Phylogenetic position and genetic features of HIV-1 in CNS
- Authors: Piterskiy M.V.1, Khodakov O.A.1, Mikheeva T.V.2, Bilalova N.V.1, Konkova-Reidman A.B.2, Zakharova Y.A.3, Semenov A.V.1
-
Affiliations:
- Federal Scientific Research Institute of Viral Infections "Virome"
- South Ural State Medical University
- F.F. Erisman Federal Scientific Centre of Hygiene
- Issue: Vol 101, No 1 (2024)
- Pages: 100-113
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/16596
- DOI: https://doi.org/10.36233/0372-9311-442
- EDN: https://elibrary.ru/wofnfq
- ID: 16596
Cite item
Abstract
Background. Due to the wide coverage with antiretroviral therapy, the life expectancy of HIV infected people has significantly increased. Against the background of a decrease in mortality from HIV infection, HIV-associated neurocognitive disorders, which develop even during effective treatment, are of high importance. The overall prevalence of this pathology among HIV-infected people reaches 42.6%.
The objective of the study was to research the genetic features and phylogenetic position of HIV-1 persisting in the central nervous system.
Materials and methods. The clinical study group consisted of 38 patients with severe neurocognitive disorders against the background of HIV infection in stage 4B. The viral load of HIV-1 in blood plasma and cerebrospinal fluid (CSF) was measured using the "AmpliSens HIV Monitor-FRT" reagents kit. Sanger sequencing was performed using the AmpliSens HIV-Resist-Seq assay kit on an Applied Biosystems 3500 analyzer. Phylogenetic analysis of the pol gene fragments of HIV-1 strains (the site encoding the viral protease and part of the reverse transcriptase) was carried out using maximum likelihood method with the GTR+G nucleotide substitution model. Comparisons of the tertiary structure of viral proteins were performed according to three-dimensional models of the protease and p51 and p66 reverse transcriptase subunits obtained by homologous reconstruction using the SWISS-MODEL tools.
Results. The viral load in the sample of patients with severe CNS lesions in blood plasma was 6.27 times higher than in CSF and amounted to 4.67 and 3.87 lg copies/ml respectively by median (p = 0,004).
Phylogenetic analysis with the use of all available HIV-1 genomes from GenBank, which differed from the studied ones by less than 5% showed close genetic relations of viruses circulating in Chelyabinsk region, apart from strains circulating in Russian Federation, with viruses circulating in neighboring countries, in most abundance — from Ukraine and Kyrgyzstan, slightly less — from Belarus, Tajikistan, Kazakhstan and Armenia and also with strains from certain foreign countries: Poland and Germany. Phylogenetic analysis of 38 HIV-1 genomes revealed significant genetic distances between HIV isolates from blood plasma and CSF in 5 patients, 4 of whom were PWID, which may indicate an event of superinfection.
The amount of independent amino acid substitutions in protease in isolates from blood plasma ranged from 1 to 3, in isolates from CSF — from 1 to 2. An amount of such substitutions in a fragment of reverse transcriptase in isolates from blood plasma ranged from 1 to 6, while in isolates from CSF, it ranged from 1 to 7. HIV isolates from blood plasma and CSF from 5 patients had differences in the tertiary structure of HIV-1 reverse transcriptase p51 subunit in amino acid positions 16–20 and 210–235. Isolates from 3 other patients differed in the tertiary structure only in amino acid positons 210–235. Isolates from 3 patients differed in the structure of HIV-1 RT p66 subunit in a non-nucleoside reverse transcriptase inhibitor binding pocket (NNRTI) region. Fixed differences in the tertiary structure of p51 subunit required at minimum only 1 amino acid substitution to emerge. Alterations in the tertiary structure of p66 subunit required at least 3 amino acid substitutions.
Conclusion. Microevolution of HIV-1 proceeds in parallel within the same patient, in different compartments, which is reflected in the accumulation of amino acid substitutions different from another compartment in the conserved pol gene. There is a weak correlation between the viral load level in plasma and in CSF. The genetic heterogeneity of HIV strains from patients of the Chelyabinsk region indicates a high frequency of reintroduction of HIV infection in the region from other countries. Differences in the tertiary structure of HIV-1 reverse transcriptase between blood plasma and CSF isolates are regularly fixed in certain domens, which also confirms the presence of parallel HIV microevolution during virus persistence in tissues separated by the blood-brain barrier which allows a better understanding of the fixation trends of individual amino acid substitutions during HIV-induced damage to central nervous system.
