Genotyping of Borrelia, Rickettsia and Anaplasma in Ixodes ricinus and Dermacentor reticulatus ticks in the Kaliningrad region
- Authors: Kartashov M.Y.1,2, Volchev E.G.3, Krivosheina E.I.1, Svirin K.A.1, Ternovoi V.A.1, Loktev V.B.1,2,4
-
Affiliations:
- State Research Center of Virology and Biotechnology "Vector", Rospotrebnadzor
- Novosibirsk National Research State University
- Institute of Living Systems, Immanuel Kant Baltic Federal University
- Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences
- Issue: Vol 101, No 2 (2024)
- Pages: 227-236
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18542
- DOI: https://doi.org/10.36233/0372-9311-503
- EDN: https://elibrary.ru/pebbmx
- ID: 18542
Cite item
Abstract
Background. Tick-borne bacterial and protozoal pathogens pose a significant public health problem. The aim of this study was to detect and genotype Borrelia, Rickettsia and Anaplasma in Ixodes ricinus and Dermacentor reticulatus ticks collected in the Kaliningrad region in 2021–2022.
Materials and methods. The study included 862 I. ricinus and 803 D. reticulatus ticks (1665 in total) collected in 33 biotopes of the Kaliningrad region. Detection of the DNA of tick-borne pathogens was carried out in individual ticks by PCR using a set of specific primers, followed by sequencing and phylogenetic analysis.
Results. The level of infection of I. ricinus ticks with Borrelia was 15.5%, and genotyping by the p66 gene sequence showed the presence of genetic material from four species: B. afzelii, B. garinii, B. valaisiana, and B. lusitaniae. In D. reticulatus ticks, no Borrelia genetic material was detected. The Rickettsia DNA has been found in both tick species. Moreover, the infection rate of I. ricinus ticks was 2.6%, and D. reticulatus — 21.2%. R. helvetica were found in I. ricinus ticks, and R. raoultii in meadow ticks when genotyping by gltA gene. Genetic markers of Anaplasma phagocytophilum have been found in I. ricinus and D. reticulatus ticks. Cases of co-infection of an individual tick have also been identified.
Conclusion. Six different species of tick-borne pathogens were found in the I. ricinus and D. reticulatus ticks collected in the Kaliningrad region and R. helvetica, R. raoultii and A. phagocytophilum were identified for the first time.
Full Text
Introduction
Ticks can be infected with pathogens of viral, bacterial and protozoan nature [1–3]. In addition to fairly well-studied pathogens, which include tick-borne encephalitis virus and ixoid borreliosis pathogens, other microorganisms that cause human diseases may be present in ticks, including in European countries [2, 4–6]. Tick-borne infections are a common group of zooanthroponotic diseases in Russia [7, 8]. The structure and characterization of tick-borne infections, including genotyping of their pathogens, in the European part of Russia is not sufficiently studied [9]. In recent years, cases of human tick-borne infections have been associated with ticks Ixodes persulcatus (Schulze, 1930), I. pavlovskyi (Pomerantzev, 1946), Dermacentor reticulatus (Fabric, 1794), D. marginatus (Sulzer, 1776), and D. nuttali (Olenev, 1928) in Siberian and Far Eastern regions of Russia [8, 10]. In the European parts of Russia, I. ricinus ticks are widely distributed (Linnaeus, 1758), which are prevalent in the western regions of the country. Interestingly, the appearance of D. reticulatus ticks is noted in urban and suburban biotopes [8, 11]. For example, in Tomsk, their numbers increased more than 200 times for urban biotopes, and the infection rate of D. reticulatus ticks was approximately 44–48% for Rickettsia spp., 0.7–0.9% for tick-borne encephalitis virus, and 0.6% for Anaplasma phagocytophilum.
Earlier in I. ricinus ticks located in the Leningrad and Kaliningrad regions, 4 species of Borrelia were presumably detected by PCR at an infection rate of 11.5% [12]. Later, taiga ticks (I. persulcatus) were detected in park areas of St. Petersburg on the Baltic Sea coast with an infection rate of 9.3% with borreliae genotyped as B. afzelii and B. garinii [13]. In Finland, the infection rate of I. ricinus and I. persulcatus infected with various tick-borne pathogens reached 30% with a significant predominance of Borrelia burgdorferi sensu lato [14]. At the same time, I. ricinus ticks were significantly more frequently infected and co-infected with various bacterial and protozoan pathogens.
