Monitoring of respiratory viral infections in Moscow during 2011–2022
- Authors: Vetrova E.N.1, Chernyshova A.I.1, Pritchina T.N.1, Isaeva E.I.1, Morozova O.V.1,2
-
Affiliations:
- National Research Center of Epidemiology and Microbiology named after N.F. Gamaleya
- Federal Research and Clinical Center of Physical-Chemical Medicine
- Issue: Vol 100, No 5 (2023)
- Pages: 328-337
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18480
- DOI: https://doi.org/10.36233/0372-9311-376
- EDN: https://elibrary.ru/tieioc
- ID: 18480
Cite item
Abstract
Introduction. Respiratory viruses (RV) circulate throughout the world and in all seasons of the year. Long-term monitoring of the distribution of respiratory pathogens is necessary to analyze the relevance of diagnostic systems to current viral isolates, to assess the risks of infection and the need for vaccine development and use, as well as to investigate the interdependence of RV reproduction in mixed infections.
Objective — to study the causative agents of acute respiratory viral infections (ARVI) in Moscow during 2011–2022 by reverse transcription with subsequent polymerase chain reaction with fluorescent hydrolysis probes detection in real-time (RT2-PCR).
Materials and Methods. Nasopharyngeal swabs from 3908 patients with acute respiratory infections were examined by the RT2-PCR.
Results. Monitoring of RV spread in Moscow showed cyclical changes in frequencies with three dominant species: influenza A virus (up to 31.3%), respiratory syncytial virus (up to 24.8%) and human rhinoviruses (up to 21.3%) in 2011–2020. The increase in the portion of unidentified clinical specimens from 1.2 to 28.5% in 2022 indicated incomplete accordance of diagnostic systems to modern RV isolates or the emergence of new species or strains of pathogens. Unidirectional changes in dynamics were registered for 5 out of 9 studied RVs with correlation coefficients of 0.43–0.79. High frequencies of mixed acute respiratory viral infections (up to 33.4%) along with unidentified samples do not allow us to accurately assess the risks of infection with various RV in Moscow, but prove the necessity of preventing infectious diseases with the most common RV.
Conclusion. Analysis of the dynamics of RV frequencies in Moscow showed the preservation of the dominant species: influenza A virus, respiratory syncytial virus and human rhinoviruses. During the period of vaccination against COVID-19, the proportion of seasonal coronaviruses increased.
Keywords
Full Text
Introduction
Acute respiratory viral infections (ARVI) account for about 90% of reported infections annually. In the world, according to the World Health Organization, 2-5 million cases of ARVI are registered annually with fatal outcomes ranging from 290,000 to 650,000 [1]. In Russia, according to Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing, 27.3-41.2 million cases are detected annually [2, 3]. At the same time, 99% of ARVI are diagnosed in children under 5 years of age, mainly in developing countries [4]. Influenza predominates among acute respiratory infections: 5–20% in adults and 20–30% in children [5]. Respiratory viruses (RV) enter the respiratory tract by airborne droplets, replicate in epithelial cells, and infect other organs and tissues, causing fever, headache, runny nose, sore throat, and cough. Besides fever forms of ARVI, complications in the form of encephalitis, paralysis, and death have been described [6]. The peculiarities of the course of ARVI and possible complications are determined by the properties of the RV (reproductive activity, virulence, pathogenicity), innate nonspecific resistance and specific immunity of the host [7, 8]. Frequent acute respiratory infections may suggest disorders of innate resistance and specific immunity.
Since the symptoms of ARVI are similar, detection of RV by by reverse transcription with subsequent polymerase chain reaction with fluorescent hydrolysis probes detection in real time (RT2-PCR) is the most specific, sensitive, and widely used method along with multiplex immunofluorescence analysis and isolation of infectious strains in permissive cell cultures [4]. However, the phenogenetic variability of viruses due to spontaneous mutagenesis, genetic rearrangements of viral quasispecies of RNA viruses, reassortment of segmented genomes, and the absence of viral and cellular RNA repair systems hampers the development of diagnostic systems and is the cause of false-negative results and unidentified etiologic agents of ARVI.
Currently, more than 200 species of viruses causing respiratory diseases are known, with the total number reaching 300, taking into account different serotypes [9]. Of these, 8 families of viruses are the most widespread:
- DNA viruses Adenoviridae (over 50 types of adenoviruses), Parvoviridae — bocavirus (2 types);
- RNA viruses Ortomyxoviridae — Influenza virus (types А, В, С), Paramixoviridae — parainfluenza virus (type 4);
- metapneumovirus (types А, В);
- Pneumoviridae — respiratory syncytial virus (type 2);
- Coronaviridae — coronaviruses (5 types);
- Picornoviridae — rhinoviruses (over 100 types),
- enteroviruses (over 90 types);
- Reoviridae — reovirus [6, 10].
An increase in the proportion of mixed viral and viral-bacterial associations was noted. The relative frequencies of detection of ARVI pathogens vary depending on geographic localization, age of patients, methods of clinical specimen collection, duration of investigation and methods of pathogen identification [10].
During the COVID-19 pandemic, quarantine measures, social distancing and widespread use of face masks limited the spread of all respiratory infections. There was a decrease in the proportion of samples positive in RT2-PCR for respiratory infections from 49.8% (2018–2019) to 39% (2019–2020) and then to 13.4% (2020–2021) [11].
The objective of this work was to study the causative agents of ARVI in Moscow during 2011-2022 by the RT2-PCR.