Full Text
Introduction
HIV-associated neurocognitive disorders are common among people living with HIV/AIDS despite effective antiretroviral therapy (ART). Even when the virus replication is effectively suppressed in the bloodstream, it may still be observed in the cerebrospinal fluid (CSF) of certain patients. This is because the permeability of the blood-brain barrier (BBB) to different antiretroviral drugs varies considerably, and the concentration of certain drugs in the CSF does not reach the concentrations necessary to effectively suppress HIV replication [1, 2]. Detection of HIV infection at late stages results in patients who already have clinical symptoms of central nervous system (CNS) lesions due to the damage caused by HIV replication in the CNS prior to starting ART [3–5].
It is known that HIV, in addition to affecting cells of the immune system, can penetrate into the CNS, which leads to various complications, including HIV-associated neurocognitive disorder (HAND).
It is divided into three stages according to the severity of symptoms:
- asymptomatic neurocognitive disorder;
- mild neurocognitive disorder;
- HIV-associated dementia [6].
The 2020 Global Meta Study found that the overall prevalence of HAND worldwide is 42.6% and varies by region. South Africa has the highest prevalence of HAND (as well as the highest number of HIV-positive individuals) and accounts for about 72% of all cases globally. About 88% of all HAND cases are usually in milder forms (asymptomatic or mild neurocognitive disorder), while HIV-associated dementia is quite rare [7, 8].
HIV penetration into the CNS occurs approximately 4–8th day after infection, when a person is usually not yet diagnosed with HIV infection [9]. The virus passes through the BBB with the help of infected monocytes and T lymphocytes [10], [11]. HIV provirus-containing CD14+ and CD16+ monocytes have been observed to pass through the BBB more actively than similar uninfected cells. The adhesive molecules JAM-A (junctional adhesion molecule A, or CD321) and ALCAM (activated leukocyte cell adhesion molecule, or CD166) play an important role in this process. By interacting with microvascular endothelial cells of the BBB, they allow monocytes to penetrate the barrier. Their expression is significantly increased in infected CD14+ and CD16+ monocytes. Furthermore, the number of chemokines in the CNS, in particular CCL2, increases, and the number of receptors to this chemokine increases on the surface of infected CD14+- and CD16+-monocytes [12, 13].
Upon entry into the CNS, infected monocytes may differentiate into perivascular macrophages, which will form a stable reservoir for the virus and will release viral particles for an extended period of time, infecting other CNS cells, such as macrophages, astrocytes and microglia, each of which may also act as a reservoir for the virus, even under conditions of prolonged administration of antiretroviral drugs [14]. HIV-infected cells in the nervous system produce viral proteins such as Tat and Nef, resulting in chronic low-level inflammation. And this inflammation persists even when antiretroviral drugs are administered [15, 16]. The first known longitudinal study of patients with HIV aged 50 years and older found that taking ART and regular check-ups cannot prevent the development of HAND [17].
A number of studies have found that the evolution of HIV-1 into the CNS parallels the evolution of the virus remaining outside the CNS [18–20]. For example, the env gene proteins of strains from CSF acquire specific forms that interact with CD4 and the N-terminus of CCR5 in tandem, allowing more efficient virus entry into macrophages located in the CNS that express low amounts of CD4 [21]. Genetic differences between HIV-1 populations isolated from plasma and CSF are present in other genes such as tat, nef, and pol [22–24]. Mutations associated with drug resistance can occur in both plasma and CSF strains, and in certain cases drug resistance mutations may be present in CSF strains and absent in plasma strains of patients [25].
The replication-capable HIV reservoir represents a genetically restricted and generally "younger" subset of the overall pool [26]. The stability of the HIV genome is closely related to the structural features of HIV reverse transcriptase, which, by making errors in HIV DNA synthesis, is the cause of mutations [27].
HIV-1 reverse transcriptase is an asymmetric heterodimer consisting of p51 (440 amino acids long) and p66 (560 amino acids long) subunits. Each subunit contains subdomains such as fingers (amino acids 1–85, 118–155), palm (amino acids 86–117, 156–236), thumb (amino acids 237–318), and a connecting subdomain (amino acids 319–446). The p66 subunit also includes a subdomain connecting it to RNase H (amino acids 427–560). The catalytically active center is formed by amino acids 110, 185, and 186. These two subunits have different spatial organization, and only the p66 subunit is catalytically active, while p51 plays only a structural role [28].
