Evaluation of symbiotic relationships of oral microorganisms and their effect on the development of inflammatory changes of the oral mucosa in the complete absence of teeth
- Authors: Shulyatnikova O.A.1, Yakovlev M.V.1, Godovalov A.P.1
-
Affiliations:
- E.A. Vagner Perm State Medical University
- Issue: Vol 101, No 4 (2024)
- Pages: 512-519
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18571
- DOI: https://doi.org/10.36233/0372-9311-524
- EDN: https://elibrary.ru/ioeegb
- ID: 18571
Cite item
Abstract
Introduction. By fixing on the exposed surfaces of complete removable dentures and oral soft tissues, bacteria form a biofilm, thereby increasing their overall virulence and resistance. The microorganisms that make up the biofilm are often in a symbiotic relationship, which allows them to increase their pathogenic potential and cause the development of denture stomatitis. Accordingly, when a particular strain is present in the oral cavity, the risks of symbiosis are significantly increased.
The aim of the study was to evaluate the effect of symbiotic relationships of oral bacteria on the development of inflammatory changes in the oral cavity in the absence of teeth.
Materials and methods. Two groups of patients belonging to the elderly age according to WHO systematization (60–74 years) with complete absence of teeth (K08.1) were formed, differing in the presence of clinical manifestations of inflammation (82 men and 49 women). Biological material sampled from the oral cavity of patients was studied using the culture method and RT-PCR. To quantify the interaction between members of the microbiocenosis, we used the Jaccard similarity coefficient.
Results. Coagulase-negative and coagulase-positive staphylococci, Neisseria, Candida spp., Enterobacterales and F. nucleatum were more frequently found in patients with complete absence of teeth. Expressed symbiotic relations between microorganisms of the Enterobacterales order, Lactobacillus, Neisseria and Corynebacterium genera, as well as S. salivarius, C. albicans, F. nucleatum were established. The nature of these relations depended on the presence of inflammatory changes in the oral mucosa and, in turn, influenced the development of the latter. Thus, in the absence of inflammation, Corynebacterium, Lactobacillus and S. salivarius showed stable synergism. In case of inflammation, the association between these bacteria was accompanied by the introduction of F. nucleatum and displacement of S. salivarius.
Conclusion. Thus, conditionally pathogenic microorganisms, forming microbial associations with multidirectional symbiotic relations increase their virulence, which allows them to occupy free niches in the oral cavity and subsequently trigger the development of pathological process of inflammatory character of prosthetic bed tissues.
Full Text
Introduction
A comprehensive approach to dental treatment requires a detailed diagnosis of pathologies of the dento-mandibular system. Treatment of patients with complete absence of teeth in most clinical cases, in addition to the main dental disease, is accompanied by several concomitant pathologies that are associated with acute, subacute or chronic inflammatory process in the soft tissues of the denture bed [1]. The development of denture stomatitis is caused by both general and local etiological factors, often contributing to the rapid chronicization of the pathological process. Among such factors, the representatives of the oral microbiome play a decisive role.
Currently, various authors consider the oral cavity as a functionally and morphologically limited ecosystem, the main part of which are microorganisms (MO) [2, 3]. At the same time, direct contact with the external environment creates conditions for the establishment of an extensive range of transient microbes, many of which are fixed on open tissues and subsequently populate the oral cavity, becoming part of the permanent microbiota of the biotope [4, 5]. It should be noted that the differences in microanatomy, humidity, mobility and aeration of individual structures of the oral cavity, as well as the presence of dental structures and fillings in it contribute to the emergence of comfortable niches for the attachment and reproduction of opportunistic pathogenic microbes with both anaerobic and aerobic type of metabolism [6]. The latter, in turn, possess an extensive spectrum of pathogenicity factors, one of which is adhesive ability. Fixing on open surfaces of hard and soft tissues of the oral cavity, as well as artificial solid media, bacteria form a biofilm through cooperation and complex interaction, due to which their general virulence and resistance increase [7]. This spatial and structural association of individual strains of MO in the extracellular polysaccharide matrix is the main factor in the occurrence of the overwhelming spectrum of pathological processes of inflammatory character observed in the oral cavity [8, 9]. The latter, in turn, are often induced by exo- and endotoxins released by microbial cells and representing activators of mediated action on the macroorganism. The danger of such a bacterial ecosystem lies not only in resistance to most antibacterial drugs, but also in resistance to the factors of cellular and humoral immunity of the macroorganism, which is especially relevant for elderly people [10].