In the Kaliningrad region in 2022, 5379 people sought medical help due to tick bites1. Only cases of viral tick-borne encephalitis and ixoid tick-borreliosis are diagnosed annually in patients who are traditionally associated with I. ricinus ticks: 3 cases of tick-borne encephalitis and 49 cases of tick-borreliosis were reported in 2022. The circulation of other tick-borne pathogens and their species affiliation have not been described.
The aim of this study was to detect, study species affiliation and genotyping of Borrelia, Rickettsia and Anaplasma detected in ixodid ticks collected in different biotopes of the Kaliningrad region.
Materials and methods
Mites were collected from vegetation using the "per flag" method in different biotopes of the Kaliningrad region in 2021–2022. Geographical coordinates of biotopes and the number of mites collected in biotopes are presented in the Appendix on the journal's website. Species identification of ticks was carried out by morphological method [15].
Nucleic acid isolation
Ticks were treated twice with 70% ethanol to inactivate infectious agents and washed with phosphate- buffered saline. Homogenization of the obtained samples was performed using a laboratory homogenizer TissueLyserLT (Qiagen) in 300 μl of sterile physiological saline. The nucleic acid isolation was performed from 100 µl of homogenate using the AmpliPrime RIBO-prep reagent kit (NextBio) according to the manufacturer's instructions.
PCR testing
Screening of the obtained samples for the presence of genetic markers of the studied pathogens was carried out by PCR using specific primers (Table) on a thermocycler T-1000 (Bio-Rad) in 25 μl of the reaction mixture of the following composition: 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 0.1% Triton X-100, 2 mM MgCl2, 0.2 mM of each dNTP, 0.25 mM of each primer, 1.5 units of HS-Taq polymerase activity (Eurogen) and 1-100 ng of DNA matrix. The following temperature regimes were used for PCR: preliminary activation of polymerase — 95ºC for 5 min; 38 cycles: 95ºC — 20 s, Tanneal — 20 s, 72ºC — 1 min; final elongation at 72ºC — 4 min.
Primers using for isolation gene fragments of Borrelia, Rickettsia and Anaplasma from ixodes ticks
Primer | Primer sequence (5′→3′) | Temperature, ºС | Size, bp | Reference |
Borr2rF | CGAATTAGGCAAAGACGATCC | 56 | 548 | [8] |
Borr2rR | TTTCCATAAGCTCCTGATAAGCCA | |||
CS409d | CCTATGGCTATTATGCTTGC | 56 | 769 | [16] |
RP1258n | ATTGCAAAAAGTACAGTGAACA | |||
MSP2-3f | CCAGCGTTTAGCAAGATAAGAG | 55 | 334 | [17] |
MSP2-3r | GCCCAGTAACAACATCATAAGC |
The amplicons were detected by gel electrophoresis in 2% agarose gel in Tris-acetate buffer containing 0.1% ethidium bromide. Amplification products were purified from agarose gel using a microcolumn-based kit (Biosilica).
Sequencing and nucleotide sequence analysis
Sanger sequencing was performed using the BigDye Terminator v.3.1 Cycle Sequencing Kit (Applied Biosystems). Nucleotide sequences were determined by capillary electrophoresis using a 3130xl Genetic Analyzer automatic sequencer (Applied Biosystems). Analysis of the obtained nucleotide sequences was performed using the UniproUGENE v. 1.46 program. The obtained nucleotide sequences were compared with previously published sequences in GenBank using the BLAST search application. The nucleotide sequences were aligned using the MUSCLE algorithm in the MEGA X software. Phylogenetic analysis of nucleotide sequences was performed by the maximum likelihood method using the Tamura-Nei evolution model to analyze genetic relationships/clustering of nucleotide sequences. The statistical reliability indices of phylogenetic tree nodes were calculated by bootstrap analysis using 1000 random replicates.
Deposit of nucleotide sequences
The following nucleotide sequences were deposited in the GenBank database: fragments of the msp2 gene of A. phagocytophilum (OR488786-OR488799); p66 gene fragments of Borrelia: B. afzelii (OR488840-OR488890), B. garinii (OR488891-OR488929), B. valaisiana (OR488930-OR488948), B. lusitaniae (OR48898949-OR488967); Rickettsiae gltA gene fragments: R. helvetica (OR496610-OR496611) and R. conorii subsp. raoultii, hereafter as the basionym of R. raoultii (OR496612-OR496613).
The studies were conducted in compliance with the biosafety rules regulated in MU 1.3.2569-09, SP 1.3.3118-13, SP 3.1.3310-15.