Materials and methods
Nasopharyngeal swabs from 3908 patients which were collected no later than in 7 days postinfection from the onset of ARVI in the period from 2011 to 2022 were kindly provided by the head of the infectious diseases department, Prof. L.V. Kolobukhina (Clinical Hospital of Infectious Diseases No. 1, Moscow) and Dr. M.S. Savenkova (Morozov Children's City Clinical Hospital and Pirogov Russian National Research Medical University, Moscow) with the patients' informed consent. Ethical approval from the Ethics Committee of the National Research Center of Epidemiology and Microbiology of N.F. Gamaleya of the Russian Ministry of Health, Moscow, Russia was taken before starting the research (Approval Code # 18 2022-02-21).
RV were identified by RT2-PCR using test systems approved by the Federal Service for Surveillance in Healthcare of Russia. Total nucleic acids were isolated by lysis of clinical samples in guanidinium isothiocyanate (GITC) solution followed by alcohol precipitation using the reagent kit "PREP-NA" ("DNA-Technology"). For reverse transcription with a random hexameric oligodeoxyribonucleotides, the reagent kit "Reverta-L" ("AmpliSens") was used. RNA of respiratory syncytial virus (hRSv), metapneumovirus (hMpv), parainfluenza viruses of types 1, 2, 3 and 4 (hPiv), coronaviruses (hCov), human rhinoviruses (hRv), DNA of adenoviruses of groups B, C and E (hAdv), human bocavirus (hBov) were detected using "AmpliSens ARVI-screen-FL" kit; RNA of influenza viruses A and B (Central Research Institute of Epidemiology) — "Amplisens Influenza virus A/B-FL" kit; RNA of SARS-CoV-2 coronavirus — "RealBest RNA SARS-CoV-2" kit ("VectorBest") on DT-32 and DT-lite thermocyclers with fluorescence detection in real time ("DNA-Technology"). In case of simultaneous detection of genome fragments of more than one RV in a nasopharyngeal swab, viral infections were considered as co-infections (mixed).
In total, 3908 nasopharyngeal swabs (1 from each patient) collected in Moscow were analyzed. SARS-CoV-2 RNA detection was performed only in 2022 due to the isolation of COVID-19 patients from ARVI patients in hospitals in 2020-2021 and the need for an additional sanitary and epidemiologic permission for the research.
Statistical analysis of RV distribution was performed using the software "Microsoft Excel". Differences were considered significant at p ≤ 0.05 [12].
Results
To analyze the distribution of ARVI etiological agents, 3908 patients were examined in Moscow in the period from 2011 to 2022. The frequency of detection of respiratory syncytial virus, metapneumovirus, parainfluenza viruses, seasonal coronaviruses, rhinoviruses, adenoviruses, bocavirus, influenza A and B viruses, co-infections with the detection of at least 2 RV in a sample and unidentified samples in 2011-2022 are presented in Table 1.
Table 1. Monitoring of ARVI pathogens in Moscow in 2011–2022 by RT2-PCR, frequency of occurrence, % of total number of samples
Virus | Year | |||||||||||
2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
Amount of studied samples | 478 | 437 | 369 | 383 | 376 | 339 | 405 | 215 | 240 | 310 | 113 | 243 |
hRSv | 11,1 | 20,4 | 19,2 | 14,1 | 4,5 | 24,8 | 10,4 | 2,9 | 3,2 | 12,7 | 0,0 | 0,0 |
hMpv | 13,4 | 3,2 | 7,0 | 5,5 | 1,6 | 1,8 | 3,7 | 6,6 | 6,6 | 5,2 | 4,4 | 0,0 |
hPiv | 3,8 | 5,0 | 3,8 | 6,0 | 13,6 | 1,5 | 7,9 | 6,0 | 6,7 | 1,3 | 6,2 | 2,7 |
hCov | 4,0 | 2,5 | 6,5 | 9,9 | 1,3 | 1,2 | 3,0 | 1,9 | 3,9 | 3,6 | 19,5 | 0,0 |
hRV | 14,9 | 21,3 | 18,4 | 13,6 | 2,1 | 11,5 | 12,3 | 7,9 | 8,8 | 15,2 | 7,1 | 0,0 |
hAdv | 13,4 | 1,1 | 6,0 | 8,6 | 25,0 | 2,9 | 4,4 | 10,3 | 8,3 | 6,5 | 5,3 | 5,9 |
hBov | 2,9 | 2,1 | 4,6 | 4,7 | 0,8 | 11,2 | 6,2 | 2,3 | 2,6 | 10,7 | 0,9 | 0,0 |
INF-A | 19,2 | 34,3 | 10,0 | 14,9 | 21,3 | 20,4 | 15,3 | 17,3 | 19,0 | 12,7 | 1,8 | 1,2 |
INF-B | 1,7 | 0,5 | 4,1 | 1,0 | 2,1 | 8,3 | 4,4 | 0,8 | 1,2 | 3,2 | 0,0 | 1,2 |
Mix | 12,1 | 7,8 | 17,3 | 19,3 | 25,5 | 15,3 | 26,7 | 29,9 | 33,4 | 24,1 | 31,0 | 28,8 |
N/I | 3,6 | 1,8 | 3,0 | 2,3 | 2,1 | 1,2 | 5,7 | 14,1 | 6,3 | 4,8 | 23,8 | 28,5 |
Note. Mix — mixed infections; N/I — not identified samples.