In the mid-1970s, the plaque-specific theory was formulated from the standpoint of clinical microbiology, which adheres to the concept of monoethiology of infectious and inflammatory diseases [11]. According to this theory, the development of the inflammatory process should be associated with the presence or relative predominance either in the biofilm composition or in the planktonic state of one etiologically significant MO species. However, due to the high contamination of the oral cavity and the presence of “comfortable” conditions for the formation of bacterial films, the doctrines described above somewhat lose their relevance.
It has been confirmed [12, 13] not only the importance of the bacterial composition of biofilms formed in the oral cavity on the surface of hard tissues of teeth and elements of dental structures in the development of inflammatory pathologies of the oral mucosa (OM), but also the amount of dental plaque and the time of its stay in direct contact with the soft tissues of the biotope in question.
Due to the formation of a close spatial-structural association, the bacteria included in the biofilm are often in symbiotic relations, which allows them to increase their pathogenic potential. At the same time, the introduction of a particular type of MO into such a symbiosis can both significantly change the orientation of symbiotic relations and influence the manifestations of the clinical picture. It is of interest to study the nature of interaction of oral cavity MOs in the presence and absence of inflammatory process.
The aim of the study was to evaluate the effect of symbiotic relations of oral cavity MOs on the development of inflammatory changes in the oral cavity in the absence of teeth.
Materials and methods
The basis for the formation of patient groups for the study was the assessment of the state of denture bed tissues in the complete absence of teeth. The study included individuals who, according to the World Health Organization systematization, belonged to the elderly age group (60-74 years old; Table 1).
Table 1. Study group composition
Participants | Group 1 | Group 2 | ||
n | age, years | n | age, years | |
Males | 40 | 64.3 ± 1.2 | 42 | 66.1 ± 1.1 |
Females | 26 | 65.7 ± 1.4 | 23 | 64.8 ± 1.5 |
In order to determine the changes in the interspecies relationships of the oral cavity MO in the presence/absence of inflammatory changes in the denture bed tissues, the patients were divided into two groups. The first group (n = 66) included patients who had been using previously fabricated complete removable plate prostheses made of acrylic plastic Etacryl-02 for at least 6 months before the examination, who had passed the adaptation period and had no clinical signs of inflammatory phenomena in the oral cavity and periodontal tissues. The second group (n = 65) included persons who had been using complete removable plate prostheses made of acrylic plastic Etacryl-02 for at least 6 months before the study, who had passed the adaptation period and whose objective clinical examination revealed signs of inflammation of soft tissues of the prosthetic bed (chronic prosthetic stomatitis), the bacterial etiology of which was confirmed by microbiological analysis.
The clinical and experimental studies were approved at the meeting of the local ethical committee of the E.A. Vagner Perm State Medical University (protocol No. 9 from 30.09.2021).
The material for the study was obtained from the OM of the denture bed in the area of the apex of the alveolar process of the maxilla (projection of the 1st and 2nd premolars on the maxilla - taking into account the outlet of the duct of the parotid salivary gland) using a swab-probe and Amies transport medium. After preliminary dilution of the material, the contents were sown on blood agar, Endo and Sabouraud media, selective media for streptococci isolation. Incubation was carried out at 37°C in a humid atmosphere under microaerophilic conditions. The isolated strains were identified by culture, tinctorial and biochemical characteristics.
DNA of periodontal pathogens was detected and quantified in biological material using the Dentoscreen reagent kit (Litech Co. Ltd.) by real-time polymerase chain reaction with hybridization-fluorescence detection.