Results
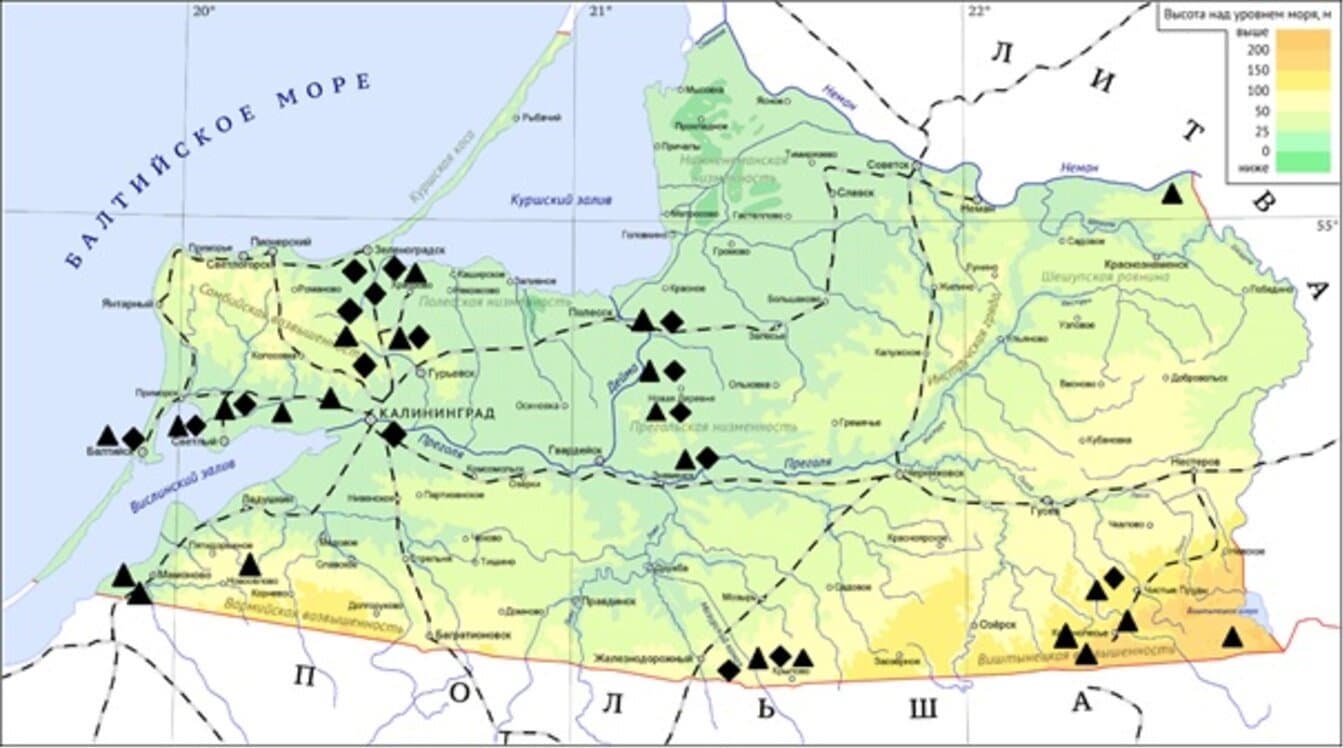
We collected and analyzed 1665 individual samples of nymphs and imago of I. ricinus (n = 862) and D. reticulatus (n = 803) ticks from 33 urban, suburban and characteristic natural biotopes of the Kaliningrad region (Fig. 1). The biotopes studied were subdivided according to the species of ticks collected in them as follows: 11 biotopes in which only I. ricinus ticks, 9 biotopes with D. reticulatus ticks and 13 biotopes with 2 tick species. Quite unusually, in fact, half of all collected mites were attributed to the meadow mite, which was found in 2/3 of the biotopes studied and absolutely prevailed in 9 of them.
Fig. 1. Locations of biotopes in the Kaliningrad region, where ixodid ticks were collected in 2021–2022.
The infection rate of I. ricinus Borrelia infection was 15.5% (128/862; 95% CI 13.2–18.1). Determination of the nucleotide sequence of a fragment of the p66 gene with a length of about 560 bp among 128 samples revealed Borrelia of four species from the B. burgdorferi s.l. complex: in 51 ticks, DNA of B. afzelii (39.9%; 95% CI 31.8–48.5), in 39 — B. garinii (30.5%; 95% CI 23.2–38.5), in 19 — B. valaisiana (14.8%; 95% CI 9.7–22.0), in 19 — B. lusitaniae (14.8%; 95% CI 9.7–22.0) were detected. No Borrelia genetic material was detected among the studied samples of ticks of D. reticulatus species. Phylogenetic analysis showed that Borreliae detected in I. ricinus ticks in the territory of the Kaliningrad region cluster with prototypic sequences isolated primarily in European countries (Fig. 2). Sequence analysis of the fragment of the p66 gene of B. afzelii revealed 6 alleles of this gene, for B. garinii isolates 8 allelic variants differing from each other by 1-14 nucleotide substitutions were detected, and B. valaisiana and B. lusitaniae isolates had 2 and 4 substitutions.
