Limitations in creating artificial populations in agent-based epidemic modeling: a systematic review
- Authors: Maslova I.I.1, Manolov A.I.1, Glushchenko O.E.1, Kozlov I.E.1, Tsurkis V.I.1, Popov N.S.1, Samoilov A.E.1, Lukashev A.N.1, Ilina E.N.1
-
Affiliations:
- Research Institute of Systems Biology and Medicine
- Issue: Vol 101, No 4 (2024)
- Pages: 530-545
- Section: REVIEWS
- URL: https://microbiol.crie.ru/jour/article/view/18491
- DOI: https://doi.org/10.36233/0372-9311-467
- EDN: https://elibrary.ru/goxztx
- ID: 18491
Cite item
Abstract
Introduction. The key step in agent-based modeling of epidemics, which allows researchers to take into account individual characteristics of people, is the creation of an artificial population. The main difficulty of this procedure is finding a balance between the detail of the population description and the computational efficiency of the calculations.
The aim and objectives of the review: Critically analyze and summarize the current evidence on how to create artificial populations; evaluate the limitations and advantages of available approaches in solving various problems in epidemiology.
Materials and methods. An analysis of literature sources devoted to agent-based modeling has been performed. The analysis is focused on algorithms for creating an artificial population with a given level of detail for modeling human respiratory infections.
Results. The approaches to the creation of artificial populations are generalized. The main principles of realization of interaction between agents are revealed: by means of networks of contacts between agents and on the basis of taking into account the movement of agents between locations. The first approach is the most computationally efficient and simple; the second approach allows to better take into account the change in the behavior of agents during the development of the epidemic process.
Conclusion. Agent-based modeling is an optimal tool for selecting the best scenario for epidemic control and investigating the role of individual characteristics of people in the development of epidemics. When creating an artificial population, it is important to include in the model factors that can be targeted for control. A significant limitation is the lack of factual data on population structure, but this can be overcome by using indirect data.
Full Text
Introduction
Since the early 2000s, humanity has faced a number of viral epidemics, including Severe Acute Respiratory Syndrome (SARS, 2002-2003), Influenza A(H1N1)-California (swine flu) (2009), Middle East Respiratory Syndrome (MERS, 2012), Ebola outbreaks (2014-2016), Zika fever (2015-2016) and finally the COVID-19 pandemic caused by the novel SARS-CoV-2 coronavirus (2019-present). The COVID-19 pandemic has sparked the interest of epidemiology and public health professionals in using computational tools to predict epidemics and select optimal anti-epidemic measures. These tools include machine learning methods and computational epidemiologic models.
Computer simulations in epidemiology are designed to reproduce the dynamics of infectious disease spread, taking into account population demographics [1–3], contact network structure [4] and information on intervention strategies [5, 6]. These models provide a virtual laboratory to study hypothetical scenarios, evaluate the effectiveness of different interventions, and anticipate outbreak trajectories.
Numerical solution of ordinary differential equations and agent-based modeling (ABM) are the two most common modeling approaches in epidemiology [7, 8]. The first approach includes various compartmental models, such as the susceptible-infected-uninfected model [9] and its modifications; the second approach includes agent-based models, which take into account the heterogeneity of a population by modeling the actions and interactions of individual agents (people) within it [3, 4, 10].
Agent-based models consider each person as an autonomous agent with characteristics that determine his/her behavior and social interactions. The semantic blocks into which any synthetic population can be divided are presented in Fig. 1.
Fig. 1. The artificial population consists of agents with different demographic characteristics (block A). These agents are assigned specific tasks to perform at specific locations and times. This determines a network that connects agents to locations throughout the day, creating a person-location network (Block В). The person-to-person contact network (Block Б ) is developed based on the interactions obtained from the person-to-location graph.
The agent-based approach is applicable for studying epidemic control measures [11–13], assessing the effectiveness of interventions on different populations [14], and conducting sensitivity analysis of modeling results to changes in parameter values [15]. The main goals of public health applications of ABM are to analyze and predict the public health consequences of proposed interventions, taking into account aspects of complex social structure. ABM-based models help to understand the underlying mechanisms that determine the dynamics and outcomes of epidemics. ABMs can be used for virtual experiments exploring different intervention strategies and other interventions to reduce morbidity in the population [16]. All this makes ABMs an important research and training tool for public health professionals.
The main difficulty in using ABM as a tool for social, political, and economic research lies in the proper matching of the purpose of modeling and the level of detail of the model [17]. The disadvantage of ABM can be excessive detail, which complicates the overall modeling task and leads to the creation of overly complex models with redundant parameters that do not contribute significantly to the modeling results [18].
Finding a balance in the choice of considered parameters and complexity when creating an artificial population (AP) for ABM is an open question facing researchers. This systematic review aims to identify the most common approaches to creating AP in agent-based modeling and to specify their limitations.
Materials and methods
This systematic review is based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. A systematic literature search was conducted using the PubMed database. The search was performed using the keywords: "agent-based" AND "epidemiology". Full-text articles published between 2020 and 2024 were considered. The initial assessment selected studies that used agent-based modeling, studied respiratory viral infections, and had a sufficiently detailed description of the model.
Fig. 2. Publication selection scheme for the systematic review.
Papers studying the behavior of the virus in a single organism, as well as studies on modeling animal infections, were excluded from the study.
According to the search methodology, 144 studies published in international journals in English were selected and used for further analysis. No Russian-language publications meeting the selection criteria were found. The selected publications were systematized according to the ways of setting the AP by the criteria "location" (space consideration) and "agent properties". The agent's properties included such characteristics as gender, age, field of activity, ethnicity, income, and the like — i.e., characteristics determined on the basis of demographic and statistical data. We considered that the model accounted for locations if the probability of transmission depended on the agent's spatial location. This property of the model can be realized both by tracking the coordinates of each agent in the modeled space and by modeling individual spatial entities (e.g., store, school) that may house agents.
Results
In 2020-2024, the greatest interest of researchers was focused on modeling the spread of SARS-CoV-2 virus, the causative agent of COVID-19, in the population: 129 (89%) papers out of 144 selected modeled the spread of this virus, 10 (11%) papers modeled the spread of influenza virus. In several studies, researchers presented their models as suitable for studying several respiratory diseases (Table 1).
Table 1. Distribution of the articles according to the pathogen
Pathogen | Publication amount | |
n | % | |
SARS-CoV-2 | 129 | 89 |
Influenza | 10 | 6,9 |
Measles | 1 | 0,7 |
MERS-CoV | 1 | 0,7 |
Unspecified respiratory diseases | 4 | 2,7 |
To systematize the types of APs used in the models, we analyzed the presence of agent properties and the consideration of their location. Fig. 3 shows the distribution of publications considered in the review according to the type of APs described in them.
Fig. 3. Distribution of publications by artificial population type. *Аt the same time, agents can be endowed with an individual level of protection against the pathogen (immunity) and the level of viral load. **Тhis group also includes papers that consider the spatial location of buildings and/or agents.
We can distinguish 4 variants of AP construction, based on combinations of presence and absence of consideration of agents' properties and consideration of locations.
Approaches to AP creation without consideration of location and agent properties (12 articles)
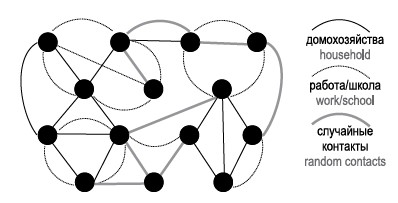
An artificial population without taking into account spatial localization and demographic properties of agents represents a graph — a network of contacting agents (Fig. 4). The stochasticity of such models is created by generating individual sets of connections at each node (agent) based on given probability distributions of the number of contacts.
Fig. 4. A network of contacts without considering the properties of agents and spatial characteristics is illustrated. Each node represents an agent, and the edges between nodes indicate a contact on one of the layers.
At the same time, contacts or social ties can be the same or differ in the strength and frequency of interaction. In 6 (50%) out of 12 reviewed works all contacts are the same. In another 5 (41.7%) papers contacts are divided into 3 categories: close, permanent (family, friends) and casual, not close (contacts on the street, work, school). In 1 (8.3%) article, the division of interactions by types is more complex.
For example, in a study conducted by J. Whitman et al., the interactions are divided into two levels: intra-cohort (strong ties, high probability of transmission) and inter-cohort (weak ties, rare cases of virus transmission, number of ties is smaller) [19]. This allowed us to account for the presence of clusters in the distribution of contacts and to reproduce the repetitive behavior of peaks in disease spread with significant stochasticity. Using this model, the researchers studied the behavior of the reproductive number at different values of the initial immune profile of the population, as well as the dynamics of the infection time series when the population size and contact matrix change.