To quantify the interaction between members of the microbiocenosis, the Jaccard similarity coefficient (q) was used, calculated by the following formula:
q = c/(a + b – c) × 100,
where a — number of observations with type a; b — number of observations with type b; c — number of observations containing both types of MO.
If q ≤ 30% — conditions in the biotope are antagonistic, with q from 30 to 70% bacteria are capable of coexistence, and their ecological commonality is great (synergism), q ≥ 70% — only joint existence of bacteria is possible (a state close to mutualism).
Statistical analysis of the data was performed using four-field conjugation tables and χ2-criterion.
Results
In patients with the complete absence of teeth, the microbial associations were characterized by a high diversity of MO species and complex relationships between them. Thus, in patients with complete absence of teeth, a significant proportion of coagulase-negative and coagulase-positive Staphylococcus spp. other than S. aureus, as well as Neisseria spp., yeast fungi of the Candida genus, Enterobacterales, and Fusobacterium nucleatum were found in the microbiota (Table 2). Among the representatives of the Enterobacterales order, representatives of the Klebsiella spp. and Enterobacter spp. genera were often found. Among the commensal species Streptococcus spp. strains with a wider set of pathogenicity factors, S. pyogenes were dominant.
Table 2. Frequency of MO detection in the oral mucosa in the denture bed of patients (% of cases)
МО | Group 1 (n = 66) | Group 2 (n = 65) | p between groups | |
Staphylococcus spp. | 95.5 | 98.5 | 0.32 | |
Coagulase-positive staphylococci | 33.3 | 76.9 | 0.19 | |
including: | S. aureus | 45.5 | 66.0 | 0.001 |
S. intermedius | 4.5 | 32.0 | 0.001 | |
S. hyicus | 50.0 | 2.0 | 0.003 | |
Coagulase-negative staphylococci | 98.9 | 75.4 | 0.001 | |
Streptococcus spp. | 83.3 | 50.8 | 0.001 | |
S. salivarius | 33.3 | 6.2 | 0.001 | |
S. pyogenes | 15.2 | 52.3 | 0.001 | |
Neisseria spp. | 48.5 | 53.8 | 0.54 | |
Candida spp. | 46.9 | 78.5 | 0.001 | |
including: | C. albicans | 48.4 | 39.2 | 0.3 |
Enterobacterales | 66.7 | 50.8 | 0.065 | |
including: | E. coli | 11.4 | 45.5 | 0.027 |
Klebsiella spp. | 25.0 | 51.5 | 0.19 | |
Enterobacter spp. | 47.7 | 48.5 | 0.36 | |
Lactobacillus spp. | 33.3 | 55.4 | 0.012 | |
Corynebacterium spp. | 34.8 | 49.2 | 0.096 | |
Enterococcus spp. | 18.2 | 26.2 | 0.27 | |
F. nucleatum | 28.8 | 76.9 | 0.001 | |
T. denticola | 0 | 0 | ||
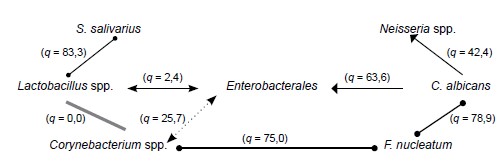
S. salivarius | Neisseria spp. | |||
(q = 83,3) | (q = 42,4) | |||
Lactobacillus spp. | (q = 2,4) | Enterobacterales | (q = 63,6) | C. albicans |
(q = 0,0) | (q = 25,7) | (q = 78,9) | ||
Corynebacterium spp. | (q = 75,0) | F. nucleatum | ||
According to the conducted research, the development of inflammatory complications of OM in the denture bed of patients with complete absence of teeth corresponded to colonization of the biotope by coagulase-positive species of Staphylococcus genus, increase in the share of S. pyogenes and decrease — of S. salivarius, increase in the occurrence of yeast fungi of Candida genus and Escherichia coli. Furthermore, it was found that more frequent detection of F. nucleatum markers was associated with the presence of denture stomatitis.