Fig. 2. Dendrogram of nucleotide sequences of the p66 gene fragment for detected borrelia isolates. Black squares — sequences derived from I. ricinus tick.
The rickettsial infection rate in ticks was 11.5% (191/1665; 95% CI 10.2–13.1%). Among I. ricinus ticks, Rickettsia DNA was detected in 22 samples and the infection rate was 2.6% (22/862; 95% CI 1.7–3.8%). All identified Rickettsia isolates from I. ricinus ticks were attributed to R. helvetica by a fragment of the citrate synthase gene (gltA). When analyzing the nucleotide sequences of the gltA gene, two main genetic variants of R. helvetica circulating in the Kaliningrad region were identified. They differ from each other by 2 synonymous nucleotide substitutions (the level of homology between the genetic variants is 99.8%). One of the genetic variants corresponds to the previously described variants of R. helvetica found in the Komi Republic and Omsk region [9, 18], the other genetic variant differs from the known sequences.
In 21.1% of D. reticulatus ticks, Rickettsia DNA was detected (169/803; 95% CI 18.4–24.0%), which was genotyped as R. raoultii. Phylogenetic analysis of R. raoultii revealed the existence of two variants differing by a single synonymous substitution (Fig. 3). In general, these genetic variants correspond to a wide range of R. raoultii isolates circulating in Europe, Russia and China [19, 20].
Fig. 3. Dendrogram of nucleotide sequences of the gltA gene fragment for identified Rickettsia isolates. Black triangles — sequences derived from D. reticulatus tick; black squares — sequences derived from I. ricinus tick.
A. phagocytophilum DNA was detected by PCR in 12 samples of ticks of the species I. ricinus (1.4%; 95% CI 0.8–2.5%) and in 2 samples of D. reticulatus ticks (0.2%; 95% CI 0.1–0.9%). The nucleotide sequences of the msp2 gene fragment of approximately 340 bp in length were determined in the detected A. phagocytophilum isolates, followed by phylogenetic analysis (Fig. 4). Three A. phagocytophilum genetic variants with a homology level of 98.6%, identical or closest to A. phagocytophilum isolates circulating in Norway and Poland, were detected in the Kaliningrad region.
Fig. 4. Dendrogram of nucleotide sequences of the msp2 gene fragment for identified A. phagocytophilum isolates. Black triangles — sequences derived from D. reticulatus ticks; black squares — sequence derived from I. ricinus ticks
Two tick samples contained simultaneously genetic material of B. afzelii and R. helvetica, one tick sample contained DNA of B. valaisiana and A. phagocytophilum.
Discussion
The results of regular long-term field observations show that the main recreational landscapes of the Kaliningrad region, including landscapes of the Baltic Sea coast, have established populations of ixodid ticks. The activity of ixodids in zones with pronounced anthropogenic load is significantly higher than in similar landscapes with insignificant anthropogenic load. Thus, in recent years, 4194–7300 people seek medical help for tick bites. Every year 2-16 cases of viral tick-borne encephalitis and 35-132 cases of ixoid tick-borne borreliosis are diagnosed. This repeatedly increases the risks of human contact with ixodid ticks, which can lead to human infection with various tick-borne pathogens.
Tick-borne borrelioses occupy an important place in the structure of infectious pathology in the Kaliningrad region. In ticks of the Kaliningrad region we detected and genotyped B. afzelii, B. garinii and B. lusitaniae, which are considered pathogenic for humans, and B. valaisiana, the pathogenicity of which is under discussion [21]. The infection rate (15.5%) correlates with earlier studies in the Leningrad and Kaliningrad regions [12]. B. afzelii and B. garinii are the most common pathogens of tick-borne borreliosis in humans and are most often found in I. ricinus ticks. Circulation of B. lusitaniae was shown for the first time in the territory of the Kaliningrad region. It should be noted that B. lusitaniae is mainly distributed in the countries of the Mediterranean region, such as Portugal, Morocco and Tunisia. In more northern latitudes, this pathogen was detected in Austria, Slovakia, Ukraine and Latvia [22].