The study of differences in the three-dimensional structure of protease and reverse transcriptase of HIV strains separated by the BBB is of interest to reveal the direction of adaptive changes of the virus associated with the presence in a certain compartment.
Materials and methods
Between January 2018 and March 2022, 38 HIV-infected patients from the Chelyabinsk region with neurocognitive disorders and severe immunodeficiency were examined.
The patients' age averaged 39 years (mean square deviation σ = 7, Shapiro–Wilk coefficient W = 0.917 at p = 0.008). Among the subjects, 20 were men (52.63%; 95% confidence interval (CI) 37.3–67.5) and 18 were women (47.37%, 95% CI 32.5–62.7). All patients were in stage 4B HIV infection. The study was conducted with the informed consent of the patients. The study was approved by the local Ethics Committee of the Federal Scientific Research Institute of Viral Infections "Virome" (protocol No. 2, May 26, 2022) and by the local Ethics Committee of the Yekaterinburg Research Institute of Viral Infections (protocol No. 3, June 17, 2016).
CD4-cell counts were measured using a BD FACSCanto II flow cytometer and BD Tritest CD4/CD8/CD3 reagent kit (Becton Dickinson). Viral load level was determined using the AmpliSense HIV-Monitor-FRT reagent kit (CRIE Rospotrebnadzor), sequencing — using the AmpliSense HIV-Resist-Seq reagent kit (CRIE Rospotrebnadzor). Electrophoregrams were processed and consensus sequences were obtained using Deona 1.7.0 software (RMBit Company).
A total of 57 nucleotide sequences of the pol HIV-1 gene were obtained, including paired samples from blood plasma and CSF from 19 patients, HIV RNA in sufficient quantity for sequencing was isolated either from blood plasma or CSF of 19 other patients. All nucleotide sequences have been deposited in the international GenBank database (Accession numbers: OR260480-OR260536).
With respect to the nucleotide sequences under study, we searched for the closest related HIV genomes using the NCBI BLAST service [29]. A data set comprising HIV genomes with 95% or higher sequence similarity to the original sequences was formed (n = 2929). Multiple alignment was performed using the ClustalOmega algorithm on the EMBL online service [30]. Subtyping, detection of drug resistance mutations and other mutations were performed using the Stanford University HIV drug resistance database (HIVdb Program: Mutations Analysis, program version 3.4.3; algorithm version 9.4) [31].
For phylogenetic analysis, the selection of the most appropriate nucleotide substitution model for the available data was performed using the FindModel online service [32]. Phylogenetic trees were constructed using the maximum likelihood method with the GTR+G (General Time Reversible + gamma) nucleotide substitution model using the "MEGA X" software [33]. 500 bootstrap replications were used as a measure of statistical support.
For phylogenetic analysis of paired genome samples of HIV strains isolated from blood plasma and CSF of 19 patients, HIV genomes representing different subtypes were added as a reference sequences: A6 (EU861977, KU749403, KT983615, JX500694), B (JX500708) and CRF63_02A6 (JN230353). The above strains are the reference strains for subtypes and circulating recombinant HIV strains according to Los Alamos HIV databases [34].
Statistical processing of data was carried out using the Statistica v. 12 software product (StatSoft Russia). Confidence intervals were calculated using Wilson's method for the level of type 1 error 0.05 [35]. Nonparametric statistics criteria (χ2, Fisher's exact test, Mann–Whitney test) were used to confirm statistically significant difference.
Three-dimensional models of viral proteins (protease and reverse transcriptase) were obtained by homology construction method. For the protease, the crystal structure model of HIV-1 protease, subtype A (PDB ID: 3ixo) was used. This model was chosen because it had the highest similarity to our amino acid sequences among HIV-1 protease models that were not associated with inhibitors and did not have drug resistance mutations, and it also belonged to the closest subtype A.
For reverse transcriptase, the p51 subunit model of HIV-1 reverse transcriptase (PDB ID: 3kjv) and the HIV-1 reverse transcriptase/RNase H model (PDB ID: 4icl) were used. These models have the highest primary structure similarity to our sequences among models that are not in complex with inhibitors and have no drug resistance mutations.
Three-dimensional protein models were built and their structures were compared using the SWISS- MODEL online service [36, 37].
