out of 98), followed by injection drug users (17.9%, 12 out of 67). Drug-resistant viruses were least frequently detected among patients infected during heterosexual contact (11.9%, 97 of 817).
Fig. 2. The dynamics of HIV-1 drug resistance by the year of the first positive immune blot.
At the final stage, the prevalence of HIV-1 DR to antiretroviral drugs in different regions of Armenia was analyzed. All of them were divided into three groups: Yerevan city; regions adjacent to Yerevan; regions distant from Yerevan. The results are presented in Table 3.
Table 3. Prevalence of HIV-1 DR in the regions of Armenia
Region | Patients count | Patients with drug resistance count | Prevalence, % |
Yerevan | 278 | 47 | 16.9 |
Nearby regions | |||
Aragatsonion | 34 | 4 | 11.8 |
Armavir | 79 | 12 | 15.2 |
Ararat | 94 | 11 | 11.7 |
Kotay | 77 | 9 | 11.7 |
Remote regions | |||
Vayodzor | 18 | 1 | 5.6 |
Gegharkunik | 96 | 10 | 10.4 |
Lori | 91 | 11 | 12.1 |
Syunik | 68 | 12 | 17.6 |
Tavush | 30 | 6 | 20.0 |
Shirak | 117 | 13 | 11.1 |
In certain regions, such as Syunik and Tavush, the prevalence of HIV-1 DR was the highest. However, on average, the prevalencee rates in nearby and remote regions were lower than in the capital city, being 12.7% and 12.6%, respectively.
Discussion
This study is a national epidemiologic investigation of HIV-1 DR in the Republic of Armenia using a large sample of naive patients. The overall prevalence of DR to antiretroviral drugs was 13.8% and was slightly higher than described in other studies. Research groups that have previously studied HIV resistance in patients with no prior experience of taking ART have shown prevalence ranging from 5.5% to 9.2% [9–11]. However, it should be taken into account that in these studies, DR was not assessed to all antiretroviral drugs. In particular, according to previous studies, resistance to antiretroviral drugs of the NNRTI group ranged from 4.4% to 6.0%; in our study, this indicator increased to 11.2% [9, 11]. This significant difference was obtained due to the fact that the HIV-1 DR to rilpivirine was taken into account in our study. In the previous study [11], the resistance rates to EFV and NVP were 6% and 6.8%, while in the current study they were 3.5% and 4.2%, respectively [9, 11].
Antiretroviral drugs of the NNRTI group have a low genetic barrier, and a single point substitution in reverse transcriptase can lead to the development of high-level resistance and subsequently to the transmission of resistant variants with alterations into the population. Therefore, mutations that significantly reduce sensitivity to nevirapine and efavirenz continued to persist among patients in this class. As primary resistance testing is not routinely performed in the region, and due to the 2018 WHO guidelines, a combination of 2 NRTIs + 1 INI was started in the first-line therapy regimen. Nevirapine was removed from the list of drugs for HIV-1 treatment in Armenia, and efavirenz was recommended for prescription as part of an alternative regimen.
At the same time, a low prevalence of DR was recorded in relation to NRTI, PI and INI classes. The rate of DR to NRTI drugs decreased significantly from 5.0% to 1.4% [10, 11]. The most frequently detected substitutions in patients were those causing high levels of DR to lamivudine and emtricitabine.
In our study, for the first time in the Republic of Armenia, the efficacy of INI class drugs in those who have not undergone ART was evaluated and resistance mutations were identified that significantly reduced sensitivity to raltegravir and partially to elvitegravir.
The results showed that drug combinations used in 1st line therapy are effective, and this is supported by data on the increase in undetectable viral load in individuals receiving ART from 68% in 2016 to 86% in 202013. However, regimens containing efavirenz and nevirapine should be cautiously prescribed or minimized, as the proportion of circulating drug-resistant variants of the virus to each of the drugs is quite high, at approximately 4%14.
The overall prevalence of mutations important for surveillance did not exceed 5% for any of the antiretroviral drugs classes individually and was highest for the NNRTI class (3.1%).
In our study, associations between the presence of drug resistance and various virus and patient characteristics were analyzed. It was found that the probability of resistance was much higher in case of infection caused by subtype B virus. Drug-resistant variants were registered more often in male patients, from the risk group of men having sexual contacts with men, living in Yerevan city.
The prevalence of HIV-1 variants with high-level resistance increases the risk of primary DR transmission. Therefore, estimation of the prevalence of transmitted DR is an objective necessity not only for successful prescription of therapy, but also for minimizing the risk of transmission of DR variants of the virus.
Conclusion
In our study, the rate of HIV-1 DR to antiretroviral drugs in patients with no history of therapy was 13.8%. However, it was mainly due to resistance to NNRTI class of antiretroviral drugs. These results suggest that the currently recommended antiretroviral drugs of NNRTI, PI and INI classes are likely to be effective, and that viral resistance will have a low negative impact on achievement of the goals of the UNAIDS "95-95-95" strategy in Armenia. The study was carried out on a sample of 982 HIV-infected patients and allowed to assess HIV-1 DR in more than 20% of the country's citizens diagnosed with HIV infection.