No genetic markers of borreliosis could be detected in D. reticulatus ticks, although a very representative sample of ticks of this species was examined. Previously, a similar situation was recorded in Tomsk, where 315 ticks of this species collected in urban biotopes were individually examined [11]. In Tomsk urban biotopes, a more than 200-fold increase in the number of D. reticulatus ticks was actually detected during 2015. It was the explosive increase in the abundance of D. reticulatus which allowed to collect a significant number of these ticks in 2016–2017 and assess their role in the transmission of tick-borne infections in Tomsk.
Rickettsiae carried by ixodid ticks are infectious agents capable of causing human disease. In the Kaliningrad region, we managed for the first time to establish the fact of circulation of two species of Rickettsia from the tick-borne spotted fever group: R. helvetica and R. raoultii. The registered level of infection of I. r. raoultii ticks with R. helvetica in I. ricinus ticks amounted to 2.6%. In other countries of the Baltic region it ranges from 5 to 10% [23]. However, ticks of D. reticulatus species were infected with R. raoultii in 21.2% of cases. At the same time, in Lithuania and Latvia the similar indicator reaches 38%, in Germany — 80% [24, 25]. In Russia, the level of infection of D. reticulatus with this Rickettsia species can vary in different regions from 21.9% to 45%. R. helvetica DNA was found in I. persulcatus, I. ricinus, I. pavlovskyi and I. trianguliceps ticks in different regions of northern Eurasia [10, 26, 27]. Human cases have been described, with patients with rickettsiosis caused by R. helvetica showing fever, rare rashes, and cases of perimyocarditis and meningitis described.
R. conorii subsp. raoultii was described as a new Rickettsia species in 2008 after the study of the prototype strain of R. raoultii isolated in 2005 in Kharbarovsk from D. silvarum ticks in Khabarovsk Krai [28]. In subsequent studies, R. raoultii was detected in D. reticulatus, D. marginatus and D. nuttalli ticks in a number of regions of the Asian part of Russia (Omsk region, Republic of Buryatia), Kazakhstan, China, and Mongolia [19, 20]. Rickettsiae genetically similar to R. raoultii have been identified in Haemaphysalis hystricis ticks in Japan and in H. ornithophila, H. shimoga, and H. lagrangei ticks in Thailand, as well as in D. marginatus ticks in Georgia, Turkey and European countries [29].
Serologic methods and DNA detection in the blood of patients confirmed the role of R. raoultii along with R. slovaca as etiologic agents of TIBOLA syndrome, which is associated with Dermacentor spp. genus ticks [30]. Patients develop an asthenic syndrome, and fever (> 38ºC) is observed in a quarter of cases. For most patients, erythema persists for up to 1-2 months. If the tick bite is localized in the scalp, about one third of patients develop persistent baldness at the site of bite healing. At the same time, no cases of human rickettsioses in Kaliningrad region have been described so far.
The presence of A. phagocytophilum genetic material in I. ricinus and D. reticulatus ticks, revealed for the first time in this work, shows the active circulation of this pathogen in the Kaliningrad region. The level of infection of D. reticulatus and I. ricinus ticks (0.2% and 1.4%, respectively) corresponds to similar indicators for such countries as Denmark, Sweden, Norway and Germany, where the pathogen is found in 1–5% of ixodid ticks [23]. Phylogenetic analysis shows simultaneous circulation of at least 3 genetic variants of A. phagocytophilum. Human granulocytic anaplasmosis (HGA) was first described in the Russian Far East in 2000. Later confirmed cases of the disease were reported in Perm and Novosibirsk regions and in Altai. The clinical course of HGA is very polymorphic: from mild, subclinical forms to extremely severe, fatal cases, which account for 0.5–1.0% and are usually associated with the development of secondary infections. The disease is characterized by the appearance of headaches and muscle aches, the development of fever. Less than half of patients may have nausea, vomiting, anorexia, diarrhea, abdominal and joint pain, cough. In most cases, leukopenia, thrombocytopenia, and elevated serum levels of liver aminotransferases and C-reactive protein are noted in HGA patients. HGA disease has not been registered in the Kaliningrad region.
High level of infection of ixodal ticks with Rickettsia and Anaplasma in the Kaliningrad region, presence of constant contacts of population with ticks allows to expect occurrence of cases of infection of people with Rickettsia spp. of tick-borne spotted fever group and Anaplasma spp. Diagnosis of these diseases may be difficult due to the imperfection of their laboratory diagnostics. These infectious agents are not cultured by classical microbiological methods, and the genetic material of the pathogens can be detected in clinical material from patients over a very narrow time range. This actualizes studies on seroepidemiological monitoring of these infections in the population living in the Kaliningrad region to clarify their current distribution.