The relative frequencies of each respiratory virus varied over the 12 years of observation. Before the COVID-19 pandemic in 2011–2020, three respiratory viruses (INF-A, hRSv, and hRv) predominated, then their relative frequencies decreased significantly. Despite mass immunization against influenza with trivalent and quadrivalent vaccines containing IFN-A and IFN-B antigens in Moscow since 2017, the detection rate of the influenza A virus in nasopharyngeal swabs of ARVI patients remained high until 2020. A significant decrease in the portion of influenza A virus was observed only in 2021–2022, which could probably be due to both quarantine measures and mask regime, and the coverage of more than half of the Russian population with vaccination against influenza [5]. Noteworthy the non-synchronous and not unidirectional changes in the percentages of influenza A and B viruses. INF-B detection frequencies remained in the range from 0.5% to 4.4% throughout the entire observation period with the only exception with growth of the influenza B virus rate up to 8.3% in 2016.
A high incidence of SARS-CoV-2 (31.7%) was registered in 2022, despite nonspecific and specific prophylaxis, as well as sanitary and educational work. The use of vector vaccines against COVID-19, in particular, the "Sputnik-V" vaccine based on Ad5 and Ad26 adenoviruses, did not lead to a significant decrease in the percentage of adenoviruses in the total etiologic structure of ARVI morbidity in Moscow (Table 1). In the absence of vaccines against most ARVI, the relative portions of the other studied RV remained minor. The maximum frequency of hMpv (13.4%) was registered in 2011 and in subsequent years did not exceed 7%. There was an increase in the frequency of mixed infections, which could be due to both disorders of innate and adaptive immunity among patients with ARVI and independent spread and increased reproduction of viruses.
In recent years, the relative rate of unidentified samples among ARVI patients with clinical symptoms has increased to 28.5% (Table 1), indicating an incomplete correspondence between licensed diagnostic test systems and circulating respiratory pathogens, the genetic diversity of strains of known viruses, and the possible emergence of new pathogens.
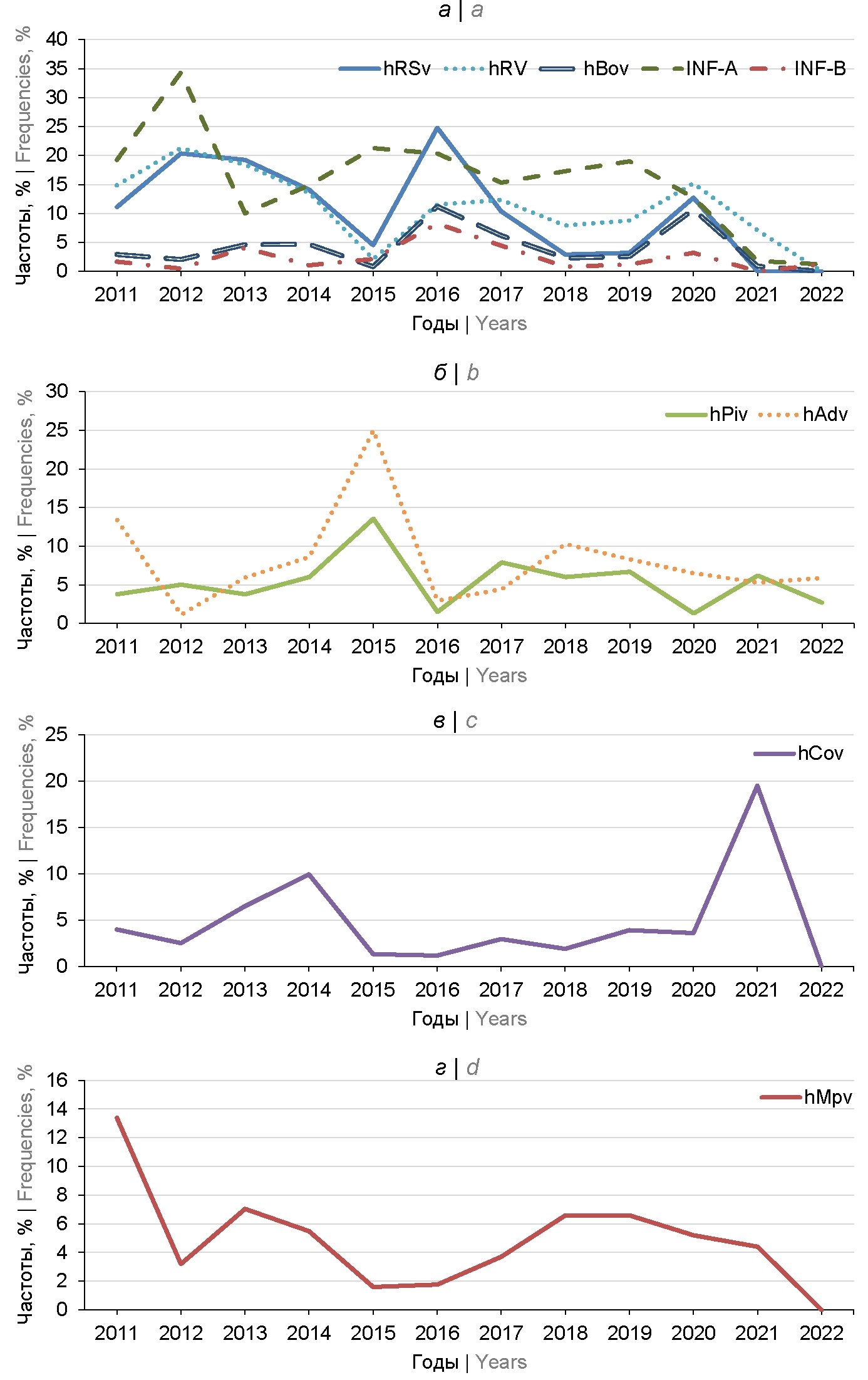
Analysis of the dynamics of the spread of ARVI etiological agents showed high correlation coefficients (r) between hRSv and hRv (r = 0.77), hRSv and hBov (r = 0.66); hRSv and INF-B (r = 0.64); hRSv and INF-A (r = 0.52); hBov and INF-B (r = 0.79), suggesting the existence of the first group of RV (Table 2; Figure, a). The second group of RV with similar distribution dynamics included hPiv and hAdv with r = 0.70 (Table 2; Figure, b).