1 UNAIDS. Global HIV & AIDS statistics — Fact sheet 2022. URL: https://www.unaids.org/ru/resources/fact-sheet
2 UNAIDS. Global HIV & AIDS statistics — Fact sheet 2019. URL: https://www.unaids.org/ru/resources/fact-sheet
3 Joint United Nations Programme on HIV/AIDS 2021. URL: https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf
4 UNAIDS DATA, 2021. URL: https://www.unaids.org/en/resources/documents/2021/2021_unaids_data
5 Monitoring for procurement of drugs for HIV and HCV treatment; development of solutions to optimize the situation in order to promote uninterrupted access to drugs in the Republic of Armenia, 2020. URL: https://itpc-eeca.org/wp-content/uploads/2020/11/armenia_monitoring_final_05.11.2020.pdf
6 World Health Organization. Global action plan on HIV drug resistance 2017–2021: 2018 progress report, July 2018: executive summary. URL: https://apps.who.int/iris/handle/10665/273049
7 Stanford University. REGA HIV-1 Subtyping Tool — Version 3.0. URL: http://dbpartners.stanford.edu:8080/RegaSubtyping/stanford-hiv/typingtool/
8 Stanford University. HIV Drug Resistance Database. URL: https://hivdb.stanford.edu/
9 HIV databases. URL: https://www.hiv.lanl.gov/content/index
10 URL: https://bioedit.software.informer.com
11 URL: https://sequenceqc.bccfe.ca/who_qc
12 URL: https://hivdb.stanford.edu/cpr
13 European Centre for Disease Prevention and Control Continuum of HIV care. Monitoring Implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2018 Progress Report. Stockholm: ECDC. 2018. URL: https://www.ecdc.europa.eu/en/publications-data/continuum-hiv-care-monitoring-implementation-dublin-declaration-2018-progress (Accessed: 05.08.2022); European Centre for Disease Prevention and Control Continuum of HIV Care. Monitoring Implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia: 2020 Progress Report. Stockholm: ECDC. 2021. URL: https://www.ecdc.europa.eu/en/publications-data/hiv-continuum-care-monitoring-implementation-dublin-declaration (Accessed: 05.08.2022).
14 International Treatment Preparedness Coalition Monitoring the Procurement of Drugs for the Treatment of HIV Infection and HCV. Development of Solutions to Optimize the Situation in Order to Promote Uninterrupted Access to Drugs in the Republic of Armenia, 2018–2019. URL: https://itpc-eeca.org/wp-content/uploads/2019/11/Monitoring-zakupok-preparatov-Armeniya_2018-2019.pdf (Accessed: 05.08.2022)
About the authors
Olga A. Osadchaya
Central Research Institute of Epidemiology
Email: osadchaya96@yandex.ru
ORCID iD: 0000-0003-1543-5579
junior researcher, Laboratory of diagnostics and molecular epidemiology of HIV-infection
Russian Federation, MoscowDmitry E. Kireev
Central Research Institute of Epidemiology
Email: osadchaya96@yandex.ru
ORCID iD: 0000-0002-7896-2379
Cand. Sci. (Biol.), researcher, Laboratory of diagnostics and molecular epidemiology of HIV-infection
Russian Federation, MoscowDaria V. Saleeva
Central Research Institute of Epidemiology
Email: osadchaya96@yandex.ru
ORCID iD: 0000-0002-5870-5594
researcher, Laboratory of diagnostics and molecular epidemiology of HIV-infection
Russian Federation, MoscowAlina A. Kirichenko
Central Research Institute of Epidemiology
Email: osadchaya96@yandex.ru
ORCID iD: 0000-0002-7116-0138
researcher, Laboratory of diagnostics and molecular epidemiology of HIV-infection
Russian Federation, MoscowIlya A. Lapovok
Central Research Institute of Epidemiology
Email: osadchaya96@yandex.ru
ORCID iD: 0000-0002-6328-1415
Cand. Sci. (Biol.), senior researcher, Laboratory of diagnostics and molecular epidemiology of HIV-infection
Russian Federation, MoscowAlexey E. Lopatukhin
Central Research Institute of Epidemiology
Email: osadchaya96@yandex.ru
ORCID iD: 0000-0002-2826-699X
researcher, Laboratory of diagnostics and molecular epidemiology of HIV-infection
Russian Federation, MoscowAnastasia V. Shlykova
Central Research Institute of Epidemiology
Email: osadchaya96@yandex.ru
ORCID iD: 0000-0002-1390-8021
researcher, Laboratory of diagnostics and molecular epidemiology of HIV-infection
Russian Federation, MoscowLeila F. Makhmudova
Central Research Institute of Epidemiology
Email: osadchaya96@yandex.ru
ORCID iD: 0009-0002-8168-9497
laboratory assistant researcher, Laboratory of diagnostics and molecular epidemiology of HIV-infection
Russian Federation, MoscowNatalia N. Ladnaya
Central Research Institute of Epidemiology
Email: osadchaya96@yandex.ru
ORCID iD: 0000-0003-2994-151X
Cand. Sci. (Biol.), senior researcher, Federal Scientific and Methodological Center for the Prevention and Control of AIDS
Russian Federation, MoscowErmis M. Hovakimyan
National Center for Infectious Diseases
Email: osadchaya96@yandex.ru
Head, Clinical department
Armenia, YerevanSiranush V. Martoyan
National Center for Infectious Diseases
Email: osadchaya96@yandex.ru
ORCID iD: 0000-0002-2067-9503
epidemiologist
Armenia, YerevanHovsep K. Kazaryan
National Center for Infectious Diseases
Email: osadchaya96@yandex.ru
ORCID iD: 0000-0001-5612-8933
Cand. Sci. (Biol.), scientific researcher
Armenia, YerevanTamara V. Hovsepyan
National Center for Infectious Diseases
Author for correspondence.