A study by X. Guo et al. presented a multilevel model of the relationship between disease transmission and emotional stress in society [20]. In this paper, two independent networks of contacts are superimposed. Each node represents some group of people, infection and information exchange occurs through the edges of these nodes. Each node, in turn, models a set of individuals in each node, which increases the accuracy of the results.
In the study conducted by N.N. Chung et al. presents a contact network consisting of a set of overlapping networks (households, dormitories, workplaces, dynamic crowd network, dynamic social gathering network) [21].
Agent-based modeling based on the construction of AP without taking into account the spatial localization and demographic properties of agents makes it possible to solve a fairly wide range of problems without additional complication of the model. This approach was used to study the influence of such factors as population size, immunity parameters, the number and nature of agent relationships, and population density on the modeling results. This approach also allows us to analyze quarantine and testing strategies, the nature of repeated peaks of incidence, the dynamics of mutant infections, and the role of super-spreaders (agents with a large number of linkages).
The lack of detailing the properties of agents when creating the AP allows us to simplify the computational model and increase its interpretability. At the same time, the main limitations of the AP considered in this section are the lack of the possibility of introducing adjustable clustering (for example, separating pensioners into a separate group) and taking into account the behavior of the population, as well as the inappropriateness of such models to study the physical impact of social interactions.
Approaches to creating an AP that takes into account properties of agents without considering locations (64 studies)
APs in which agents with demographic, biological, and social properties interact with each other in an unstructured space are the most common in agent-based modeling. Many authors consider this type of AP to be optimal from the point of view of accuracy/performance balance. This approach is also popular due to the fact that high computational efficiency allows the agents to be endowed with an extensive set of parameters.
The construction of a network of contacts in the considered type of AP is often based on the creation of 4 main layers: households, work, schools and kindergartens, and society. In more complex models, up to 30 layers can be overlaid.
The considered agent-based models based on the formation of AP, taking into account the properties of agents without taking into account locations, according to the nature of the realization of social ties were distributed as follows:
- Uniform contact— 11 (17.2%) publications;
- Close/long distance contact — 1 (1.5%);
- Three or more types of contact — 52 (81.3%).
The most common agent characteristics include age (64/64) and sex (9/64). Age groups may differ in the likelihood of infection and the development of more severe cases of disease. The age structure of the population also affects the properties of contact networks between agents. For example, in models with homogeneous contacts, the network of interactions is built based on age-specific contact matrices [22, 23]. Work contacts may be excluded for the older generation, and some models construct additional blocks of contact networks for elderly care facilities [24-30].
The number and nature of contacts between agents may depend on the agent's occupation/profession. In the simplest case, professions such as teacher and hospital employee are modeled. Such an approach allows modeling elements of temporal dynamics of agents' interaction, e.g., five-day working day, possibility of vacation and skipping school/work, division of contact networks into daytime (school, work) and evening/nighttime (home, community) ones.
About 20% of the publications reviewed in this section use the Covasim environment for AP construction and modeling [10]. In its basic version, Covasim is an open-source modeling environment adapted to study the dynamics of the COVID-19 pandemic. The AP embedded in Covasim represents a set of people, each with attributes such as age, gender, and social status (Figure 5). In modeling the spread of infection, the model takes into account the frequency of contacts, the infectiousness of the virus, and the susceptibility of agents.
Fig. 5. Inter-agent interactions under the assumption that agents do have properties. Constant (solid lines) and dynamic (dashed lines) contact networks are modelled.
Using the open source agent-based modeling environment Covasim, researchers can explore different epidemic scenarios by changing infection parameters and modeling various interventions such as social distancing, isolation, testing, contact tracing, and vaccination campaigns. In a study conducted by A. Cattaneo et al., the Covasim environment was used to evaluate the effectiveness and optimization of a COVID-19 vaccination campaign in the Italian region of Lombardy [31]. The age structure of the population and the household characteristics were matched with data from the Italian National Institute of Statistics, while the rest of the contact network variables were constructed based on the default parameters embedded in Covasim. Different levels of constraints were modeled by reducing the number of contacts in the school, work and social interaction strata, and by varying the probability of transmission between household members. The Covasim environment also allows for the specification and tracking of dynamic characteristics of agent immunity. For example, vaccination, as well as disease, affects the dynamics of neutralizing antibodies and the level of protection of agents; cross-immunity with a given degree of effectiveness is realized when different strains of the virus are present in the population. In the study of A. Cattaneo et al., the Covasim model showed results consistent with the registered cases of COVID-19 infection, detection and mortality, the most effective vaccination strategy was determined and age priorities for vaccination were proposed [31].
In general, agent-based modeling on AP, which takes into account the properties of agents without taking into account their locations, is used to study the development of an epidemic taking into account various demographic data, as well as to assess the effects of diseases on public health and the economy. In particular, such modeling makes it possible to assess the effectiveness of quarantine measures, analyze vaccination scenarios (including those targeting different age groups of the population), calculate the economic cost of introducing restrictive measures, and build population immunity.
One of the main limitations of this type of AP is the simplified representation of the network of contacts [32], as well as the idealization of individuals' activities during the day [33]. The authors also emphasize the potential importance of additional properties of agents, which are not taken into account in this approach to modeling [24, 34].
Approaches to agent location-aware and agent property-aware AP (12 studies)
The main purpose of AP modeling with and without taking into account the spatial movements of agents is to reflect both the mobility of agents and the spatial dynamics of their movements during the spread of an epidemic.
The most common tool for this approach is the NetLogo software. In this environment, a map of a closed space is represented either by a coordinate grid or by a set of cells, and agents move randomly across the map or according to specified movement patterns (Fig. 6) [35-37]. Infection in this type of representation is possible if the agents (infected and susceptible) collide, converge to some threshold distance, or fall into one cell.
Fig. 6. Representation of an artificial population accounting for the movement of identical agents. A contact is defined as a collision, approaching a critical distance, and/or agents entering the same cell.
In the agent-based models we have considered, based on the formation of AP with and without taking into account the location of agents, social ties were analyzed as follows:
- Uniform contact — 6 (50%) publications;
- Close/long distance contact — 4 (34%);
- Three or more types of contact — 2 (16%).
A good example of this approach is shown in the study by T. Daghriri et al., in which several ways of distancing were modeled and the movements of agents resulting from different scenarios were visually represented [35]. The model took into account the possibility of a part of the agents not respecting the distancing. The authors showed the importance of compliance with restrictive measures and depicted the correlation between the strictness of the social distancing policy and the spread of the disease.
The two main models describing the movement of agents in the environment are random walks and the gravity model, according to which the strength of interaction (intensity of flows) depends on the importance (magnitude) of objects and the distance between them. For example, the study conducted by N. Kishore et al. showed that a densely populated center has a higher probability of being visited by agents [38].
The main goals of research in this approach are to study distancing strategies, the effectiveness of restrictive quarantine measures, the role of geographical factors in the spread of disease, and the role of super-spreaders. Such modeling also allows direct tracking of contacts of individuals in a population. However, it is not possible to model the implementation of anti-epidemic measures in different age and social groups of the population.
Approaches to creating an AP that takes into account the location and properties of the agents (56 studies)
When modeling with both geographic and demographic data, researchers try to achieve the closest approximation to the real population, with the goal of creating a digital twin. Typically, contact networks are divided into households, schools, workspace, and community, and geographic features are taken into account in two ways: modeling agents' movements on a map or capturing the location of buildings and determining the probability of agents visiting them. However, if in the group of APs that take into account the location of agents without taking into account their properties, the more common was the mapping of terrain, then in the works that take into account both the properties of agents and the properties of places, the division of the model space into conditional locations in which an agent can be located was more often used in the creation of APs (Fig. 7).
Fig. 7. Artificial population taking into account the location and characteristics of agents. It is possible to overlay a network of contacts on a map or to simulate the movements and contacts of agents.
The most common framework for this type of model is FRED (a Framework for Reconstructing Epidemic Dynamics) [39]. FRED uses synthetic populations based on census data that reflect the demographic and geographic heterogeneity of the population. Each agent has associated demographic and socioeconomic information (e.g., age, gender, race, family income). Race, along with sex and age, can be used to account for known disease prevalence. Households, educational and health institutions, places of work, and some other locations are georeferenced to a spatial grid of coordinates (at 1 km resolution). When calculating the probability of visiting different geographic locations, the agent's household income is taken into account. One of the features of this model is the ability to take into account the dynamic demography of agents, including aging, fertility and mortality. Works [40-43] have been performed on the basis of this model. Currently, FRED continues to be actively used to study seasonal influenza.