Table 2. Correlation coefficients of the dynamics of RV frequencies
Virus | hRSv | hMpv | hPiv | hCov | hRv | hAdv | hBov | INF-A | INF-B |
hRSv | 1,00 | 0,05 | –0,41 | –0,20 | 0,77 | –0,40 | 0,66 | 0,52 | 0,64 |
hMpv | 0,05 | 1,00 | –0,15 | 0,19 | 0,45 | 0,14 | –0,01 | 0,11 | –0,20 |
hPiv | –0,41 | –0,15 | 1,00 | 0,05 | –0,38 | 0,70 | –0,52 | 0,21 | –0,31 |
hCov | –0,20 | 0,19 | 0,05 | 1,00 | 0,07 | –0,16 | –0,17 | –0,45 | –0,35 |
hRv | 0,77 | 0,45 | –0,38 | 0,07 | 1,00 | –0,47 | 0,43 | 0,49 | 0,17 |
hAdv | –0,40 | 0,14 | 0,70 | –0,16 | –0,47 | 1,00 | –0,36 | 0,09 | –0,20 |
hBov | 0,66 | –0,01 | –0,52 | –0,17 | 0,43 | –0,36 | 1,00 | 0,12 | 0,79 |
INF-A | 0,52 | 0,11 | 0,21 | –0,45 | 0,49 | 0,09 | 0,12 | 1,00 | 0,09 |
INF-B | 0,64 | –0,20 | –0,31 | –0,35 | 0,17 | –0,20 | 0,79 | 0,09 | 1,00 |
Note. Values corresponding to high correlation coefficients (r ≥ 0.43) are in italic; data corresponding to correlation coefficients r ≈ 0 are in bold.
Dynamics of the spread of respiratory viruses in 2011–2022.
Based on the correlation analysis of the dynamics of distribution (Table 2), 4 groups of RV were identified (Figure). Unidirectional changes were detected for 5 RV, including respiratory syncytial virus, human rhinoviruses, human bocavirus, influenza A and B viruses. Maximum values of detection frequencies of hRSv and human hRv were registered in 2012, 2016 and 2020, which suggests cyclic changes with rises every 4 years. For INF-A, high frequencies were found in 2012, 2015–2016 and 2019. This group is distinguished by similar but not identical dynamics for another group consisting of hAdv and hPiv, with a maximum in 2015 (Figure, b). For seasonal coronaviruses, low forward and inverse correlation coefficients (Table 2) indicated independent distribution with two peaks in 2014 and 2021 (Figure, c). The hMpv detection frequencies were also inconsistent with the other groups with a peak in 2011 and subsequent fluctuations at a relatively low level (Figure, d).
A comparative analysis of the annual mean values of RV frequencies in 5 Russian cities (Moscow, Yekaterinburg, Kazan, Rostov-on-Don, St. Petersburg) in 2014-2018, in Moscow during the years 2011–2022, and in the world (Table 3) showed the prevalence of 3 types of RV: INF-A, hRv, and hRSv. The dominance of RV in the world did not depend on specific prophylaxis, since the existing vaccines against influenza include INF-A and INF-B strains, vaccines against other acute respiratory viral infections are at the stage of clinical trials and are not used for mass immunization. Minimal average annual frequencies were detected for hMPV both in the world and in most regions of Russia, with the exception of Saint Petersburg (Table 3). At the same time, the portion of hMPV in Saint Petersburg (3.0%) was comparable to other regions of Russia. Human bocavirus was also rarely detected in most Russian cities, but global trends are not known [4].
Table 3. Comparison of annual mean frequencies of viral infections in Russia and in the world, %
Virus | Russiа | World [4] | |||||
Moscow | Ekaterinburg [13] | Kazan [13] | Rostov-on-Don [13] | St. Petersburg [13] | |||
2011–2022 | 2014–2018 [13] | ||||||
hRSv | 10,3 | 4,6 | 11,9 | 16,0 | 10,1 | 13,4 | 26,0 |
hMpv | 4,9 | 0,4 | 3,3 | 1,3 | 3,2 | 3,0 | 3,0 |
hPiv | 5,4 | 25,5 | 7,1 | 5,7 | 8,5 | 5,3 | 8,0 |
hCov | 4,8 | 0,5 | 3,9 | 1,8 | 2,6 | 1,4 | – |
hRv | 11,1 | 2,4 | 24,6 | 26,0 | 19,6 | 15,4 | 35,0 |
hAdv | 8,2 | 6,8 | 4,1 | 5,7 | 18,2 | 6,7 | 4,0 |
hBov | 4,1 | 0,1 | 1,6 | 4,2 | 3,9 | 2,5 | – |
INF-A | 15,6 | 49,5 | 38,9 | 25,8 | 25,8 | 32,4 | 10,0 |
INF-B | 2,4 | 10,1 | 4,6 | 13,5 | 8,1 | 20,0 | 5,0 |
Note. Cells corresponding to the dominant RV species are marked with bold font.
RT2-PCR provides quantitative estimates of genome-equivalents in the reaction mixture. However, clinical samples of nasopharyngeal swabs are not standardized, that excludes the possibility of calculation the number of virions per unit of volume or per host cell. Low viral loads were a peculiarity of the results of RT2-PCR of nasopharyngeal swabs of patients with ARVI.