Results and discussion
Based on patient history, the duration of HIV infection from diagnosis to hospitalization was found to be 81 months on average (σ = 64; W = 0.924; p = 0.01). The majority of patients, 30 of 38 (79.0%; 95% CI 63.7–88.9), had no history of ART, and the remainder were on therapy for a median of up to 29 months (interquartile range (IQR) 9–35 months), but with low adherence, self-interrupting prescribed courses of treatment. Given that all patients were hospitalized at stage 4B of HIV, their infection occurred long before diagnosis. Thus, it was the long-term course of HIV infection without ART that caused CNS damage.
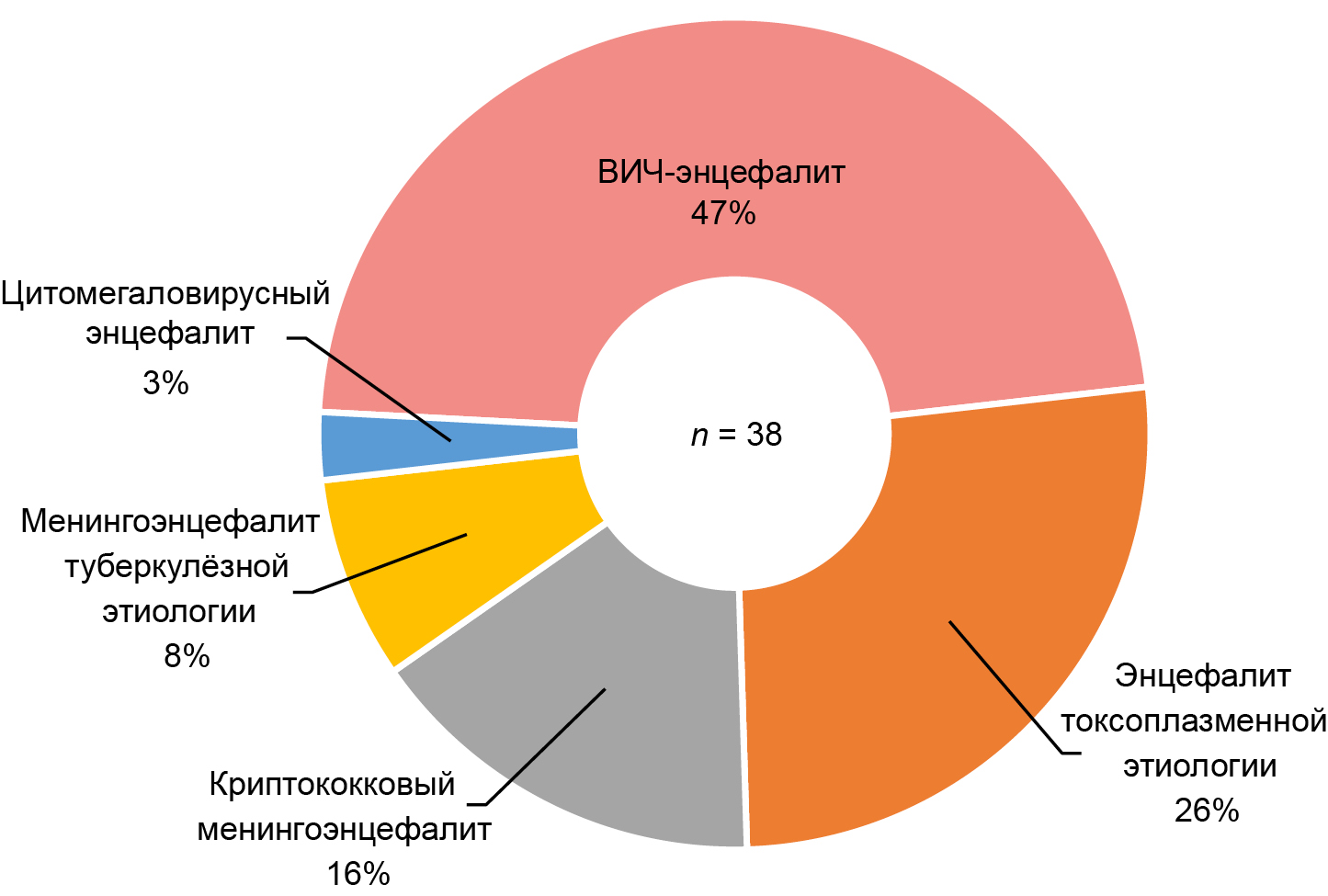
Among the patients studied (n = 38), the most common brain lesions were meningoencephalitis and encephalitis associated with opportunistic infections and tuberculosis, 20 out of 38 cases (52.6%; 95% CI 37.3–67.5), among which encephalitis of toxoplasma etiology predominated, 10 out of 20 cases (50.0%; 95% CI 29.9–70.1). HIV encephalitis was identified in 18 of 38 cases (47.4%; 95% CI 32.5–62.7; Figure 1).