M.G. Krauland et al. studied the effect of a decrease in population immunity caused by the restriction of virus activity on its dynamics in subsequent years [43]. Modeling was conducted for a population representing Allegheny County (Pennsylvania, USA) with a population of about 1.2 million. This county includes both urban and suburban areas and is large enough to investigate influenza patterns. According to the results, a decrease in the incidence rate in the first season will lead to an increase in the incidence rate in the second season. Compensating for the decline in population immunity may help to increase vaccination. Depending on cross-immunity from previous infection and the transmissibility of the strain, the incidence rate could increase by up to 50%.
Many of the publications reviewed in this section describe complicated models where additional parameters have been added to the basic version of the AP. In particular, A. Truszkowska et al. modified the basic version of the model by adding the division of the able-bodied population into spheres of activity [44]. This allowed the model to reflect the complex structure of employment. And in the study conducted by C. Fosco et al., the labor force was divided into 4 groups according to different mobility in case of quarantine measures [45].
A number of works paid more attention to the division of the day into time segments. In 24 models, the temporal characteristics of agents' mobility were taken into account (taking into account the schedule, division of the day).
The goals of approaches that take into account both agent and location properties include:
- management decision analysis;
- finding the optimal approach to implementing non-pharmaceutical interventions;
- study of infection spread using GPS;
- studying the spread of the pathogen in its early stages;
- studying the distribution of different strains;
- modeling contact tracing and virus transmission;
- studying the spread of the virus in different countries/cities;
- exploring vaccination strategies;
- studying the protection of the population depending on the past season.
In generating this type of AP, model developers often resort to various simplifications to allow for the inclusion of additional characteristics that they consider crucial [46]. Some assumptions exceed the current understanding of the mechanisms of epidemic development, allowing them to be included in the study only in an approximate form [47]. It is common practice to use updated real-world data as the basis for the creation of an AP-digital twin, which is then projected onto a sample of smaller size than the general population. Even if the sample replicates the structure of the real population, the results obtained for it may not fully reflect the situation in the real population [48].
Increasing complexity of AP formation
When creating a realistic population for epidemiologic studies, an extensive set of parameters is required, each of which cannot be taken into account at the moment. Basic versions of models allow describing the epidemiologic process in a general way and investigating regularities and trends in the dynamics of epidemics.
In order to make it more plausible, some authors have resorted to complicating the AP by introducing the following parameters:
- seasonality;
- comorbidities;
- dynamic immunity;
- ethnicity;
- profits;
- transport flows.
The heterogeneity of the population in terms of the susceptibility of individuals to the virus and the severity of the disease can be accounted for by a co-morbidity factor. In the simplest version, co-morbidities can be taken into account thanks to the binary parameter (present or absent) [28]. In a more complex version, by introducing an additional module for calculating the risk factor, it is possible to take into account both specific diseases (type 1 and type 2 diabetes, hypertension, cardiovascular diseases, etc.) and risk factors related to lifestyle (smoking, physical activity, increased body mass index, etc.) [49].
In certain studies, dynamic immunity modeling has been performed. A popular framework for accounting for immunity has been the Covasim model [50–52], which provides the possibility of dynamically changing the values of the level of specific immune defense for each agent and the population as a whole.
Seasonality can affect both the properties of the pathogen (mainly used in modeling seasonal influenza) and other parameters (effect of average daily temperature on susceptibility, effect of season on the contact network with sex distribution, etc.). [43, 53–58].
If appropriate data are available, it is possible to add sociological parameters of agents — income level and ethnicity, and these characteristics can be reflected in the model in different ways. In the study conducted by M.D. Patel et al., people of different nationalities had different susceptibility to the virus and tolerated the disease differently [59]. In the study conducted by C. Fosco et al., income level influenced the ability of workers to stay at home during the epidemic [45]. In the study conducted by M. Thakur et al. income was directly correlated with decreased vaccination rates [60].
Modeling of transport flows within the AP was used in 15 (10%) papers, 8 of which considered geographic and demographic population data, 7 — only demographic data.
Representation of transport was possible in the form of:
- an additional random network of contacts;
- more transportation stops/blocks;
- addition of common agent routing.
- Some researchers have resorted to dividing transportation into modes:
- automobiles, hitchhiking, public transport, walking, etc. (with the possibility of getting infected only in automobiles and public transportation) [25];
- metro, bus, shuttle bus [61].
Conclusion
AP formation is a key point in the construction of predictive agent-based models. The use of ABM allows us to consider the population at the level of individual representatives, which opens new opportunities for studying the development of epidemics and analyzing measures to prevent the spread of infection.
In our review, based on the analysis of 144 original studies, we consider 4 variants of AP construction with different degrees of detail. We intentionally used the PubMed database exclusively for the literature search because it is focused on biomedical research, including epidemiology. This choice allowed us to analyze the main publications published in ranked peer-reviewed journals in the field of interest, but it is possible that some part of the available publications was not considered. The review also considered articles published since the beginning of the COVID-19 pandemic. This allowed us to analyze the most relevant cross-section of papers, focusing on the demanded solutions in AP formation, while the review did not include the previously published EpiSimS [62] and TRANSIMS models [63].
It should be noted that all the considered variants of AP construction turned out to be suitable for solving the list of tasks in the field of infectious disease epidemiology stated by the developers. The limitations of the present study are dictated by the impossibility of experimental confirmation of the success of the implementation of the presented ABM to achieve the goals and objectives in the reviewed studies. In most cases, there is no possibility to critically conceptualize the model due to the availability of a general, often superficial description of its device given in the publication and the lack of access to the source code of the model. The selected literature was analyzed largely on the basis of the authors' evaluation of the results of the papers. In most cases, the authors do not provide an analysis of the sensitivity of the result to the parameters of the modeled pathogen and AP. Such analysis is an important feature of complex models and can show the real importance of parameters, and this review revealed a systematic shortcoming of a large part of the analyzed papers.
Among the identified limitations in AP creation, the most significant are the insufficiency and anachronism of real demographic and statistical data required for further accounting in the model. Works that take into account the properties of agents in the AP, as a rule, rely on census data or sociological surveys, which do not always have the required detail. Models that incorporate the movement of agents on a city map use information from specialized applications, databases and mapping services such as Google Maps and OpenStreetMap. Obtaining this data and incorporating it into the model can be challenging, so simplified models based on assumptions about agent behavior and interactions were used in some cases.
The use of complex and diverse real demographic and statistical data is possible when studying small groups (at the level of a room, building), but for larger studies, the computational complexity in case of increasing the number of parameters or population size may exceed the technical capabilities of the calculation and lead to unreliable or uninterpretable results.
Further research on the creation and use of AP in agent-based modeling can be focused on optimizing methods of model parameterization and finding a balance between model detail and interpretability to achieve maximum accuracy and precision of results. When creating a AP, it is important to consider the factors that can be targeted for control. This will improve the quality of public health decision-making and increase the effectiveness of epidemic response.
About the authors
Irina I. Maslova
Research Institute of Systems Biology and Medicine
Email: paraslonic@gmail.com
ORCID iD: 0009-0000-0849-9123
student
Russian Federation, MoscowAleksandr I. Manolov
Research Institute of Systems Biology and Medicine
Author for correspondence.