Discussion
ARVI are dangerous due to their ubiquitous spread, year-round circulation, high contagiousness and risk of complications, including pneumonia, acute respiratory failure, infectious toxic shock, meningitis, encephalitis, paralysis, acute distress syndrome, as well as associated chronic diseases — bronchial asthma, chronic obstructive pulmonary disease, liver, kidney, cardiovascular diseases, leading to fatal outcomes [9]. Therefore, monitoring the distribution of etiologic agents of acute respiratory infections is necessary to improve diagnostic systems, to control their compliance with modern RV, to assess the risks of infection of the population in different regions and socio-demographic groups, the need to develop vaccines and their economically justified large-scale application.
The comparative analysis of RV distribution in Moscow showed periodic variations with preservation of dominance of three types: INF-A, hRSv and hRv, which corresponded to the spectra of etiological agents of ARVI in 5 cities of Russia [13, 14] and all over the world [4] (Table 3), due to globalization and determined the necessity of common measures of prevention, diagnostics and treatment. Analysis of the dynamics of RV distribution in Central Europe during 10 years also shows that influenza viruses were detected in almost half of the samples regardless of influenza immunization and quarantine restrictions during the COVID-19 pandemic [15]. Despite reports of an increase in the percentage of influenza B virus in 2019-2022 in Russia and in other countries [5, 16], the results of RV monitoring in Moscow (Table 1) showed no changes in the dominance structure. During the period of a significant decrease in the frequency of INF-A in recent years, no increase in the proportion of INF-B was found. One of the possible reasons is the coverage of the Moscow population with immunization against influenza. In Russia, the decree of the Chief State Sanitary Doctor for the 2020–2021 season sets the goal of influenza vaccination coverage of at least 60% of the population and 75% of citizens in risk groups [17]. Starting in 2017, Russia vaccinated more than 40% of the population annually, in 2019 — 50.5% of the population [5], in 2020 there was a decrease to 41.2%, with only 6.9% of children with laboratory-confirmed influenza needing hospital treatment [17]. In 2020, immunization with the Russian seasonal trivalent inactivated vaccine showed a decrease in antibody levels to all vaccine components 2.6–3.5 times below the protective level (titer in hemagglutination inhibition reaction < 1/40) in 6–7 months after vaccination, indicating instability of vaccine-induced humoral immunity against INF-A and INF-B viruses; dependence on the age of vaccinated persons (children 3–14 years old had a more active response compared to older persons, despite the immaturity of the immune system of children); insufficient immunogenicity of vaccine components of influenza B viruses, which led to an increase in the incidence of influenza B virus among etiological agents of ARVI (Table 1) and an increase in the incidence of influenza B, as well as an inverse correlation between the level of pre-existing antibodies to influenza viruses and the secretion of antibodies to new vaccine strains, which is due to the known effect of "original antigenic sin" [18]. One should note that the growth of influenza incidence to the maximum value in 2021 (in Russia — 14.96 per 100 thousand of the population; in Moscow — 65.99 per 100 thousand of the population) during the COVID-19 pandemic with the immune layer of more than 60% with the dominance of antibodies against the influenza B virus. The persistence of influenza A virus in the aquatic environment, among aquatic and near-water mammals and birds, determines the risks of the emergence of new mutant variants that escape from host immune system [19].
Monitoring of RV distribution in Japan showed periodic changes in hRSv frequencies with a temporary decrease during the COVID-19 pandemic similar to our observations and high frequencies of hRV occurrence [20]. RT2-PCR screening in 26 regions of Russia in 2020 showed a maximal rhinovirus detection rate of 7.32% both in the interepidemic period and at the beginning of the epidemic season [21, 22], which corresponded to a significant portion of rhinoviruses in the etiologic structure of ARVI in Moscow in 2020 (Table 1). The proportion of individuals seropositive to rhinovirus at the beginning of 2022 was 77.8% [16], which could be due to high frequencies of human rhinovirus spread in the previous 2 years (Table 3). In Saint Petersburg, a seasonal cyclicity of circulation was revealed for the prevalent hRSv and hRV species [14]. The observed differences in frequencies are probably determined by the limited sample size, geographical and socio-demographic differences, and are a consequence of the lack of standardization of nasopharyngeal swab collection methods, instability of viral genomic RNA during storage, and rapid evolutionary variability of RV. Currently, additional methods of multiplex analysis are being developed for RV monitoring with high sensitivity and specificity based on microfluidic technologies, biosensors, liquid chromatography with mass spectrometry, etc. [23]. In addition to traditional laboratory methods, it has been proposed to use Internet-of-Things computer technologies, which, by analyzing large amounts of data on human behavior and the environment using mathematical and statistical models, increase the probability of detecting epidemic outbreaks at early stages [23].
Parainfluenza viruses in 2020 in Russia occurred with similar frequencies both in the interepidemic period (1.09%) and at the beginning of the epidemic season (1.05%). The prevalence of other etiological agents of ARVI and influenza A and B viruses did not exceed 1% on average in Russia [21]. In 2021–2022, the prevalence of SARS-CoV-2 was 15%, of human coronaviruses — 12%, the prevalence of other RV — from 0.05 to 9% [22].
In 2020–2021, with low activity of influenza viruses, the etiological role of bocavirus and metapneumovirus was found to increase, while the frequency of isolation of parainfluenza viruses, adenoviruses, rhinoviruses and respiratory syncytial virus decreased [24]. Our data (Fig. 1) also show a significant increase in the frequency of bocavirus to 10.7% in 2020. For metapneumovirus, a peak in prevalence was recorded in the previous 2018–2019, followed by a gradual decline (Fig. 1). Detection frequencies of hAdv depended on the sample studied, as adenovirus infection was prevalent in organized collectives [14].