Conclusion
In the collection of I. ricinus and D. reticulatus ticks, collected in 33 different biotopes in the Kaliningrad region in 2021–2022, DNA of 6 different species of tick-borne pathogens of bacterial and protozoan nature was detected. Sequencing of genome fragments of these pathogens and their phylogenetic analysis allowed to identify and genotype the following species of tick-borne pathogens: B. afzelii, B. garinii, B. valaisiana, B. lusitaniae, R. helvetica, R. raoultii and A. phagocytophilum. R. helvetica, R. raoultii and A. phagocytophilum were detected for the first time in this region in both I. ricinus and D. reticulatus ticks. The obtained data confirm the necessity of continuous monitoring for circulation of pathogens of borreliosis, rickettsioses and anaplasmosis in natural foci of tick-borne infections in Kaliningrad region, further improvement of methods of diagnostics and prevention of these infections, including detection of possible cases of human cases of rickettsioses and HGA.
1 State report “On the state of sanitary and epidemiological changes in the population in the Kaliningrad region in 2022.” Kaliningrad; 2023. 238 p. URL: https://39.rospotrebnadzor.ru/sites/default/files/doklad_o_goskontrole_za_2022_kaliningradskaya.pdf
About the authors
Mikhail Yu. Kartashov
State Research Center of Virology and Biotechnology "Vector", Rospotrebnadzor; Novosibirsk National Research State University
Email: valeryloktev@gmail.com
ORCID iD: 0000-0002-7857-6822
Cand. Sci. (Biol.), senior researcher, Department of molecular virology for flaviviruses and viral hepatitis
Russian Federation, Novosibirsk Region, Koltsovo; NovosibirskEvgenii G. Volchev
Institute of Living Systems, Immanuel Kant Baltic Federal University
Email: valeryloktev@gmail.com
ORCID iD: 0000-0002-7401-3678
postgraduate student, Institute of Living Systems
Russian Federation, KaliningradEkaterina I. Krivosheina
State Research Center of Virology and Biotechnology "Vector", Rospotrebnadzor
Email: valeryloktev@gmail.com
ORCID iD: 0000-0001-5181-0415
junior researcher, Department of molecular virology for flaviviruses and viral hepatitis
Russian Federation, Novosibirsk Region, KoltsovoKirill A. Svirin
State Research Center of Virology and Biotechnology "Vector", Rospotrebnadzor
Email: valeryloktev@gmail.com
ORCID iD: 0000-0001-9083-1649
junior researcher, Department of molecular virology for flaviviruses and viral hepatitis
Russian Federation, Novosibirsk Region, KoltsovoVladimir A. Ternovoi
State Research Center of Virology and Biotechnology "Vector", Rospotrebnadzor
Email: valeryloktev@gmail.com
ORCID iD: 0000-0003-1275-171X
Cand. Sci. (Biol.), leading researcher, Head, Department of molecular virology for flaviviruses and viral hepatitis
Russian Federation, Novosibirsk Region, KoltsovoValery B. Loktev
State Research Center of Virology and Biotechnology "Vector", Rospotrebnadzor; Novosibirsk National Research State University; Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences
Author for correspondence.