Fig. 1. Structure of brain lesions among the examined patients.
In this study, the groups of patients with brain lesions caused by opportunistic infections and the group of patients with HIV encephalitis had no statistically significant differences in terms of ART experience, sex, age and other indicators, which is possibly due to the small sample size. Previous studies have shown that the spectrum of neurological disorders in patients with HIV infection depends significantly on the availability of therapy and its timely initiation. In countries where ART is widely available, neurological symptoms in patients with HIV infection are often due to HIV encephalitis. On the other hand, in developing countries where access to HIV treatment still needs to be improved, neurological deterioration is often associated with opportunistic CNS infections such as toxoplasmosis and cryptococcosis [38].
Low CD4 count and high viral load are key factors determining the development of HIV encephalitis [39]. The level of HIV viral load in plasma was at a median of 4.67 (ICI 4.19–5.40) lg copies/mL and was statistically significantly higher than that in CSF, at a median of 3.87 (ICI 2.73–4.66) lg copies/mL, by 0.8 lg, or 6.27 times (U = 442; z = 2.904; p = 0.004).
The CD4-lymphocyte content in plasma was at a median of 41.0 (ICI 21.3–66.5) lg copies/mL, with no correlation with the level of viral load (Fig. 2).
Fig. 2. HIV viral load in blood plasma and cerebrospinal fluid and an amount of CD4 cells among the examined patients (n = 38). The dotted line indicates the median.
The higher HIV viral load in CSF compared to plasma is quite common among patients not on effective ART and among ART-naïve patients, which was also shown in a cross-sectional multicenter study conducted in large European cities between 1982 and 2017 [40]. In this study, the difference in viral load level was 1.0 lg copies/mL, and there was a correlation between these values in plasma and CSF. In our study, there was a weak direct correlation between HIV viral load levels in plasma and CSF: Spearman coefficient Rs = 0.38, p = 0.019.
Among 57 genotyped samples from 38 patients, 54 representatives of subtype A6 were identified (94.7%; 95% CI 85.6–98.2), 2 samples from 1 patient contained recombinant strain CRF63_02A6 (3.5%; 95% CI 1.0–11.9) and 1 strain isolated from plasma contained subtype B (1.8%; 95% CI 0.3–9.3), whereas a strain isolated from the same patient's CSF belonged to HIV-1 subtype A6.
To determine the phylogenetic position of the studied strains, HIV genomes with 95% or higher similarity were found in each of the 57 sequences using the NCBI BLAST service. After excluding repetitive records, synthetic sequences, and records in which the country of origin of the strain could not be determined, 2872 nucleotide sequences remained, with a median identity of 95.97% (ICI 95.52–96.51%). Together with the studied samples, the genotypic structure was represented by 2508 strains of subtype A6 (85.5%; 95% CI 84.1–86.7), 397 CRF63_02A6 (13.8%; 95% CI 12.6–15.1), 14 subtype B (0.5%; 95% CI 0.3–0.8), and 7 CRF02_A6G (0.2%; 95% CI 0.1–0.5) among the 2929 strains analyzed.
Phylogenetic analysis of 2929 HIV-1 pol gene fragments encoding a protease and part of a reverse transcriptase was performed by maximum likelihood analysis using the GTR+G nucleotide substitution model and 500 bootstrap replications. As a result, 26 clusters were formed, 11 of which included 57 HIV strains isolated from the studied patients (Fig. 3).
Fig. 3. Maxim-likehood phylogenetic tree derived from 2929 HIV-1 pol gene (fragment encoding viral protease and part of reverse transcriptase) sequences which had 95% or more identity with studied ones.
Strains from patients in the study sample were included in 11 clusters. Among strains from patients from near abroad countries, strains circulating in Ukraine and Kyrgyzstan (6 out of 11 clusters each), Belarus, Tajikistan, Kazakhstan, Armenia (5 out of 11 clusters), and Poland and Germany (5 out of 11 clusters) were the most frequently found in clusters with the samples under study.