Despite all non-specific and specific COVID-19 prevention measures in 2020–2022, the part of samples containing SARS-CoV-2 was 31.7% in 2022 (Table 1). However, seasonal coronaviruses were not detected in the absence of other RV, although the portion was 19.5% in 2021. Possible reasons were the phenogenotypic variability of the RNA-containing coronaviruses of the four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Deltacoronavirus, among which α-coronaviruses HCoV-229E and HCoV-NL63, β-coronaviruses HCoV-OC43, HCoV-HKU1 are detected in humans with signs of acute respiratory infections, escape mutant virus variants from the host immune response and persistence of viruses in the environment, among wild reservoir hosts, domestic and farm animals. The natural reservoirs of Alphacoronavirus and Betacoronavirus are rats, birds, mammals and fish. Persistent infection with fecal excretion of viruses has been detected in poultry and pigs [19]. In addition to the widespread occurrence of coronaviruses in humans worldwide, as confirmed by retrospective serologic analysis, they are common in bats: the level of homology of amino acid sequences of SARS-CoV-2 and RaTG13 (coronavirus from bats and reptiles) is 97% [25]. Stabilty of parasitary systems is known to be supported by the diversity of reservoir hosts, differences in their population dynamics [26] and the persistence of viable viruses in the environment. To assess epidemiologic risks, RV monitoring by RT2-PCR with high sensitivity and specificity not only in clinical samples but also in aerosols is proposed [27].
The specific immune response induced by vaccines leads to selection of new variants of viral quasispecies of RNA-containing viruses resistant to neutralization by specific antibodies and causes acceleration of molecular evolution of viruses.
In the absence of vaccine prophylaxis against most minor ARVI, their relative portions remain stably low (Table 3), and new dangerous virus variants have no selective advantage. Prolonged co-evolution of humans and most RV has led to weakening of pathogenic properties, reduction of viral loads and host survival for successful virus reproduction and spread.
During 12 years of observation, an increase of unidentified clinical specimens from patients with symptoms of acute respiratory infections from 1.2% to 28.5% was noted, which could be due to bacterial respiratory infections with similar symptoms and/or sequence mismatches between primers and probes used in RT2-PCR and genomes of current RV. High frequencies of mixed infections in the range of 7.8–33.4% may be a consequence of the lack of virus interference during reproduction in human cells, independent reproduction of RV and/or immunodeficiencies in the Moscow population. Correlation analysis of the dynamics of RV spread (Table 3) showed significant interdependence for 5 out of 9 studied etiological agents of ARVI, including all 3 dominant species — INF-A, hRSv and hRv, as well as INF-B and hBov (Figure a). Predominant coinfection with rhinoviruses and bocavirus with high correlation coefficients have also been reported in long-term monitoring in Central Europe [15]. The second group included hAdv and hPiv (r = 0.7) with a peak in 2015 (Figure b). There was no significant correlation between the distribution dynamics of seasonal coronaviruses (Table 2; Fig. c) and metapneumovirus (Fig. d) and other RV analyzed. Further monitoring is needed to analyze the correlation of the distribution dynamics of seasonal hCov (Figure, c), SARS-CoV-2, and other RV [20]. The cyclical ups and downs in the ffrequencies of RV detection may be due to regular changes in herd immunity associated with varying frequencies of acute respiratory infections.
The observed frequencies of mixed infections with 2 and 3 RV were higher than theoretically expected values under the assumption of independent distribution of pathogens, indicating the absence of interference between most RV, possibly due to defects in the interferon system in etiological agents of ARVI.
One should note the part of individuals infected with viruses without symptoms of acute respiratory infections is 0.25–6.09% of the population. Asymptomatic virus carriers are involved in RV circulation and increased incidence [22, 27].
Conclusion
During 2011–2022 in Moscow the etiologic structure of ARVI was rearranged with 3 dominant species remaining: influenza A virus, respiratory syncytial virus, and human rhinoviruses. During the period of restrictive measures of nonspecific prophylaxis and mass immunization against COVID-19, the portion of seasonal coronaviruses increased significantly, possibly due to α-coronaviruses.
About the authors
Elizaveta N. Vetrova
National Research Center of Epidemiology and Microbiology named after N.F. Gamaleya
Author for correspondence.
Email: immunol.lab@mail.ru
ORCID iD: 0000-0003-1902-5278
researcher, Laboratory of immunology, National Research Center of Epidemiology and Microbiology named after N.F. Gamaleya
Russian Federation, MoscowAlyona I. Chernyshova
National Research Center of Epidemiology and Microbiology named after N.F. Gamaleya
Email: immunol.lab@mail.ru
ORCID iD: 0000-0003-1290-4042
researcher, Laboratory of immunology, National Research Center of Epidemiology and Microbiology named after N.F. Gamaleya
Russian Federation, MoscowTatiana N. Pritchina
National Research Center of Epidemiology and Microbiology named after N.F. Gamaleya
Email: immunol.lab@mail.ru
ORCID iD: 0000-0003-3418-2394
researcher, Laboratory of immunology, National Research Center of Epidemiology and Microbiology named after N.F. Gamaleya
Russian Federation, MoscowElena I. Isaeva
National Research Center of Epidemiology and Microbiology named after N.F. Gamaleya
Email: jmei@crie.ru
ORCID iD: 0000-0002-2523-0692
Cand. Sci. (Biol.), leading researcher, Laboratory of immunology, National Research Center of Epidemiology and Microbiology named after N.F. Gamaleya
Russian Federation, MoscowOlga V. Morozova
National Research Center of Epidemiology and Microbiology named after N.F. Gamaleya; Federal Research and Clinical Center of Physical-Chemical Medicine
Email: immunol.lab@mail.ru
ORCID iD: 0000-0001-9630-0777
D. Sci. (Biol.), senior researcher, Laboratory of immunology, National Research Center of Epidemiology and Microbiology named after N.F. Gamaleya, Moscow, Russia; leading researcher, Laboratory of medical nanotechnology, Federal Research and Clinical Center of Physical-Chemical Medicine
Russian Federation, Moscow; MoscowReferences
- WHO. World Health Organization fact sheet: Influenza (Seasonal). Geneva; 2021.