Email: paraslonic@gmail.com
ORCID iD: 0000-0003-3912-429X
Cand. Sci. (Biol.), researcher
Russian Federation, MoscowOksana E. Glushchenko
Research Institute of Systems Biology and Medicine
Email: paraslonic@gmail.com
ORCID iD: 0000-0002-2028-3066
junior researcher
Russian Federation, MoscowIvan E. Kozlov
Research Institute of Systems Biology and Medicine
Email: paraslonic@gmail.com
ORCID iD: 0009-0007-2437-5527
research assistant
Russian Federation, MoscowVera I. Tsurkis
Research Institute of Systems Biology and Medicine
Email: paraslonic@gmail.com
ORCID iD: 0009-0001-5140-0990
student
Russian Federation, MoscowNikolay S. Popov
Research Institute of Systems Biology and Medicine
Email: paraslonic@gmail.com
ORCID iD: 0009-0002-6450-8594
student
Russian Federation, MoscowAndrey E. Samoilov
Research Institute of Systems Biology and Medicine
Email: paraslonic@gmail.com
ORCID iD: 0000-0001-8284-3164
researcher
Russian Federation, MoscowAlexandr N. Lukashev
Research Institute of Systems Biology and Medicine
Email: paraslonic@gmail.com
ORCID iD: 0000-0001-7365-0352
D. Sci. (Biol.), Corresponding member of RAS, chief scientist
Russian Federation, MoscowElena N. Ilina
Research Institute of Systems Biology and Medicine
Email: paraslonic@gmail.com
ORCID iD: 0000-0003-0130-5079
D. Sci. (Biol.), Сorresponding member of RAS, chief scientist, Head, Laboratory for mathematical biology and bioinformatics
Russian Federation, MoscowReferences
- Kirkeby C., Brookes V.J., Ward M.P., et al. A practical introduction to mechanistic modeling of disease transmission in veterinary science. Front. Vet. Sci. 2021;7:546651. DOI: https://doi.org/10.3389/fvets.2020.546651
- Hudson E.G., Brookes V.J., Ward M.P. Demographic studies of owned dogs in the Northern Peninsula Area, Australia, to inform population and disease management strategies. Aust. Vet. J. 2018;96(12):487–94. DOI: https://doi.org/10.1111/avj.12766
- Glushchenko O.E., Prianichnikov N.A., Olekhnovich E.I., et al. VERA: agent-based modeling transmission of antibiotic resistance between human pathogens and gut microbiota. Bioinformatics. 2019;35(19):3803–11. DOI: https://doi.org/10.1093/bioinformatics/btz154
- Decelle A., Krzakala F., Moore C., Zdeborová L. Asymptotic analysis of the stochastic block model for modular networks and its algorithmic applications. Phys. Rev. E. Stat. Nonlin. Soft Matter. Phys. 2011;84(6 Pt 2):066106. DOI: https://doi.org/10.1103/PhysRevE.84.066106
- Ackland G.J., Ackland J.A., Antonioletti M., Wallace D.J. Fitting the reproduction number from UK coronavirus case data and why it is close to 1. Philos. Trans. A. Math. Phys. Eng. Sci. 2022;380(2233):20210301. DOI: https://doi.org/10.1098/rsta.2021.0301
- Creswell R., Augustin D., Bouros I., et al. Heterogeneity in the onwards transmission risk between local and imported cases affects practical estimates of the time-dependent reproduction number. Philos. Trans. A. Math. Phys. Eng. Sci. 2022;380(2233):20210308. DOI: https://doi.org/10.1098/rsta.2021.0308
- Van Dyke Parunak H., Savit R., Riolo R.L. Agent-based modeling vs. equation-based modeling: A case study and users’ guide. Multi-Agent Systems and Agent-Based Simulation. 2010;1534:10–25. DOI: https://doi.org/10.1007/10692956_2
- Rahmandad H., Sterman J. Heterogeneity and network structure in the dynamics of diffusion: comparing agent-based and differential equation models. Management Science. 2008;54:998–1014. DOI: https://doi.org/10.1287/mnsc.1070.0787
- Shanta S.S., Biswas M.H.A. The impact of media awareness in controlling the spread of infectious diseases in terms of SIR model. Mathematical Modelling of Engineering Problems. 2020;7:368–76. DOI: https://doi.org/10.18280/mmep.070306
- Kerr C.C., Stuart R.M., Mistry D., et al. Covasim: аn agent-based model of COVID-19 dynamics and interventions. PLoS Comput. Biol. 2021;17(7):e1009149. DOI: https://doi.org/10.1371/journal.pcbi.1009149
- Gozzi N., Tizzoni M., Chinazzi M., et al. Estimating the effect of social inequalities on the mitigation of COVID-19 across communities in Santiago de Chile. Nat. Commun. 2021;12(1):2429. DOI: https://doi.org/10.1038/s41467-021-22601-6
- Squazzoni F., Polhill J.G., Edmonds B., et al. Computational models that matter during a global pandemic outbreak: a call to action. Journal of Artificial Societies and Social Simulation. 2020; 23(2). DOI: https://doi.org/10.18564/jasss.4298
- Lux T. The social dynamics of COVID-19. Physica A. 2021;567: 125710. DOI: https://doi.org/10.1016/j.physa.2020.125710
- Conte R., Paolucci M. On agent-based modeling and computational social science. Front. Psychol. 2014;5:668. DOI: https://doi.org/10.3389/fpsyg.2014.00668
- Lux T., Zwinkels R.C. Empirical Validation of Agent-Based Models. In: Handbook of Computational Economics. Volume 4. Elsevier;2018:437–88. DOI: https://doi.org/10.1016/bs.hescom.2018.02.003
- Tracy M., Cerdá M., Keyes K.M. Agent-based modeling in public health: current applications and future directions. Annu. Rev. Public Health. 2018;39:77–94. DOI: https://doi.org/10.1146/annurev-publhealth-040617-014317
- Bonabeau E. Agent-based modeling: methods and techniques for simulating human systems. Proc. Natl. Acad. Sci. U.S.A. 2002;99(Suppl. 3):7280–7. DOI: https://doi.org/10.1073/pnas.082080899
- Marks R.E. Validating simulation models: a general framework and four applied examples. Comput. Econ. 2007;30:265–90. DOI: https://doi.org/10.1007/s10614-007-9101-7
- Whitman J., Jayaprakash C. Stochastic modeling of influenza spread dynamics with recurrences. PLoS One. 2020;15(4): e0231521. DOI: https://doi.org/10.1371/journal.pone.0231521
- Guo X., Tong J., Chen P., Fan W. The suppression effect of emotional contagion in the COVID-19 pandemic: a multi-layer hybrid modelling and simulation approach. PLoS One. 2021;16(7):e0253579. DOI: https://doi.org/10.1371/journal.pone.0253579
- Chung N.N., Chew L.Y. Modelling Singapore COVID-19 pandemic with a SEIR multiplex network model. Sci. Rep. 2021;11(1):10122. DOI: https://doi.org/10.1038/s41598-021-89515-7
- Moghadas S.M., Fitzpatrick M.C., Shoukat A., et al. Simulated identification of silent COVID-19 infections among children and estimated future infection rates with vaccination. JAMA Netw Open. 2021;4(4):e217097. DOI: https://doi.org/10.1001/jamanetworkopen.2021.7097
- Sah P., Vilches T.N., Pandey A., et al. Estimating the impact of vaccination on reducing COVID-19 burden in the United States: December 2020 to March 2022. J. Glob. Health. 2022;12:03062. DOI: https://doi.org/10.7189/jogh.12.03062
- Català M., Li X., Prats C., Prieto-Alhambra D. The impact of prioritisation and dosing intervals on the effects of COVID-19 vaccination in Europe: an agent-based cohort model. Sci. Rep. 2021;11(1):18812. DOI: https://doi.org/10.1038/s41598-021-98216-0
- Truszkowska A., Thakore M., Zino L., et al. Designing the safe reopening of US towns through high-resolution agent-based modeling. Adv. Theory Simul. 2021;4(9):2100157. DOI: https://doi.org/10.1002/adts.202100157
- Truszkowska A., Zino L., Butail S., et al. Exploring a COVID-19 Endemic scenario: high-resolution agent-based modeling of multiple variants. Adv. Theory Simul. 2023;6(1):2200481. DOI: https://doi.org/10.1002/adts.202200481
- Truszkowska A., Zino L., Butail S., et al. Predicting the effects of waning vaccine immunity against COVID-19 through high-resolution agent-based modeling. Adv. Theory Simul. 2022;5(6):2100521. DOI: https://doi.org/10.1002/adts.202100521
- Hadley E., Rhea S., Jones K., et al. Enhancing the prediction of hospitalization from a COVID-19 agent-based model: A Bayesian method for model parameter estimation. PLoS One. 2022;17(3):e0264704. DOI: https://doi.org/10.1371/journal.pone.0264704
- Rodríguez J.P., Aleta A., Moreno Y. Digital cities and the spread of COVID-19: Characterizing the impact of non-pharmaceutical interventions in five cities in Spain. Front. Public Health. 2023;11:1122230. DOI: https://doi.org/10.3389/fpubh.2023.1122230