Among the 57 HIV strains isolated from patients in the study sample, 11 (19.3%; 95% CI 11.1–31.3) formed a separate cluster #3, where they accounted for 73.3% (95% CI 48–89.1). These strains were isolated from 8 patients, including 6 women and 1 man from Chelyabinsk and 1 woman from the city of Satka, Chelyabinsk region. Of the 8 patients, only 2 were infected by intravenous drug use, and the rest were sexually infected.
Most related strains originated from Russia (75.4%; 95% CI 73.8–76.9). Their proportion varied from cluster to cluster from 24% (cluster #23) to 100% (cluster #15). Strains originating from foreign countries were of greatest interest.
The largest cluster on the phylogenetic tree, cluster #13, containing 873 HIV genomes (29.8%; 95% CI 28.2–31.5), contained 16 strains from patients in the study group, as well as strains from patients of 19 foreign countries. The highest proportions of the 208 HIV strains from foreign countries in this cluster were strains circulating in Kyrgyzstan (29.3%), Belarus (23.6%), and Tajikistan (12.0%).
Phylogenetic analysis of paired samples from blood plasma and CSF from 19 patients with references of subtype A6, subtype B and CRF63_02A6 showed that in 5 patients, sequences from plasma and CSF fell into different clusters and in one of them, HIV strains belonged to different subtypes: subtype B in plasma (ID159, AN: OR260493), and subtype A6 in CSF (ID160, AN: OR260494) (Fig. 4).
Fig. 4. Phylogenetic tree of 38 HIV-1 pol gene sequences from 19 patients obtained in pairs from blood plasma and CSF. Ovals of the same color indicate paired samples that have fallen into different clusters. Red branches and clusters contain HIV-1 subtype A6 sequences.
Of the 5 patients with genetic heterogeneity of HIV genomes from different compartments, 4 were people who inject drugs (PWID). It should be noted that minimal differences in the genome of HIV strains from CSF and plasma were observed in the patient who was not a PWID. On a phylogenetic tree with 2929 HIV genomes, strains from these patients also appeared in different clusters. Significant differences in the genome of HIV persisting on different sides of the BBB may indirectly indicate superinfection of the patient, with a later-infected strain isolated from blood plasma.
To analyze amino acid substitutions and the tertiary structure of HIV protease and reverse transcriptase, strains were selected from paired samples from 13 patients out of 19. Five patients with high strain heterogeneity and suspected superinfection and one patient with identical amino acid composition of protease and reverse transcriptase in plasma and CSF strains were excluded from the comparison.
Comparative analysis of the detected amino acid substitutions in protease and reverse transcriptase of strains from plasma and CSF of 13 patients allowed us to identify independently arisen mutations that were formed and fixed in the process of microevolution of HIV strains separated by the BBB. Taking into account the extremely severe condition of the patients, in certain cases the maximum possible changes in the genome could be observed, which had time to occur during the course of HIV infection in one person without ART.
In the protease of HIV strains isolated from plasma, only 5 out of 13 samples showed amino acid substitutions that occurred independently (present only in strains from one compartment and, therefore, not inherited by a strain persisting in the CNS) from strains isolated from CSF, with the number of amino acid substitutions ranging from 1 to 3. In the strains isolated from CSF, independent amino acid substitutions were also observed in 5 of the 13 samples, and the number of amino acid substitutions ranged from 1 to 2.
In reverse transcriptase strains isolated from plasma, amino acid substitutions that occurred independently of strains isolated from CSF were detected in 10 of 13 samples, with the number of amino acid substitutions ranging from 1 to 6. In 12 samples from CSF, the number of independently occurring amino acid substitutions ranged from 2 to 7.
Comparison of the tertiary structure of proteins based on a model of the p51 subunit of HIV reverse transcriptase revealed 2 highly co-variable regions with differences between strains from plasma and those from CSF. In strains from plasma and CSF of 5 patients, the HIV-1 reverse transcriptase structures matched completely.
In strains from plasma and from CSF from the other 5 patients, differences in the reverse transcriptase structure were present at amino acid positions 16–20, which corresponds to the beginning of the fingers subdomain (Fig. 5).
Fig. 5. Difference in the tertiary structure of HIV-1 reverse transcriptase p51 subunit between strains from blood plasma and from CSF. Amino acid positions 16–20 are framed. Strain from blood plasma is shown in cyan, from CSF — in light brown. Amino acid position 18 is highlighted in red.
At the end of the palm subdomain at positions 210–235, there also appeared to be a variable region where differences between strains of both plasma and CSF were detected in 8 pairs of images, including 5 pairs that exhibited a difference in structure at positions 16–20 (Fig. 6).