- Львов Н.И., Лихопоенко В.П. Острые респираторные заболевания. Руководство по инфекционным болезням. СПб.;2011:7–122. L'vov N.I., Likhopoenko V.P. Acute Respiratory Diseases. Guide to Infectious Diseases. St. Petersburg;2011:7–122.
- Государственный доклад «О санитарно-эпидемиологической обстановке в Российской Федерации в 2010 году». М.; 2011.
- Waghmode R., Jadhav S., Nema V. The burden of respiratory viruses and their prevalence in different geographical regions of India: 1970–2020. Front. Microbiol. 2021;12:723850. DOI: https://doi.org/10.3389/fmicb.2021.723850
- Шахтахтинская Ф.Ч., Намазова–Баранова Л.С., Федосеенко М.В., Калюжная Т.А. Актуальные вопросы вакцинопрофилактики гриппа. Вопросы современной педиатрии. 2021;20(4):333–7. Shakhtakhtinskaya F.CH., Namazova-Baranova L.S., Fedoseenko M.V., Kaliuzhnaia T.A. Topical issues of influenza vaccine prevention. Current Pediatrics. 2021;20(4):333–7. DOI: https://doi.org/10.15690/vsp.v20i4.2291 EDN: https://elibrary.ru/iyloiz
- Гладких Р.А., Молочный В.П., Бутакова Л.В. и др. Клинико–этиологическая характеристика острых респираторных вирусных инфекций у госпитализированных детей города Хабаровска в эпидемических сезонах 2014–2017 гг. Детские инфекции. 2018;17(2):6–14. Gladkikh R.A., Molochny V.P., Butakovа L.V., et al. Clinical and etiological characteristics of acute respiratory viral infections in hospitalized children of the city of Khabarovsk in epidemic seasons 2014–2017. Children infections. 2018;17(2):6–14. DOI: https://doi.org/10.22627/2072-8107-2018-17-2-6-14 EDN: https://elibrary.ru/xrftet
- Львов Д.К. Рождение и развитие вирусологии — история изучения новых и возвращающихся вирусных инфекций. Вопросы вирусологии. 2012;(S1):5–20. Lvov D.K. Birth and development of virology — the history of emerging-reemerging viral infection investigation. Problems of Virology. 2012;(S1):5–20. EDN: https://elibrary.ru/qjanrj
- Железникова Г.Ф. Инфекция и иммунитет: стратегии обеих сторон. Медицинская иммунология. 2006;8(5–6):597–614. Zheleznikova G.F. Infection and immunity: strategies from both sides. Medical Immunology. 2006;8(5–6):597–614. DOI: https://doi.org/10.15789/1563-0625-2006-5-6-597-614 EDN: https://elibrary.ru/iixjnx
- Носуля Е.В. Острая респираторно-вирусная инфекция — сложности диагностики и лечения. Медицинский совет. 2013;(3–2):20–6. Nosulya E.V. Acute respiratory viral infection: challenges of diagnosis and treatment. Medical Council. 2013;(3–2):20–6. EDN: https://elibrary.ru/rveqhj
- Лазарева Н.Б., Журавлева М.В., Пантелеева Л.Р. ОРВИ: рациональная фармакотерапия с позиции клинической фармакологии. Медицинский Совет. 2016;(4):68–73. Lazareva N.B., Zhuravleva M.V., Panteleeva L.R. ARVI: Rational pharmacotherapy from the standpoint of clinical pharmacology. Medical Council. 2016;(4):68–73. DOI: https://doi.org/10.21518/2079-701X-2016-4-68-73 EDN: https://elibrary.ru/rjoikd
- Avolio M., Venturini S., De Rosa R., et al. Epidemiology of respiratory virus before and during COVID-19 pandemic. Infez. Med. 2022;30(1):104–8. DOI: https://doi.org/10.53854/liim-3001-12
- Реброва О.Ю. Статистический анализ медицинских данных. Применение пакета прикладных программ STATISTICA. М.; 2000. Rebrova O.Yu. Statistical Analysis of Medical Data. Application of the Package of Applied Programs STATISTICA. Moscow; 2000.
- Жигарловский Б.А. Эпидемиологическая характеристика и оптимизация эпидемиологического надзора за гриппом и ОРВИ: Автореф. дисс. … канд. мед. наук. М.; 2019. Zhigarlovsky B.A. Epidemiological characteristics and optimization of epidemiological surveillance of influenza and SARS: Diss. Moscow; 2019.