Email: osadchaya96@yandex.ru
Head, Diagnostic laboratory
Armenia, YerevanNarina K. Sargsiants
National Center for Infectious Diseases
Email: osadchaya96@yandex.ru
Cand. Sci. (Med.), deputy director
Armenia, YerevanVadim V. Pokrovsky
Central Research Institute of Epidemiology
Email: osadchaya96@yandex.ru
ORCID iD: 0000-0002-9514-7288
D. Sci. (Med.), Professor, Academician of the Russian Academy of Sciences, Head, Specialized research department for the prevention and control of AIDS
Russian Federation, MoscowReferences
- Saadat V.M. HIV risks, testing, and treatment in the former Soviet Union: challenges and future directions in research and methodology. Cent. Asian J. Glob. Health. 2015;4(2):225. doi: https://doi.org/10.5195/cajgh.2015.225
- Kelly S.L., Shattock A.J., Kerr C.C., et al. Optimizing HIV/AIDS resources in Armenia increasing ART investment and examining HIV programmes for seasonal migrant labourers. J. Int. AIDS Soc. 2016;19(1):20772. doi: https://doi.org/10.7448/ias.19.1.20772
- National Center for AIDS Prevention. Biological and Behavioral Surveillance Survey on Armenia, Male, Seasonal Labor Migrants in Urban Communities in Armenia; 2018.
- Mallitt K.A., Grigoryan S.R., Papoyan A.S., et al. Access to antiretroviral therapy and survival in Eastern Europe and Central Asia: a case study in Armenia. J. Int. AIDS Soc. 2014;17(1):18795. DOI: https://doi.org/10.7448/ias.17.1.18795
- Vella S., Schwartländer B., Sow S.P., et al. The history of antiretroviral therapy and of its implementation in resource-limited areas of the world. AIDS. 2012;26(10):1231–41. doi: https://doi.org/10.1097/qad.0b013e32835521a3
- Seble G.K., Geoffrey B., Jagadish C.S., et al. HIV-1 transmitted drug resistance surveillance shifting trends in study and prevalence estimates. J. Int. AIDS Soc. 2020;23(9):e25611. DOI: https://doi.org/10.1002/jia2.25611
- WHO. HIV Drug Resistance Report 2017. Geneva; 2017.
- Laga V., Vasilyev A., Lapovok I., et al. HIV type 1 subtype A1 dominates in Armenia. Curr. HIV Res. 2015;13(3):219–25. DOI: https://doi.org/10.2174/1570162x13666150407142834
- Осадчая О.А., Ерошкин П.В., Кириченко А.А. и др. Изучение передачи лекарственной устойчивости ВИЧ-1 в Республике Армения с помощью биоинформатических методов. Эпидемиология и инфекционные болезни. Актуальные вопросы. 2021;11(4):53–60. Osadchaya O.A., Eroshkin P.V., Kirichenko A.A., et al. Studying the transmission of HIV-1 drug resistance in the Republic of Armenia using bioinformatics methods. Epidemiology and Infectious Diseases. Current Items. 2021;11(4):53–60. doi: https://doi.org/10.18565/epidem.2021.11.4.53-60 EDN: https://elibrary.ru/zylroa
- Kirichenko A., Kireev D., Lopatukhin A., et al. Prevalence of HIV-1 drug resistance in Eastern European and Central Asian countries. PLoS One. 2022;17(1):e0257731. doi: https://doi.org/10.1371/journal.pone.0257731
- Kirichenko A., Kireev D., Lapovok I., et al. Prevalence of pretreatment HIV-1 drug resistance in Armenia in 2017–2018 and 2020–2021 following a WHO survey. Viruses. 2022;14(11):2320. DOI: https://doi.org/10.3390/v14112320