Email: valeryloktev@gmail.com
ORCID iD: 0000-0002-0229-321X
D. Sci. (Biol.), Professor, chief researcher, Department of molecular virology for flaviviruses and viral hepatitis
Russian Federation, Novosibirsk Region, Koltsovo; Novosibirsk; NovosibirskReferences
- Charrel R.N., Attoui H., Butenko A.M., et al. Tick-borne virus diseases of human interest in Europe. Clin. Microbiol. Infect. 2004;10(12):1040–55. doi: https://doi.org/10.1111/j.1469-0691.2004.01022.x
- Defaye B., Moutailler S., Pasqualini V., Quilichini Y. A systematic review of the distribution of tick-borne pathogens in wild animals and their ticks in the mediterranean Rim between 2000 and 2021. Microorganisms. 2022;10(9):1858. doi: https://doi.org/10.3390/microorganisms10091858
- Ni X.B., Cui X.M., Liu J.Y., et al. Metavirome of 31 tick species provides a compendium of 1,801 RNA virus genomes. Nat. Microbiol. 2023;8(1):162–73. doi: https://doi.org/10.1038/s41564-022-01275-w
- Kiewra D., Krysmann A. Interactions between hard ticks (Ixodidae) and bacterial tick-borne pathogens. Ann. Parasitol. 2023;69(1):7–16. DOI: https://doi.org/0.17420/ap6901.502
- Hansford K.M., Wheeler B.W., Tschirren B., Medlock J.M. Questing Ixodes ricinus ticks and Borrelia spp. in urban green space across Europe: A review. Zoonoses Public Health. 2022;69(3):153–66. DOI: https://doi.org/10.1111/zph.12913
- Moraga-Fernández A., Muñoz-Hernández C., Sánchez-Sánchez M., et al. Exploring the diversity of tick-borne pathogens: The case of bacteria (Anaplasma, Rickettsia, Coxiella and Borrelia) protozoa (Babesia and Theileria) and viruses (Orthonairovirus, tick-borne encephalitis virus and louping ill virus) in the European continent. Vet. Microbiol. 2023;286:109892. doi: https://doi.org/10.1016/j.vetmic.2023.109892
- Alekseev A.N., Dubinina H.V., Jushkova O.V. First report on the coexistence and compatibility of seven tick-borne pathogens in unfed adult Ixodes Persulcatus Schulze (Acarina:Ixodidae). Int. J. Med. Microbiol. 2004;293(Suppl. 37):104–8. doi: https://doi.org/10.1016/s1433-1128(04)80015-9
- Карташов М.Ю., Кривошеина Е.И., Свирин К.А. и др. Генотипирование возбудителей клещевых инфекций и определение видового состава клещей, нападающих на людей в г. Новосибирске и его пригородах. Инфекция и иммунитет. 2022;12(6):1103–12. Kartashov M.Yu., Krivosheina E.I., Svirin K.A., et al. Genotyping of tick-borne pathogens and determination of human attacking tick species in Novosibirsk and its suburbs. Russian Journal of Infection and Immunity. 2022;12(6):1103–12. doi: https://doi.org/10.15789/2220-7619-GOT-1979
- Kartashov M.Yu., Glushkova L.I., Mikryukova T.P., et al. Detection of Rickettsia helvetica and Candidatus R. tarasevichiae DNA in Ixodes persulcatus ticks collected in Northeastern European Russia (Komi Republic). Ticks Tick Borne Dis. 2017;8(4):588–92. doi: https://doi.org/10.1016/j.ttbdis.2017.04.001
- Rar V., Livanova N., Tkachev S., et al. Detection and genetic characterization of a wide range of infectious agents in Ixodes pavlovskyi ticks in Western Siberia, Russia. Parasit. Vectors. 2017;10(1):258. doi: https://doi.org/10.1186/s13071-017-2186-5
- Карташов М.Ю., Микрюкова Т.П., Кривошеина Е.И., и др. Генотипирование возбудителей клещевых инфекций в клещах Dermacentor reticulatus, собранных в городских биотопах г. Томска. Паразитология. 2019;53(5):355–69. Kartashov M.Yu., Mikryukova T.P., Krivosheina E.I., et al. Genotyping of tick-borne infections in Dermacentor reticulatus ticks collected in urban foci of Tomsk. Parazitologiya. 2019;53(5):355–69. doi: https://doi.org/10.1134/S0031184719050016 EDN: https://elibrary.ru/xodhop
- Alekseev A.N., Dubinina H.V., Van De Pol I., Schouls L.M. Identification of Ehrlichia spp. and Borrelia Burgdorferi in ixodes ticks in the Baltic regions of Russia. J. Clin. Microbiol. 2101;39(6):2237–42. doi: https://doi.org/10.1128/jcm.39.6.2237-2242.2001
- Панферова Ю.А., Ваганова А.Н., Фрейлихман О.А., и др. Распространенность генетических маркеров Borrelia burgdorferi sensu lato у кровососущих клещей в парковых зонах Санкт-Петербурга. Инфекция и иммунитет. 2020;10(1): 175–9. Panferova Yu.A., Vaganova A.N., Freilikhman O.A., et al. Prevalence of Borrelia burgdorferi sensu lato genetic markers in blood-sucking ticks in suburban park zones in Saint Petersburg. Russian Journal of Infection and Immunity. 2020;10(1):175–9. doi: https://doi.org/10.15789/2220-7619-POB-806
- Laaksonen M., Klemola T., Feuth E., et al. Tick-Borne pathogens in Finland: comparison of Ixodes ricinus and I. persulcatus in sympatric and parapatric areas. Parasit. Vectors. 2018;11(1):556. doi: https://doi.org/10.1186/s13071-018-3131-y
- Филиппова Н.А. Иксодовые клещи подсемейства Ixodinae. Ленинград;1977. Filippova N.A. Ixodid Ticks Subfamily Ixodinae. Leningrad;1977.