- Писарева М.М., Едер В.А., Бузицкая Ж.В. и др. Этиологическая структура гриппа и других ОРВИ в Санкт-Петербурге в эпидемические сезоны 2012–2016 гг. Вопросы вирусологии. 2018;63(5):233–9. Pisareva M.M., Eder V.A., Buzitskaya Zh.V., et al. Etiological structure of influenza and other ARVI in St. Petersburg during epidemic seasons 2012–2016. Problems of Virology. 2018;63(5):233–9. DOI: http://doi.org/10.18821/0507-4088-2018-63-5-233-239 EDN: https://elibrary.ru/sllvnj
- Horemheb-Rubio G., Eggeling R., Schmeiβer N., et al. Respiratory viruses dynamics and interactions: ten years of surveillance in Central Europe. BMC Public Health. 2022;22(1):1167. DOI: https://doi.org/10.1186/s12889-022-13555-5
- Готвянская Т.П., Мукашева Е.А., Ноздрачева А.В. и др. Заболеваемость и популяционный иммунитет к гриппу и ОРВИ в условиях пандемии COVID–19. Санитарный врач. 2023;(3):153–63. Gotvyanskaya T.P., Mukasheva E.A., Nozdracheva A.V., et al. Incidence and population immunity to influenza and ARVI in the context of the COVID-19 pandemic. Sanitary Doctor. 2023;(3):153–63. DOI: https://doi.org/10.33920/med-08-2303-03
- Заплатников А.Л., Гирина А.А., Бурцева Е.И., Свинцицкая В.И. Вакцинация против сезонного гриппа и пандемия COVID-19: не упустить последнюю возможность. Медицинское обозрение. 2020;4(11):687–90. Zaplatnikov A.L., Garina A.A., Burtseva E.I., Svinitskaya V.I. Seasonal flu shot and the COVID-19 pandemic: don’t miss the last chance. Russian Medical Inquiry. 2020;4(11):687–90. DOI: https://doi.org/10.32364/2587-6821-2020-4-11-687-690 EDN: https://elibrary.ru/ybtpty
- Кривицкая В.З., Кузнецова Е.В., Майорова В.Г. и др. Влияние вакцинации против гриппа на уровень специфического гуморального иммунитета здоровых лиц. Инфекция и иммунитет. 2022;12(1):127–41. Krivitskaya V.Z., Kuznecova E.V., Maiorova V.G., et al. Influenza vaccination influencing level of specific humoral immunity in healthy individuals. Russian Journal of Infection and Immunity. 2022;12(1):127–41. DOI: https://doi.org/10.15789/2220-7619-IVI-1750 EDN: https://elibrary.ru/kkhkqd
- Львов Д.К., ред. Вирусы и вирусные инфекции человека и животных. М.; 2013. Lvov D.K., ed. Viruses and Viral Infections of Humans and Animals. Moscow; 2013.
- Hamamoto I., Shimasaki N. The importance of monitoring viral respiratory infections during the COVID-19 crisis. J. Disaster Res. 2022;17(1):73–81. DOI: https://doi.org/10.20965/jdr.2022.p0073
- Яцышина С.Б., Мамошина М.В., Елькина М.А. и др. Распространённость возбудителей ОРВИ, гриппа и COVID-19 у лиц без симптомов респираторной инфекции. Журнал микробиологии, эпидемиологии и иммунобиологии. 2021;98(4):383–96. Yatsyshina S.B., Mamoshina M.V., Elkina M.A., et al. Prevalence of ARVI, Influenza, and COVID-19 pathogens in individuals without symptoms of respiratory infection. Journal of Microbiology, Epidemiology and Immunobiology. 2021;98(4):383–96. DOI: https://doi.org/10.36233/0372-9311–152 EDN: https://elibrary.ru/skidig
- Мамошина М.В., Яцышина С.Б., Акимкин В.Г. Анализ результатов мониторинга возбудителей ОРВИ, гриппа и COVID-19 у бессимптомных лиц. Эпидемиология и инфекционные болезни. Актуальные вопросы. 2023;(2):63–9. Mamoshina M.V., Yatsyshina S.B., Akimkin V.G. Analysis of the results of monitoring the pathogens of acute respiratory viral infection, influenza, and COVID-19 in asymptomatic individuals. Epidemiology and Infectious Diseases. Current Items. 2023;(2):63–9. DOI: https://doi.org/10.18565/epidem.2023.13.2.63-9
- Gradisteanu Pircalabioru G., Iliescu F.S., Mihaescu G., et al. Advances in the rapid diagnostic of viral respiratory tract infections. Front. Cell Infect. Microbiol. 2022;12:807253. DOI: https://doi.org/10.3389/fcimb.2022.807253
- Гирина А.А., Заплатников А.Л., Бурцева Е.И. и др. Иммунопрофилактика гриппа, острых и рекуррентных респираторных инфекций в период пандемии COVID-19. Медицинский Совет. 2021;(17):111–20. Girina A.A., Zaplatnikov A.L., Burtseva E.I., et al. Immunoprophylaxis of influenza, acute and recurrent respiratory infections during the COVID-19 pandemic. Medical Council. 2021;(17):111–20. DOI: https://doi.org/10.21518/2079-701X-2021-17-111-120 EDN: https://elibrary.ru/tqaabm
- Fischer H., Tschachler E., Eckhart L. Pangolins lack IFIH1/MDA5, a cytoplasmic RNA sensor that initiates innate immune defense upon coronavirus infection. Front. Immunol. 2020;11:939. DOI: https://doi.org/10.3389/fimmu.2020.00939
- Bakhvalova V.N., Panov V.V., Morozova O.V. Tick-Borne encephalitis virus quasispecies rearrangements in ticks and mammals. In: Růžek D., ed. Flavivirus Encephalitis. InTech; 2011. DOI: https://doi.org/10.5772/20744
- Yadana S., Coleman K.K., Nguyen T.T., et al. Monitoring for airborne respiratory viruses in a general pediatric ward in Singapore. J. Public Health Res. 2019;8(3):1407. DOI: https://doi.org/10.4081/jphr.2019.1407
Supplementary files