In the present study, no MO strains were isolated as a monovariant, and the minimum number of associates was at least 3. At the same time, no significant difference in the number of associates in microsymbioses among the compared groups was found. When evaluating pairwise relationships by Jaccard's coefficient, pronounced symbiotic relationships were found between the MOs of the Enterobacterales order, Lactobacillus, Neisseria and Corynebacterium genera, as well as S. salivarius, C. albicans, F. nucleatum. The nature of these relationships depended on the presence of inflammatory changes in the oral cavity. Among coagulase-negative staphylococci inhabiting the oral cavity of group 2 patients, it was noted that S. epidermidis showed the ability to coexist with S. pyogenes (q = 50), and S. schleiferi – with bacteria of the genus Lactobacillus (q = 50). In group 1, coagulase-negative staphylococci showed antagonistic properties against E. coli, S. mitis and Neisseria spp.
Corynebacterium spp. and Lactobacillus spp. with active participation of S. salivarius (Fig. 1) are characteristic symbionts for the oral cavity mucosa of group 1 patients, which are found in the vast majority of cases, and mutualistic relationships are formed between them and S. salivarius (q > 70). The formation of such an association allows participants to exert an antagonistic effect on representatives of the order Enterobacterales, the most common in dry mucosa of the oral cavity [14]. A negative point in group 1 patients should be recognized as a persistent ecological commonality between Corynebacterium spp. and Fusobacterium spp. (q = 75), which may be due to the syntrophy of these bacteria. Thus, Corynebacterium spp. synthesizes several free fatty acids essential for Fusobacterium spp. which, in turn, facilitates the availability of Corynebacterium amino acids [15]. The associations of these species, according to some authors, are most frequently registered in oral squamous cell cancer [16].
Fig. 1. The nature of the symbiotic relationship of the oral MOs of group 1 patients.
Yeast fungi of the Candida genus were found in 46.9% of cases in group 1 patients, which showed synergism with Neisseria spp. (q = 42.4) and Enterobacterales (q = 63.6). Neisseria spp. probably adapted to coexist with Candida spp. in this community because fungi are unique biochemical transformers, and the products of their metabolism are convenient for utilization by Neisseria spp. [17]. The synergism between fungi of the Candida and Enterobacterales is due more to increased antimicrobial resistance [18].
S. salivarius strains isolated from group 1 patients were found to exhibit pronounced (up to mutualistic) symbiotic relationships with lactic acid-producing Lactobacillus. However, as shown in the study [19], Streptococcus spp. bacteriocins can inhibit the production of this metabolite by Lactobacillus spp.
S. salivarius, which exhibited pronounced (up to mutualistic) symbiotic relationships with Lactobacillus spp. in group 1, do not participate in the formation of a persistent association of MO in the complete absence of teeth and accession of inflammation (Fig. 2). This situation leads to the formation of synergism not only between Corynebacterium spp. and Fusobacterium spp. (q = 60.8), but also between Lactobacillus spp. and Fusobacterium spp. (q = 62.3), indicating a closer incorporation of Fusobacterium spp. into the oral microbiocenosis and requiring a comprehensive targeting approach to oral antibacterial treatment before prosthodontics.
Fig. 2. Nature of symbiotic relationship of oral MOs of group 2 patients.
Synergism between Enterobacterales, fungi of the Candida genus and Neisseria spp. (q = 30–70) in group 2 patients persists and leads to the displacement of S salivarius. Furthermore, F. nucleatum forms more divergent relationships than strains of the same species isolated from group 1 patients. Thus, if in group 1 patients the neutrality between Fusobacterium spp. and Neisseria spp. was revealed, in group 2 patients these species show synergism (q = 66.7). The nature of relationships in the pair of Fusobacterium spp. and C. albicans changes in the case of inflammatory changes in the mucosa to antagonistic (q = 18.6), which, at first glance, does not seem logical, but is quite explainable by the fact that in antagonistic relationships between bacteria the spectrum of their metabolite changes, which is reflected in the clinical picture. Antagonistic relations between bacteria of the Enterobacterales order and the Lactobacillus genus are preserved in patients with and without clinical markers of inflammatory process.