Fig. 6. Difference in the tertiary structure of HIV-1 reverse transcriptase p51 subunit between strains from blood plasma and from CSF. Amino acid positions 210–235 are framed. Strain from blood plasma is shown in cyan, from CSF — in light brown. Amino acid position 220 is highlighted in red.
Other studies have observed that a site in the region of amino acid positions 219–230, which forms a disordered loop in the p51 subunit of HIV-1 reverse transcriptase, plays an important role in the dimerization process of the 2 subunits of the enzyme [41].
When comparing the tertiary structure of the p66 subunit of HIV-1 reverse transcriptase in 10 out of 14 patients, the structures of the enzyme in plasma and CSF strains coincided completely, while in another 3 patients there were regular repeated differences in amino acid positions 187–190 (immediately after catalytically active positions 185, 186) (Fig. 7).
Fig. 7. Difference in the tertiary structure of HIV-1 reverse transcriptase p66 subunit between strains from blood plasma and from CSF. Amino acid positions 187–190 are framed. Strain from blood plasma is shown in cyan, from CSF — in light brown. Amino acid position 187 is highlighted in red.
This region is included in the binding site of NNRTIs to the enzyme [42].
As a result of even single amino acid substitutions in the microevolution process, the tertiary structure of reverse transcriptase changed, adapting to specific conditions of functioning. Such adaptation occurred independently in different compartments separated by the BBB. At the same time, only those amino acid substitutions that are most optimal for the functioning environment of each HIV strain became fixed. Analysis of amino acid substitutions in the p51 and p66 subunits of reverse transcriptase demonstrated their relationship to differences in tertiary structure (Table).
Amino acid substitutions, associated with HIV-1 reverse transcriptase (RT) tertiary structure alterations in strains from CSF and blood plasma
Patient ID | Accession number | Locus | Amino acid substitutions relative to HXB2 reference sequence | Differences in the tertiary structure of RT p51 subunit in positions 16–20 | Differences in the tertiary structure of RT p51 subunit in positions 210–235 | Differences in the tertiary structure of RT p66 subunit in positions 187–190 |
p8 | OR260486 | Blood plasma | T69S, Q242K | + | + | |
OR260531 | CSF | E6D, K20E, E28K | ||||
p11 | OR260523 | Blood plasma | T39D | + | + | |
OR260482 | CSF | T39N | ||||
p26 | OR260512 | Blood plasma | _ | + | + | |
OR260533 | CSF | E28K, K64R | ||||
p27 | OR260521 | Blood plasma | K11A, T39K, V60I | + | + | |
OR260480 | CSF | K11T, E36D, T39E, K64R | ||||
p53 | OR260516 | Blood plasma | _ | + | ||
OR260535 | CSF | K64R, A158S | ||||
p59 | OR260517 | Blood plasma | V35K, T39K, I47M | + | + | |
OR260528 | CSF | T39R, K64R, D67N, T200A | ||||
p95 | OR260507 | Blood plasma | E40D, D86N, L214F | + | + | + |
OR260508 | CSF | _ | ||||
p96 | OR260509 | Blood plasma | V35T, T39M, S162H | + | ||
OR260510 | CSF | V35I, T39K, V60I, S162Y | ||||
p46 | OR260513 | Blood plasma | V35K, F116Y | + | ||
OR260534 | CSF | T27P, V35T, S162C, D177N |
The tertiary structure of the p51 subunit of HIV-1 reverse transcriptase turned out to be the most variable, with at least 2 evolutionary events related to amino acid substitutions, 1 in each strain, required for the emergence of conformational differences between proteins from blood plasma and CSF strains. The p51 subunit is catalytically inactive and plays only a structural role in the reverse transcription complex. Similar amino acid substitutions in the p66 subunit of the reverse transcriptase, which does have catalytic functions, did not result in changes in the tertiary structure. In 3 cases, differences in the tertiary structure of the p66 subunit were present but were minimal and covered a region 3 amino acids in length. Differences in the tertiary structure of the p66 reverse transcriptase subunit were associated with at least 3 evolutionary events in one of the strains or with 5 events in strains from CSF and plasma.
Conclusion
A statistically significant weak correlation between the HIV viral load level in plasma and CSF was revealed. The HIV viral load in blood plasma exceeded the values in CSF by 0.8 lg, or 6.3 times.