- Chiba A. Modeling the effects of contact-tracing apps on the spread of the coronavirus disease: Mechanisms, conditions, and efficiency. PLoS One. 2021;16(9):e0256151. DOI: https://doi.org/10.1371/journal.pone.0256151
- Cattaneo A., Vitali A., Mazzoleni M., Previdi F. An agent-based model to assess large-scale COVID-19 vaccination campaigns for the Italian territory: The case study of Lombardy region. Comput. Methods Programs Biomed. 2022;224:107029. DOI: https://doi.org/10.1016/j.cmpb.2022.107029
- Krivorotko O., Sosnovskaia M., Vashchenko I., et al. Agent-based modeling of COVID-19 outbreaks for New York state and UK: Parameter identification algorithm. Infect. Dis. Model. 2022;7(1):30–44. DOI: https://doi.org/10.1016/j.idm.2021.11.004
- Koichubekov B., Takuadina A., Korshukov I., et al. The epidemiological and economic impact of COVID-19 in Kazakhstan: An agent-based modeling. Healthcare (Basel). 2023;11(22):2968. DOI: https://doi.org/10.3390/healthcare11222968
- Hinch R., Probert W.J.M., Nurtay A., et al. OpenABM-COVID19 – an agent-based model for non-pharmaceutical interventions against COVID-19 including contact tracing. PLoS Comput. Biol. 2021;17(7):e1009146. DOI: https://doi.org/10.1371/journal.pcbi.1009146
- Daghriri T., Ozmen O. Quantifying the effects of social distancing on the spread of COVID-19. Int. J. Environ. Res. Public Health. 2021;18(11):5566. DOI: https://doi.org/10.3390/ijerph18115566
- Li H., Zhang H. Cost-effectiveness analysis of COVID-19 screening strategy under China's dynamic zero-case policy. Front. Public Health. 2023;11:1099116. DOI: https://doi.org/10.3389/fpubh.2023.1099116
- Wang Q., Shi N., Huang J., et al. Cost-effectiveness of public health measures to control COVID-19 in China: A microsimulation modeling study. Front. Public Health. 2022;9:726690. DOI: https://doi.org/10.3389/fpubh.2021.726690
- Kishore N., Kahn R., Martinez P.P., et al. Lockdowns result in changes in human mobility which may impact the epidemiologic dynamics of SARS-CоV-2. Sci. Rep. 2021;11(1):6995. DOI: https://doi.org/10.1038/s41598-021-86297-w
- Grefenstette J.J., Brown S.T., Rosenfeld R., et al. FRED (a Framework for Reconstructing Epidemic Dynamics): an open-source software system for modeling infectious diseases and control strategies using census-based populations. BMC Public Health. 2013;13:940. DOI: https://doi.org/10.1186/1471-2458-13-940
- Krauland M.G., Zimmerman R.K., Williams K.V., et al. Agent-based model of the impact of higher influenza vaccine efficacy on seasonal influenza burden. Vaccine X. 2023;13:100249. DOI: https://doi.org/10.1016/j.jvacx.2022.100249
- Williams K.V., Krauland M.G., Harrison L.H., et al. Can a two-dose influenza vaccine regimen better protect older adults? An agent-based modeling study. Vaccines (Basel). 2022;10(11):1799. DOI: https://doi.org/10.3390/vaccines10111799
- Woodul R.L., Delamater P.L., Woodburn M. Validating model output in the absence of ground truth data: a COVID-19 case study using the Simulator of Infectious Disease Dynamics in North Carolina (SIDD-NC) model. Health Place. 2023;83:103065. DOI: https://doi.org/10.1016/j.healthplace.2023.103065
- Krauland M.G., Galloway D.D., Raviotta J.M., et al. Impact of low rates of influenza on next-season influenza infections. Am. J. Prev. Med. 2022;62(4):503–10. DOI: https://doi.org/10.1016/j.amepre.2021.11.007
- Truszkowska A., Fayed M., Wei S., et al. Urban determinants of COVID-19 spread: a comparative study across three cities in New York state. J. Urban. Health. 2022;99(5):909–21. DOI: https://doi.org/10.1007/s11524-022-00623-9
- Fosco C., Zurita F. Assessing the short-run effects of lockdown policies on economic activity, with an application to the Santiago Metropolitan Region, Chile. PLoS One. 2021;16(6):e0252938. DOI: https://doi.org/10.1371/journal.pone.0252938
- Maziarz M., Zach M. Agent-based modelling for SARS-CоV-2 epidemic prediction and intervention assessment: A methodological appraisal. J. Eval. Clin. Pract. 2020; 26(5): 1352–60. DOI: https://doi.org/10.1111/jep.13459
- Gupta P., Maharaj T., Weiss M., et al. Proactive contact tracing. PLOS Digit. Health. 2023;2(3):e0000199. DOI: https://doi.org/10.1371/journal.pdig.0000199
- Staffini A., Svensson A.K., Chung U.I., Svensson T. An agent-based model of the local spread of SARS-CоV-2: modeling study. JMIR Med. Inform. 2021;9(4):e24192. DOI: https://doi.org/10.2196/24192
- Mintram K., Anagnostou A., Anokye N., et al. CALMS: Modelling the long-term health and economic impact of COVID-19 using agent-based simulation. PLoS One. 2022;17(8):e0272664. DOI: https://doi.org/10.1371/journal.pone.0272664
- Silva M.E.P., Fyles M., Pi L., et al. The role of regular asymptomatic testing in reducing the impact of a COVID-19 wave. Epidemics. 2023;44:100699. DOI: https://doi.org/10.1016/j.epidem.2023.100699
- Pham Q.D., Stuart R.M., Nguyen T.V., et al. Estimating and mitigating the risk of COVID-19 epidemic rebound associated with reopening of international borders in Vietnam: a modelling study. Lancet Glob. Health. 2021;9(7):e916–24. DOI: https://doi.org/10.1016/s2214-109x(21)00103-0
- Sanz-Leon P., Hamilton L.H.W., Raison S.J., et al. Modelling herd immunity requirements in Queensland: impact of vaccination effectiveness, hesitancy and variants of SARS-CоV-2. Philos. Trans. A. Math. Phys. Eng. Sci. 2022;380(2233):20210311. DOI: https://doi.org/10.1098/rsta.2021.0311
- Pais C.M., Godano M.I., Juarez E., et al. City-scale model for COVID-19 epidemiology with mobility and social activities represented by a set of hidden Markov models. Comput. Biol. Med. 2023;160:106942. DOI: https://doi.org/10.1016/j.compbiomed.2023.106942
- Reguly I.Z., Csercsik D., Juhász J., et al. Microsimulation based quantitative analysis of COVID-19 management strategies. PLoS Comput. Biol. 2022;18(1):e1009693. DOI: https://doi.org/10.1371/journal.pcbi.1009693
- Barthe G., Viti R., Druschel P., et al. Listening to Bluetooth beacons for epidemic risk mitigation. Sci. Rep. 2022;12(1):5558. DOI: https://doi.org/10.1038/s41598-022-09440-1
- Bicher M., Rippinger C., Schneckenreither G., et al. Model based estimation of the SARS-CоV-2 immunization level in austria and consequences for herd immunity effects. Sci. Rep. 2022;12(1):2872. DOI: https://doi.org/10.1038/s41598-022-06771-x
- Nagpal S., Kumar R., Noronha R.F., et al. Seasonal variations in social contact patterns in a rural population in north India: Implications for pandemic control. PLoS One. 2024;19(2):e0296483. DOI: https://doi.org/10.1371/journal.pone.0296483
- Souther A., Chang M.H., Tassier T. It's worth a shot: urban density, endogenous vaccination decisions, and dynamics of infectious disease. J. Econ. Interact. Coord. 2023;18(1):163–89. DOI: https://doi.org/10.1007/s11403-022-00367-4
- Patel M.D., Rosenstrom E., Ivy J.S., et al. Association of simulated COVID-19 vaccination and nonpharmaceutical interventions with infections, hospitalizations, and mortality. JAMA Netw. Open. 2021;4(6):e2110782. DOI: https://doi.org/10.1001/jamanetworkopen.2021.10782
- Thakur M., Zhou R., Mohan M., et al. COVID's collateral damage: likelihood of measles resurgence in the United States. BMC Infect. Dis. 2022;22(1):743. DOI: https://doi.org/10.1186/s12879-022-07703-w
- Rykovanov G.N., Lebedev S.N., Zatsepin O.V., et al. Agent-based simulation of the COVID-19 epidemic in Russia. Her. Russ. Acad. Sci. 2022;92(4):479–87. DOI: https://doi.org/10.1134/s1019331622040219
- Stroud P., Del Valle S., Sydoriak S., et al. Spatial dynamics of pandemic influenza in a massive artificial society, journal of artificial societies and social simulation. J. Artif. Soc. Soc. Simul. 2007; 10(4): 1–9.
- Barrett C.L., Beckman R.J., Berkbigler K.P., et al. TRANSIMS: Transportation analysis simulation system. In: Portland Study Reports. Los Alamos, NM;1999.