Discussion
The oral microbiome is populated by representatives of more than 300 species of bacterial taxa alone. Within such a system, complex relationships are formed between individual members of associations, often not always mutualistic or synergistic. Different techniques have been proposed to study bacterial relationships, which, however, are characterized by complexity of replication and difficulty of interpretation. In the present study, we propose to use an index approach based on the Jaccard coefficient, which allows to reveal not only the directionality of the relationship, but also partly its expression.
Among all oral cavity MOs, S. salivarius, which belongs to the autochthonous symbionts of this biotope, should be emphasized. It has been shown that this species has a pronounced antimicrobial and antibiofilm activity [20], which was confirmed in the present study, when in patients without inflammatory changes in OM this species enhances antagonistic properties at the expense of Lactobacillus spp. and Corynebacterium spp. However, in the case of inflammatory changes, which are probably due to changes in the oral microbiome, an almost complete displacement of this species by Streptococcus spp. was observed.
According to the results of the study, Enterobacterales are allochthonous microbes that retain antagonistic relationships with autochthonous MOs (Lactobacillus spp., Corynebacterium spp.) and synergism with yeast fungi of the Candida genus both in the absence of clinical manifestations of the inflammatory process (prosthetic stomatitis) and in their presence. Such a picture indicates that bacteria with a wide range of pathogenicity factors and, accordingly, more pronounced virulence (e.g., Enterobacterales), which are not characteristic for the oral cavity, by fixation on the structural material of removable dental prostheses occupy free niches, and later, with a decrease in the activity of the immune system, poor hygiene or other provoking factors, it is these taxa that ensure the development of inflammatory processes in the soft tissues of the denture bed, together with autochthonous conditionally pathogenic MOs (Candida spp., etc.). At the same time, a change in the orientation of the relationship between Enterobacterales and F. nucleatum from mutualistic to antagonistic in the presence of inflammatory phenomena of denture bed tissues in patients with complete absence of teeth is likely to correlate with the worsening of the clinical picture when the association of these MOs is detected.
Conclusion
The obtained results allow us to consider S. pyogenes, E. coli, F. nucleatum and Candida spp. as initiators of pathological changes of inflammatory character in soft tissue periodontal tissues of persons using complete removable plate prostheses with a base made of acrylic polymer Etacryl-02. Conditionally pathogenic MOs, forming microbial associations with multidirectional symbiotic relations can increase their virulence, which allows them to occupy free niches in the oral cavity, and subsequently trigger the development of pathological process of inflammatory character of denture bed tissues.
About the authors
Oksana A. Shulyatnikova
E.A. Vagner Perm State Medical University
Email: agodovalov@gmail.com
ORCID iD: 0000-0002-2033-5903
D. Sci. (Med.), Associate Professor, Department of orthopedic dentistry
Russian Federation, PermMikhail V. Yakovlev
E.A. Vagner Perm State Medical University
Email: agodovalov@gmail.com
ORCID iD: 0000-0002-2895-387X
Cand. Sci. (Med.), orthopedic dentist
Russian Federation, PermAnatoly P. Godovalov
E.A. Vagner Perm State Medical University
Author for correspondence.