- Roux V., Rydkina E., Eremeeva M., Raoult D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int. J. Syst. Bacteriol. 1977;47(2):252–61. doi: https://doi.org/10.1099/00207713-47-2-252
- Levin M.L., Ross D.E. Acquisition of different isolates of Anaplasma phagocytophilum by Ixodes scapularis from a model animal. Vector Borne Zoonotic. Dis. 2004;4(1):53–9. doi: https://doi.org/10.1089/153036604773082997
- Igolkina Y.P., Rar V.A., Yakimenko V.V., et al. Genetic variability of Rickettsia spp. in Ixodes persulcatus/Ixodes trianguliceps sympatric areas from Western Siberia, Russia: identification of a new Candidatus Rickettsia species. Infect. Genet. Evol. 2015;34:88–93. doi: https://doi.org/10.1016/j.meegid.2015.07.015
- Speck S., Derschum H., Damdindorj T., et al. Rickettsia raoultii, the predominant Rickettsia found in Mongolian Dermacentor nuttalli. Ticks Tick Borne Dis. 2012;3(4):227–31. doi: https://doi.org/10.1016/j.ttbdis.2012.04.001
- Wen J., Jiao D., Wang J.H., et al. Rickettsia raoultii, the predominant Rickettsia found in Dermacentor silvarum ticks in China–Russia border areas. Exp. Appl. Acarol. 2014; 63(4):579–85. doi: https://doi.org/10.1007/s10493-014-9792-0
- Strle F., Stanek G. Clinical manifestations and diagnosis of Lyme borreliosis. Curr. Probl. Dermatol. 2009;37:51–110. DOI: https://doi.org/10.1159/000213070
- Norte A.C., Boyer P.H., Castillo-Ramirez S., et al. The population structure of Borrelia lusitaniae is reflected by a population division of its Ixodes vector. Microorganisms. 2021;9(5):933. DOI: https://doi.org/10.3390/microorganisms9050933
- Quarsten H., Henningsson A., Krogfelt K.A., et al. Tick-borne diseases under the radar in the North Sea Region. Ticks Tick Borne Dis. 2023;14(4):102185. doi: https://doi.org/10.1016/j.ttbdis.2023.102185
- Arz C., Król N., Imholt C., et al. Spotted fever group rickettsiae in ticks and small mammals from grassland and forest habitats in Central Germany. Pathogens. 2023;12(7):933. doi: https://doi.org/10.3390/pathogens12070933
- Răileanu C., Tauchmann O., Silaghi C. Sympatric occurrence of Ixodes ricinus with Dermacentor reticulatus and Haemaphysalis concinna and the associated tick-borne pathogens near the German Baltic coast. Parasit. Vectors. 2022;15(1):65. doi: https://doi.org/10.1186/s13071-022-05173-2
- Shpynov S., Fournier P.E., Rudakov N., et al. Detection of members of the genera Rickettsia, Anaplasma, and Ehrlichia in ticks collected in the Asiatic part of Russia. Ann. NY Acad. Sci. 2006;1078:378–83. doi: https://doi.org/10.1196/annals.1374.075
- Igolkina Y., Bondarenko E., Rar V., et al. Genetic variability of Rickettsia ssp. in Ixodes persulcatus ticks from continental and island areas of the Russian Far East. Ticks Tick Borne Dis. 2016;7(6):1284–9. doi: https://doi.org/10.1016/j.ttbdis.2016.06.005
- Mediannikov O., Matsumoto K., Samoylenko I., et al. Rickettsia raoultii sp. nov., a spotted fever group rickettsia associated with Dermacentor ticks in Europe and Russia. Int. J. Syst. Evol. Microbiol. 2008;58(Pt. 7):1635–9. doi: https://doi.org/10.1099/ijs.0.64952-0
- Guccione C., Colomba C., Iaria C., Cascio A. Rickettsiales in the WHO European Region: an update from a One Health perspective. Parasit. Vectors. 2023;16(1):41. doi: https://doi.org/10.1186/s13071-022-05646-4
- Oteo J.A., Aránzazu P. Tick-borne rickettsioses in Europe. Ticks Tick Borne Dis. 2012;3(5-6):271–8. doi: https://doi.org/10.1016/j.ttbdis.2012.10.035
Supplementary files