- Kaxiras E., Neofotistos G. Multiple epidemic wave model of the COVID-19 pandemic: modeling study. J. Med. Internet Res. 2020;22(7):e20912. DOI: https://doi.org/10.2196/20912
- Quilty B.J., Clifford S., Hellewell J., et al. Quarantine and testing strategies in contact tracing for SARS-CоV-2: a modelling study. Lancet Public Health. 2021;6(3):e175–83. DOI: https://doi.org/10.1016/s2468-2667(20)30308-x
- Gwizdałła T. Viral disease spreading in grouped population. Comput. Methods Programs Biomed. 2020;197:105715. DOI: https://doi.org/10.1016/j.cmpb.2020.105715
- Sood M., Sridhar A., Eletreby R., et al. Spreading processes with mutations over multilayer networks. Proc. Natl. Acad. Sci. U.S.A. 2023;120(24):e2302245120. DOI: https://doi.org/10.1073/pnas.2302245120
- Streilein W., Finklea L., Schuldt D., et al. Evaluating COVID-19 exposure notification effectiveness with SimAEN: A simulation tool designed for public health decision making. Public Health Rep. 2022;137(2_suppl):83S–9S. DOI: https://doi.org/10.1177/00333549221116361
- Kim Y., Ryu H., Lee S. Effectiveness of intervention strategies on MERS-CoV transmission dynamics in South Korea, 2015: Simulations on the network based on the real-world contact data. Int. J. Environ. Res. Public Health. 2021;18(7):3530. DOI: https://doi.org/10.3390/ijerph18073530
- Kwon O., Son W.S., Kim J.Y., Kim J.H. Intervention effects in the transmission of COVID-19 depending on the detection rate and extent of isolation. Epidemiol. Health. 2020;42:e2020045. DOI: https://doi.org/10.4178/epih.e2020045
- Tatsukawa Y., Arefin M.R., Kuga K., Tanimoto J. An agent-based nested model integrating within-host and between-host mechanisms to predict an epidemic. PLoS One. 2023;18(12):e0295954. DOI: https://doi.org/10.1371/journal.pone.0295954
- Stapelberg N.J.C., Smoll N.R., Randall M., et al. A Discrete-Event, Simulated Social Agent-Based Network Transmission (DESSABNeT) model for communicable diseases: Method and validation using SARS-CоV-2 data in three large Australian cities. PLoS One. 2021;16(5):e0251737. DOI: https://doi.org/10.1371/journal.pone.0251737
- Thompson J., McClure R., Blakely T., et al. Modelling SARS-CоV-2 disease progression in Australia and New Zealand: an account of an agent-based approach to support public health decision-making. Aust. N. Z. J. Public Health. 2022;46(3):292–303. DOI: https://doi.org/10.1111/1753-6405.13221
- Hinch R., Panovska-Griffiths J., Probert W.J.M., et al. Estimating SARS-CоV-2 variant fitness and the impact of interventions in England using statistical and geo-spatial agent-based models. Philos. Trans. A. Math. Phys. Eng. Sci. 2022;380(2233):20210304. DOI: https://doi.org/10.1098/rsta.2021.0304
- Tatapudi H., Das R., Das T.K. Impact assessment of full and partial stay-at-home orders, face mask usage, and contact tracing: An agent-based simulation study of COVID-19 for an urban region. Glob. Epidemiol. 2020;2:100036. DOI: https://doi.org/10.1016/j.gloepi.2020.100036
- Jahn B., Sroczynski G., Bicher M., et al. Targeted COVID-19 vaccination (TAV-COVID) considering limited vaccination capacities – an agent-based modeling evaluation. Vaccines (Basel). 2021;9(5):434. DOI: https://doi.org/10.3390/vaccines9050434
- Tatapudi H., Das T.K. Impact of school reopening on pandemic spread: a case study using an agent-based model for COVID-19. Infect. Dis. Model. 2021;6:839–47. DOI: https://doi.org/10.1016/j.idm.2021.06.007
- Romero-Brufau S., Chopra A., Ryu A.J., et al. Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: simulation agent based modeling study. BMJ. 2021;373:n1087. DOI: https://doi.org/10.1136/bmj.n1087
- Goldenbogen B., Adler S.O., Bodeit O., et al. Control of COVID-19 outbreaks under stochastic community dynamics, bimodality, or limited vaccination. Adv. Sci. (Weinh). 2022;9(23):e2200088. DOI: https://doi.org/10.1002/advs.202200088
- Moghadas S.M., Vilches T.N., Zhang K., et al. Evaluation of COVID-19 vaccination strategies with a delayed second dose. PLoS Biol. 2021;19(4):e3001211. DOI: https://doi.org/10.1371/journal.pbio.3001211
- Keskinocak P., Oruc B.E., Baxter A., et al. The impact of social distancing on COVID19 spread: State of Georgia case study. PLoS One. 2020;15(10):e0239798. DOI: https://doi.org/10.1371/journal.pone.0239798
- Berec L., Diviák T., Kuběna A., et al. On the contact tracing for COVID-19: A simulation study. Epidemics. 2023;43:100677. DOI: https://doi.org/10.1016/j.epidem.2023.100677
- Moghadas S.M., Fitzpatrick M.C., Sah P., et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proc. Natl. Acad. Sci. U.S.A. 2020;117(30):17513–5. DOI: https://doi.org/10.1073/pnas.2008373117
- Ben-Zuk N., Daon Y., Sasson A., et al. Assessing COVID-19 vaccination strategies in varied demographics using an individual-based model. Front. Public Health. 2022;10:966756. DOI: https://doi.org/10.3389/fpubh.2022.966756
- Nguyen Q.D., Prokopenko M. A general framework for optimising cost-effectiveness of pandemic response under partial intervention measures. Sci. Rep. 2022;12(1):19482. DOI: https://doi.org/10.1038/s41598-022-23668-x
- Abdollahi E., Haworth-Brockman M., Keynan Y., et al. Simulating the effect of school closure during COVID-19 outbreaks in Ontario, Canada. BMC Med. 2020;18(1):230. DOI: https://doi.org/10.1186/s12916-020-01705-8
- Zhang K., Vilches T.N., Tariq M., et al. The impact of mask-wearing and shelter-in-place on COVID-19 outbreaks in the United States. Int. J. Infect. Dis. 2020;101:334–41. DOI: https://doi.org/10.1016/j.ijid.2020.10.002
- Shoukat A., Wells C.R., Langley J.M., et al. Projecting demand for critical care beds during COVID-19 outbreaks in Canada. CMAJ. 2020;192(19):E489–96. DOI: https://doi.org/10.1503/cmaj.200457
- Eilersen A., Sneppen K. Cost-benefit of limited isolation and testing in COVID-19 mitigation. Sci. Rep. 2020;10(1):18543. DOI: https://doi.org/10.1038/s41598-020-75640-2
- Hotton A.L., Ozik J., Kaligotla C., et al. Impact of changes in protective behaviors and out-of-household activities by age on COVID-19 transmission and hospitalization in Chicago, Illinois. Ann. Epidemiol. 2022;76:165–73. DOI: https://doi.org/10.1016/j.annepidem.2022.06.005
- Aleta A., Martín-Corral D., Bakker M.A., et al. Quantifying the importance and location of SARS-CоV-2 transmission events in large metropolitan areas. Proc. Natl. Acad. Sci. U.S.A. 2022;119(26):e2112182119. DOI: https://doi.org/10.1073/pnas.2112182119
- Bicher M., Rippinger C., Zechmeister M., et al. An iterative algorithm for optimizing COVID-19 vaccination strategies considering unknown supply. PLoS One. 2022;17(5):e0265957. DOI: https://doi.org/10.1371/journal.pone.0265957
- Nishi A., Dewey G., Endo A., et al. Network interventions for managing the COVID-19 pandemic and sustaining economy. Proc. Natl. Acad. Sci. U.S.A. 2020;117(48):30285–94. DOI: https://doi.org/10.1073/pnas.2014297117
- Weng X., Chen Q., Sathapathi T.K., et al. Impact of school operating scenarios on COVID-19 transmission under vaccination in the U.S.: an agent-based simulation model. Sci. Rep. 2023;13(1):12836. DOI: https://doi.org/10.1038/s41598-023-37980-7
- Vilches T.N., Abdollahi E., Cipriano L.E., et al. Impact of non-pharmaceutical interventions and vaccination on COVID-19 outbreaks in Nunavut, Canada: a Canadian Immunization Research Network (CIRN) study. BMC Public Health. 2022;22(1):1042. DOI: https://doi.org/10.1186/s12889-022-13432-1
- Ng V., Fazil A., Waddell L.A., et al. Projected effects of nonpharmaceutical public health interventions to prevent resurgence of SARS-CоV-2 transmission in Canada. CMAJ. 2020;192(37):E1053–64. DOI: https://doi.org/10.1503/cmaj.200990
- Scott N., Abeysuriya R.G., Delport D., et al. COVID-19 epidemic modelling for policy decision support in Victoria, Australia 2020-2021. BMC Public Health. 2023;23(1):988. DOI: https://doi.org/10.1186/s12889-023-15936-w
- Moghadas S.M., Vilches T.N., Zhang K., et al. The impact of vaccination on coronavirus disease 2019 (COVID-19) outbreaks in the United States. Clin. Infect. Dis. 2021;73(12):2257–64. DOI: https://doi.org/10.1093/cid/ciab079
- Alagoz O., Sethi A.K., Patterson B.W., et al. Effect of timing of and adherence to social distancing measures on COVID-19 burden in the United States: A simulation modeling approach. Ann. Intern. Med. 2021;174(1):50–7. DOI: https://doi.org/10.7326/m20-4096