Email: agodovalov@gmail.com
ORCID iD: 0000-0002-5112-2003
Cand. Sci. (Med.), Associate Professor, Department of microbiology and virology, research assistant, Central Research Laboratory
Russian Federation, PermReferences
- Арутюнов С.Д., Грачев Д.И., Мартыненко А.В. Медико-социальная работа с лицами пожилого и старческого возраста с полной утратой зубов. Проблемы социальной гигиены, здравоохранения и истории медицины. 2021;29(3):509–13. Arutyunov S.D., Grachev D.I., Martynenko A.V. The medical social work with individuals of elderly and senile age with total loss of teeth. Problems of Social Hygiene, Public Health and History of Medicine. 2021;29(3):509–13. DOI: https://doi.org/10.32687/0869-866X-2021-29-3-509-513 EDN: https://elibrary.ru/atnenu
- Романова Ю.М., Гинцбург А.Л. Бактериальные биопленки как естественная форма существования бактерий в окружающей среде и организме хозяина. Журнал микробиологии, эпидемиологии и иммунобиологии. 2011;88(3):99–109. Romanova Yu.M., Gintsburg A.L. Bacterial biofilms as a natural form of existence of bacteria in the environment and host organism. Journal of Microbiology, Epidemiology and Immunobiology. 2011;88(3):99–109. EDN: https://elibrary.ru/rsyplj
- Arweiler N.B., Netuschil L. The oral microbiota. Adv. Exp. Med. Biol. 2016;902:45–60. DOI: https://doi.org/10.1007/978-3-319-31248-4_4
- Вечеркина Ж.В., Шалимова Н.А., Чиркова Н.В. и др. Анализ этиопатогенеза дисбиоза в стоматологии (обзор литературы). Вестник новых медицинских технологий. 2020;27(3):11–9. Vecherkina Zh.V., Shalimova N.A., Chirkova N.V., et al. Analysis of etiopathogenesis of dysbiosis in (literature review). Journal of New Medical Technologies. 2020;27(3):11–9. DOI: https://doi.org/10.24411/1609-2163-2020-16684 EDN: https://elibrary.ru/xrebnl
- Zhang Y., Wang X., Li H., et al. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018;99: 883–93. DOI: https://doi.org/10.1016/j.biopha.2018.01.146
- Царев В.Н., Ипполитов Е.В., Трефилов А.Г. и др. Особенности адгезии анаэробных пародонтопатогенных бактерий и грибов Candida albicans к экспериментальным образцам базисной стоматологической пластмассы в зависимости от шероховатости поверхности и способа полировки. Журнал микробиологии, эпидемиологии и иммунобиологии. 2014;91(6):21–7. Tsarev V.N., Ippolitov E.V., Trefilov A.G. Features of adhesion of anaerobic periodontopathogenic bacteria and Candida albicans fungi to experimental samples of basis dental plastic depending on surface roughness and polishing method. Journal of Microbiology, Epidemiology and Immunobiology. 2014;91(6):21–7. EDN: https://elibrary.ru/tucmgf
- Marsh P.D., Zaura E. Dental biofilm: ecological interactions in health and disease. J. Clin. Periodontol. 2017;44(Suppl. 18):12–22. DOI: https://doi.org/10.1111/jcpe.12679
- Дзампаева Ж.В. Особенности этиологии и патогенеза воспалительных заболеваний пародонта. Кубанский научный медицинский вестник. 2017;24(5):103–10. Dzampaeva Zh.V. Etiology and pathogenesis features of inflammatory periodontal diseases. Kuban Scientific Medical Bulletin. 2017;24(5):103–10. DOI: https://doi.org/10.25207/1608-6228-2017-24-5-103-110 EDN: https://elibrary.ru/zsjalb
- Фукс Е.И., Карева Ю.А., Гализина О.А., Таболина Е.С. Современные аспекты этиологии и патогенеза заболеваний пародонта. Российский медико-биологический вестник имени академика И.П. Павлова. 2013;21(3):153–60. Fuks E.I., Kareva Yu.A., Galizina O.A., Tabolina E.S. Modern aspects of etiology and pathogenesis of diseases of parodont. I.P. Pavlov Russian Medical Biological Herald. 2013;21(3):153–60. EDN: https://elibrary.ru/rkxtmn