- Shi P., Yan J., Keskinocak P., et al. The impact of opening dedicated clinics on disease transmission during an influenza pandemic. PLoS One. 2020;15(8):e0236455. DOI: https://doi.org/10.1371/journal.pone.0236455
- Wu S., Huang Z., Grant-Muller S., et al. Modelling the reopen strategy from dynamic zero-COVID in China considering the sequela and reinfection. Sci. Rep. 2023;13(1):7343. DOI: https://doi.org/10.1038/s41598-023-34207-7
- Moreno López J.A., Arregui García B., Bentkowski P., et al. Anatomy of digital contact tracing: Role of age, transmission setting, adoption, and case detection. Sci. Adv. 2021;7(15):eabd8750. DOI: https://doi.org/10.1126/sciadv.abd8750
- Scott N., Palmer A., Delport D., et al. Modelling the impact of relaxing COVID-19 control measures during a period of low viral transmission. Med. J. Aust. 2021;214(2):79–83. DOI: https://doi.org/10.5694/mja2.50845
- Abeysuriya R.G., Delport D., Stuart R.M., et al. Preventing a cluster from becoming a new wave in settings with zero community COVID-19 cases. BMC Infect. Dis. 2022;22(1):232. DOI: https://doi.org/10.1186/s12879-022-07180-1
- Houdroge F., Palmer A., Delport D., et al. Frequent and unpredictable changes in COVID-19 policies and restrictions reduce the accuracy of model forecasts. Sci. Rep. 2023;13(1):1398. DOI: https://doi.org/10.1038/s41598-023-27711-3
- Sneppen K., Nielsen B.F., Taylor R.J., Simonsen L. Overdispersion in COVID-19 increases the effectiveness of limiting nonrepetitive contacts for transmission control. Proc. Natl. Acad. Sci. U.S.A. 2021;118(14):e2016623118. DOI: https://doi.org/10.1073/pnas.2016623118
- Zhang X., Chen B., Le J., Hu Y. Impact of different nucleic acid testing scenarios on COVID-19 transmission. Heliyon. 2023;10(1):e23700. DOI: https://doi.org/10.1016/j.heliyon.2023.e23700
- Li A., Wu J., Moghadas S.M. Epidemic dynamics with time-varying transmission risk reveal the role of disease stage-dependent infectiousness. J. Theor. Biol. 2023;573:111594. DOI: https://doi.org/10.1016/j.jtbi.2023.111594
- Delport D., Sacks-Davis R., Abeysuriya R.G., et al. Lives saved by public health restrictions over the Victorian COVID-19 Delta variant epidemic wave, Aug-Nov 2021. Epidemics. 2023;44:100702. DOI: https://doi.org/10.1016/j.epidem.2023.100702
- Han A.X., Girdwood S.J., Khan S., et al. Strategies for using antigen rapid diagnostic tests to reduce transmission of severe acute respiratory syndrome coronavirus 2 in low- and middle-income countries: a mathematical modelling study applied to Zambia. Clin. Infect. Dis. 2023;76(4):620–30. DOI: https://doi.org/10.1093/cid/ciac814
- Zachreson C., Fair K.M., Harding N., Prokopenko M. Interfering with influenza: nonlinear coupling of reactive and static mitigation strategies. J. R. Soc. Interface. 2020;17(165):20190728. DOI: https://doi.org/10.1098/rsif.2019.0728
- Pandey A., Fitzpatrick M.C., Moghadas S..M, et al. Modelling the impact of a high-uptake bivalent booster scenario on the COVID-19 burden and healthcare costs in New York City. Lancet Reg. Health Am. 2023;24:100555. DOI: https://doi.org/10.1016/j.lana.2023.100555
- Sanz-Leon P., Stevenson N.J., Stuart R.M., et al. Risk of sustained SARS-CоV-2 transmission in Queensland, Australia. Sci. Rep. 2022;12(1):6309. DOI: https://doi.org/10.1038/s41598-022-10349-y
- Groves-Kirkby N., Wakeman E., Patel S., et al. Large-scale calibration and simulation of COVID-19 epidemiologic scenarios to support healthcare planning. Epidemics. 2023;42:100662. DOI: https://doi.org/10.1016/j.epidem.2022.100662
- Sah P., Vilches T.N., Moghadas S.M., et al. Return on investment of the COVID-19 vaccination campaign in New York city. JAMA Netw Open. 2022;5(11):e2243127. DOI: https://doi.org/10.1001/jamanetworkopen.2022.43127
- Goldberg L.A., Jorritsma J., Komjáthy J., Lapinskas J. Increasing efficacy of contact-tracing applications by user referrals and stricter quarantining. PLoS One. 2021;16(5):e0250435. DOI: https://doi.org/10.1371/journal.pone.0250435
- Zhu Y., Shen R., Dong H., Wang W. Spatial heterogeneity and infection patterns on epidemic transmission disclosed by a combined contact-dependent dynamics and compartmental model. PLoS One. 2023;18(6):e0286558. DOI: https://doi.org/10.1371/journal.pone.0286558
- Giacopelli G. A full-scale agent-based model to hypothetically explore the impact of lockdown, social distancing, and vaccination during the COVID-19 pandemic in Lombardy, Italy: model development. JMIRx Med. 2021;2(3):e24630. DOI: https://doi.org/10.2196/24630
- Kustudic M., Niu B., Liu Q. Agent-based analysis of contagion events according to sourcing locations. Sci. Rep. 2021;11(1):16032. DOI: https://doi.org/10.1038/s41598-021-95336-5
- Lima L.L., Atman A.P.F. Impact of mobility restriction in COVID-19 superspreading events using agent-based model. PLoS One. 2021;16(3):e0248708. DOI: https://doi.org/10.1371/journal.pone.0248708
- Eilersen A., Sneppen K. SARS-CоV-2 superspreading in cities vs the countryside. APMIS. 2021;129(7):401–7. DOI: https://doi.org/10.1111/apm.13120
- Gugole F., Coffeng L.E., Edeling W., et al. Uncertainty quantification and sensitivity analysis of COVID-19 exit strategies in an individual-based transmission model. PLoS Comput. Biol. 2021;17(9):e1009355. DOI: https://doi.org/10.1371/journal.pcbi.1009355
- Wallentin G., Kaziyeva D., Reibersdorfer-Adelsberger E. COVID-19 intervention scenarios for a long-term disease management. Int. J. Health Policy Manag. 2020;9(12):508–16. DOI: https://doi.org/10.34172/ijhpm.2020.130
- Bissett K.R., Cadena J., Khan M., Kuhlman C.J. Agent-Based Computational Epidemiological Modeling. J. Indian Inst. Sci. 2021;101(3):303–27. DOI: https://doi.org/10.1007/s41745-021-00260-2
- Murakami T., Sakuragi S., Deguchi H., Nakata M. Agent-based model using GPS analysis for infection spread and inhibition mechanism of SARS-CоV-2 in Tokyo. Sci. Rep. 2022;12(1):20896. DOI: https://doi.org/10.1038/s41598-022-25480-z
- Truszkowska A., Behring B., Hasanyan J., et al. High-resolution agent-based modeling of COVID-19 spreading in a small town. Adv. Theory Simul. 2021;4(3):2000277. DOI: https://doi.org/10.1002/adts.202000277
- Dong T., Dong W., Xu Q. Agent simulation model of COVID-19 epidemic agent-based on GIS: A case study of Huangpu district, Shanghai. Int. J. Environ. Res. Public Health. 2022;19(16):10242. DOI: https://doi.org/10.3390/ijerph191610242
- Castro B.M., Reis M.M., Salles R.M. Multi-agent simulation model updating and forecasting for the evaluation of COVID-19 transmission. Sci. Rep. 2022;12(1):22091. DOI: https://doi.org/10.1038/s41598-022-22945-z
- Gostoli U., Silverman E. An agent-based model of social care provision during the early stages of Covid-19. Sci. Rep. 2022;12(1):16534. DOI: https://doi.org/10.1038/s41598-022-20846-9
- Chang S.L., Cliff O.M., Zachreson C., Prokopenko M. Simulating transmission scenarios of the delta variant of SARS-CоV-2 in Australia. Front. Public Health. 2022;10:823043. DOI: https://doi.org/10.3389/fpubh.2022.823043
- Nguyen Q.D., Chang S.L., Jamerlan C.M., Prokopenko M. Measuring unequal distribution of pandemic severity across census years, variants of concern and interventions. Popul. Health Metr. 2023;21(1):17. DOI: https://doi.org/10.1186/s12963-023-00318-6
- Zhang H., Yin L., Mao L., et al. Combinational recommendation of vaccinations, mask-wearing, and home-quarantine to control influenza in megacities: an agent-based modeling study with large-scale trajectory data. Front. Public Health. 2022;10:883624. DOI: https://doi.org/10.3389/fpubh.2022.883624
- Li Q., Huang Y. Optimizing global COVID-19 vaccine allocation: An agent-based computational model of 148 countries. PLoS Comput. Biol. 2022;18(9):e1010463. DOI: https://doi.org/10.1371/journal.pcbi.1010463
- Kirpich A., Koniukhovskii V., Shvartc V., et al. Development of an interactive, agent-based local stochastic model of COVID-19 transmission and evaluation of mitigation strategies illustrated for the state of Massachusetts, USA. PLoS One. 2021;16(2):e0247182. DOI: https://doi.org/10.1371/journal.pone.0247182
- Singh D.E., Olmedo Luceron C., Limia Sanchez A., et al. Evaluation of vaccination strategies for the metropolitan area of Madrid via agent-based simulation. BMJ Open. 2022;12(12):e065937. DOI: https://doi.org/10.1136/bmjopen-2022-065937
- Alzu'bi A.A., Alasal S.I.A., Watzlaf V.J.M. A Simulation study of coronavirus as an epidemic disease using agent-based modeling. Perspect. Health Inf. Manag. 2020;18(Winter):1g.