- Морозов А.М., Сергеев А.Н., Кадыков В.А. и др. О развитии антибиотикорезистентности в аспекте поликлинической службы. Вестник современной клинической медицины. 2021;14(5):43–50. Morozov A.M., Sergeev A.N., Kadykov V.A., et al. Development of antibiotic resistance in the aspect of outpatient services. The Bulletin of Contemporary Clinical Medicine. 2021;14(5):43–50. DOI: https://doi.org/10.20969/VSKM.2021.14(5).43-50 EDN: https://elibrary.ru/lgswxr
- Варшакидзе К.А., Гулам А., Камчибекова Н.Т., Касымахунов И.Б. Золотистый стафилококк как причина развития заболеваний слизистой оболочки полости рта и влияние антибиотикотерапии. Forcipe. 2020;3(S1):772–3. Varshakidze K.A., Gulam A., Kamchibekova N.T., Kasymakhunov I.B. Staphylococcus aureus as a cause of diseases of the oral mucosa and the effect of antibiotic therapy. Forcipe. 2020;3(S1):772–3. EDN: https://elibrary.ru/jtkehr
- Арутюнов С.Д., Царев В.Н., Ипполитов Е.В. и др. Формирование биопленки на временных зубных протезах: соотношение процессов первичной микробной адгезии, коагрегации и колонизации. Стоматология. 2012;91(5-1):5–10. Arutiunov S.D., Tsarev V.N., Ippolitov E.V., et al. Biofilm formation on temporary dentures: correlation of primary adhesion, coaggregation and colonization. Stomatology. 2012;91(5-1):5–10. EDN: https://elibrary.ru/puafwr
- Афанасьев В.В., Арутюнов С.Д., Деев М.С. и др. Клинико-микробиологические аспекты формирования микробной биопленки на конструкционных материалах, используемых для починки и перебазировки съемных зубных протезов. Российский стоматологический журнал. 2015;19(2):44–6. Afanasyeva V.V., Arutyunov D.S., Deev M.S., et al. Clinical and microbiological aspects of the formation of microbial bio-films on the structural materials used for repair and perebazirovka removable dentures. Russian Journal of Dentistry. 2015;19(2):44–6. EDN: https://elibrary.ru/twjwur
- Leung W.K., Jin L.J., Yam W.C., Samaranayake L.P. Oral colonization of aerobic and facultatively anaerobic gram-negative rods and cocci in irradiated, dentate, xerostomic individuals. Oral Microbiol. Immunol. 2001;16(1):1–9. DOI: https://doi.org/10.1034/j.1399-302x.2001.160101.x.
- Treerat P., Redanz U., Redanz S., et al. Synergism between Corynebacterium and Streptococcus sanguinis reveals new interactions between oral commensals. ISME J. 2020;14(5):1154-1169. DOI: https://doi.org/10.1038/s41396-020-0598-2
- Григорьевская З.В., Терещенко И.В., Казимов А.Э. и др. Микробиота полости рта и ее значение в генезе рака орофарингеальной зоны. Злокачественные опухоли. 2020;10(3S1):54–9. Grigor'evskaya Z.V., Tereshchenko I.V., Kazimov A.E., et al. The microbiota of the oral cavity and its significance in the genesis of cancer of the oropharyngeal zone. Malignant Tumours. 2020;10(3S1):54–9. DOI: https://doi.org/10.18027/2224-5057-2020-10-3s1-54-59 EDN: https://elibrary.ru/zjbyge
- Donati C., Zolfo M., Albanese D., et al. Uncovering oral Neisseria tropism and persistence using metagenomic sequencing. Nat. Microbiol. 2016;1(7):16070. DOI: https://doi.org/10.1038/nmicrobiol.2016.70
- Mishra K., Bukavina L., Ghannoum M. Symbiosis and dysbiosis of the human mycobiome. Front. Microbiol. 2021;12:636131. DOI: https://doi.org/10.3389/fmicb.2021.636131
- Gönczi N.N., Strang O., Bagi Z., et al. Interactions between probiotic and oral pathogenic strains. Biol. Futur. 2021;72(4):461–71. DOI: https://doi.org/10.1007/s42977-021-00091-3
- Stašková A., Sondorová M., Nemcová R., et al. Antimicrobial and antibiofilm activity of the probiotic strain Streptococcus salivarius K12 against oral potential pathogens. Antibiotics (Basel). 2021;10(7):793. DOI: https://doi.org/10.3390/antibiotics10070793