- De-Leon H., Aran D. MAM: Flexible Monte-Carlo Agent based model for modelling COVID-19 spread. J. Biomed. Inform. 2023;141:104364. DOI: https://doi.org/10.1016/j.jbi.2023.104364
- Thompson J., Wattam S. Estimating the impact of interventions against COVID-19: From lockdown to vaccination. PLoS One. 2021;16(12):e0261330. DOI: https://doi.org/10.1371/journal.pone.0261330
- Gomez J., Prieto J., Leon E., Rodríguez A. INFEKTA-An agent-based model for transmission of infectious diseases: The COVID-19 case in Bogotá, Colombia. PLoS One. 2021;16(2):e0245787. DOI: https://doi.org/10.1371/journal.pone.0245787
- Schröder M., Bossert A., Kersting M., et al. COVID-19 in South Africa: outbreak despite interventions. Sci. Rep. 2021;11(1):4956. DOI: https://doi.org/10.1038/s41598-021-84487-0.
- Hunter E., Namee B.M., Kelleher J.D. A Model for the spread of infectious diseases in a region. Int. J. Environ. Res. Public Health. 2020;17(9):3119. DOI: https://doi.org/10.3390/ijerph17093119
- Zachreson C., Chang S.L., Cliff O.M., Prokopenko M. How will mass-vaccination change COVID-19 lockdown requirements in Australia? Lancet Reg. Health West Pac. 2021;14:100224. DOI: https://doi.org/10.1016/j.lanwpc.2021.100224
- Guzmán-Merino M., Durán C., Marinescu M.C., et al. Assessing population-sampling strategies for reducing the COVID-19 incidence. Comput. Biol. Med. 2021;139:104938. DOI: https://doi.org/10.1016/j.compbiomed.2021.104938
- Gaudou B., Huynh N.Q., Philippon D., et al. COMOKIT: A modeling kit to understand, analyze, and compare the impacts of mitigation policies against the COVID-19 epidemic at the scale of a city. Front. Public Health. 2020;8:563247. DOI: https://doi.org/10.3389/fpubh.2020.563247
- Chang S.L., Harding N., Zachreson C., et al. Modelling transmission and control of the COVID-19 pandemic in Australia. Nat. Commun. 2020;11(1):5710. DOI: https://doi.org/10.1038/s41467-020-19393-6
- Harding N., Spinney R., Prokopenko M. Phase transitions in spatial connectivity during influenza pandemics. Entropy (Basel). 2020;22(2):133. DOI: https://doi.org/10.3390/e22020133
- Parisi A., Brand S.P.C., Hilton J., et al. Spatially resolved simulations of the spread of COVID-19 in three European countries. PLoS Comput. Biol. 2021;17(7):e1009090. DOI: https://doi.org/10.1371/journal.pcbi.1009090
- Germann T.C., Smith M.Z., Dauelsberg L.R., et al. Assessing K-12 school reopenings under different COVID-19 spread scenarios — United States, school year 2020/21: а retrospective modeling study. Epidemics. 2022;41:100632. DOI: https://doi.org/10.1016/j.epidem.2022.100632
- Mokhtari A., Mineo C., Kriseman J., et al. A multi-method approach to modeling COVID-19 disease dynamics in the United States. Sci Rep. 2021;11(1):12426. DOI: https://doi.org/10.1038/s41598-021-92000-w
- Gabler J., Raabe T., Röhrl K., Gaudecker H.V. The effectiveness of testing, vaccinations and contact restrictions for containing the CoViD-19 pandemic. Sci. Rep. 2022;12(1):8048. DOI: https://doi.org/10.1038/s41598-022-12015-9
- Milne G.J., Carrivick J., Whyatt D. Mitigating the SARS-CоV-2 Delta disease burden in Australia by non-pharmaceutical interventions and vaccinating children: a modelling analysis. BMC Med. 2022;20(1):80. DOI: https://doi.org/10.1186/s12916-022-02241-3
- Szanyi J., Wilson T., Howe S., et al. Epidemiologic and economic modelling of optimal COVID-19 policy: public health and social measures, masks and vaccines in Victoria, Australia. Lancet Reg. Health West Pac. 2023;32:100675. DOI: https://doi.org/10.1016/j.lanwpc.2022.100675
- Jackson M.L. Low-impact social distancing interventions to mitigate local epidemics of SARS-CоV-2. Microbes Infect. 2020;22(10):611–6. DOI: https://doi.org/10.1016/j.micinf.2020.09.006
- Gankin Y., Nemira A., Koniukhovskii V., et al. Investigating the first stage of the COVID-19 pandemic in Ukraine using epidemiological and genomic data. Infect. Genet. Evol. 2021;95:105087. DOI: https://doi.org/10.1016/j.meegid.2021.105087
- Zhang N., Jack Chan P.T., Jia W., et al. Analysis of efficacy of intervention strategies for COVID-19 transmission: a case study of Hong Kong. Environ. Int. 2021;156:106723. DOI: https://doi.org/10.1016/j.envint.2021.106723
- Nguyen Q.D., Chang S.L., Jamerlan C.M., Prokopenko M. Measuring unequal distribution of pandemic severity across census years, variants of concern and interventions. Popul. Health Metr. 2023;21(1):17. DOI: https://doi.org/10.1186/s12963-023-00318-6
- Aleta A., Martín-Corral D., Pastore Y. Piontti A., et al. Modelling the impact of testing, contact tracing and household quarantine on second waves of COVID-19. Nat. Hum. Behav. 2020;4(9):964–71. DOI: https://doi.org/10.1038/s41562-020-0931-9
- Zhao C., Zhang J., Hou X., et al. A high-frequency mobility big-data reveals how COVID-19 spread across professions, locations and age groups. PLoS Comput. Biol. 2023;19(4): e1011083. DOI: https://doi.org/10.1371/journal.pcbi.1011083
- Li K.K.F., Jarvis S.A., Minhas F. Elementary effects analysis of factors controlling COVID-19 infections in computational simulation reveals the importance of social distancing and mask usage. Comput. Biol. Med. 2021;134:104369. DOI: https://doi.org/10.1016/j.compbiomed.2021.104369
- Lei H., Zhang N., Niu B., et al. Effect of rapid urbanization in mainland China on the seasonal influenza epidemic: spatiotemporal analysis of surveillance data from 2010 to 2017. JMIR Public Health Surveill. 2023;9:e41435. DOI: https://doi.org/10.2196/41435
- Chiba A. The effectiveness of mobility control, shortening of restaurants' opening hours, and working from home on control of COVID-19 spread in Japan. Health Place. 2021;70:102622. DOI: https://doi.org/10.1016/j.healthplace.2021.102622
- Wang Y., Sun K., Pan Y., et al. A Retrospective modeling study of the targeted non-pharmaceutical interventions during the Xinfadi outbreak in the early stage of the COVID-19 pandemic — Beijing, China, 2020. China CDC Wkly. 2023;5(5):108–12. DOI: https://doi.org/10.46234/ccdcw2023.020
- Latkowski R., Dunin-Kȩplicz B. An agent-based COVID-19 simulator: extending Covasim to the polish context. Procedia Comput. Sci. 2021;192:3607–16. DOI: https://doi.org/10.1016/j.procs.2021.09.134
- Koo J.R., Cook A.R., Park M., et al. Interventions to mitigate early spread of SARS-CоV-2 in Singapore: a modelling study. Lancet Infect. Dis. 2020;20(6):678–88. DOI: https://doi.org/10.1016/s1473-3099(20)30162-6
- Rippinger C., Bicher M., Urach C., et al. Evaluation of undetected cases during the COVID-19 epidemic in Austria. BMC Infect. Dis. 2021;21(1):70. DOI: https://doi.org/10.1186/s12879-020-05737-6
Supplementary files