Phylogenetic analysis of the pol HIV-1 gene fragment encoding protease and part of reverse transcriptase demonstrates a high degree of heterogeneity, with part of the genome clustering with closely related strains circulating both in the near abroad countries of Ukraine and Kyrgyzstan, and in Central Europe: Poland and Germany. This may indicate a high frequency of HIV infections entering the region from abroad.
Comparison of HIV-1 pol gene regions (encoding protease and part of reverse transcriptase) between strains from blood plasma and from CSF revealed significant genetic distances between HIV-1 genomes in strains from 5 patients (in 1 case strains belonged to different subtypes — A6 in plasma and B in CSF).
The number of independent amino acid substitutions in the site encoding the viral protease ranged from 1 to 3 in plasma strains and from 1 to 2 in CSF strains. The number of amino acid substitutions in the site encoding the HIV-1 reverse transcriptase fragment ranged from 1 to 6 in blood plasma strains and from 1 to 7 in CSF strains.
Highly co-variable regions in the structure of the p51 subunit of HIV-1 reverse transcriptase were found at amino acid positions 16–20 and 210–235. In 5 patients, the structure of the enzyme in strains from blood plasma and CSF differed in both positions, and in 4 patients — only in positions 210–235. In 3 patients, the tertiary structure of the p66 subunit of HIV-1 reverse transcriptase differed in amino acid positions 187-190. This region is part of the NNRTI binding site. A much greater difference in the tertiary structure was observed in the p51 subunit, which is known to have no catalytic activity but plays an important structural role in the formation of the reverse transcription complex.
No differences were observed in the tertiary structure of the viral protease between strains from plasma and those from CSF. Amino acid substitutions in HIV-1 reverse transcriptase that lead to changes in the tertiary structure of one subunit will not necessarily result in changes in the other.
The described observations confirm the presence of a virus microevolution process, manifested by changes in both primary and tertiary structures of HIV-1 reverse transcriptase, proceeding in parallel and independently in the organism of one patient in different compartments separated by the BBB.
About the authors
Mikhail V. Piterskiy
Federal Scientific Research Institute of Viral Infections "Virome"
Author for correspondence.
Email: piterskiy_mv@niivirom.ru
ORCID iD: 0000-0001-5506-2389
SPIN-code: 8756-9549
Scopus Author ID: 57215819806
researcher, Ural Federal District AIDS Centre, Federal Scientific Research Institute of Viral Infections "Virome"
Russian Federation, EkaterinburgOleg A. Khodakov
Federal Scientific Research Institute of Viral Infections "Virome"
Email: hodakov_oa@niivirom.ru
ORCID iD: 0000-0003-1493-6080
SPIN-code: 4238-4150
junior researcher, Ural Federal District AIDS Centre, Federal Scientific Research Institute of Viral Infections "Virome"
Russian Federation, EkaterinburgTatyana V. Mikheeva
South Ural State Medical University
Email: micheeva74@mail.ru
ORCID iD: 0000-0003-3371-9495
Head, 1st Infectious diseases department, Clinic of the South Ural State Medical University
Russian Federation, ChelyabinskNatalia V. Bilalova
Federal Scientific Research Institute of Viral Infections "Virome"
Email: bilalova_nv@niivirom.ru
ORCID iD: 0000-0003-2693-5544
Head, Arbitration laboratory of HIV and opportunistic infections, Ural Federal District AIDS Centre, Federal Scientific Research Institute of Viral Infections "Virome"
Russian Federation, EkaterinburgAlena B. Konkova-Reidman
South Ural State Medical University
Email: konkova-reidman@mail.ru
ORCID iD: 0000-0002-6058-0997
SPIN-code: 2580-8467
D. Sci. (Med.), Professor, Department of Infectious diseases, South Ural State Medical University
Russian Federation, ChelyabinskYuliya A. Zakharova
F.F. Erisman Federal Scientific Centre of Hygiene
Email: piterskiy_mv@niivirom.ru
ORCID iD: 0000-0003-3416-0902
D. Sci. (Med.), Professor, Scientific Director, Institute of Disinfectology, F.F. Erisman Federal Scientific Centre of Hygiene
Russian Federation, MoscowAleksandr V. Semenov
Federal Scientific Research Institute of Viral Infections "Virome"
Email: semenov_av@niivirom.ru
ORCID iD: 0000-0003-3223-8219
SPIN-code: 2372-5134
Scopus Author ID: 56988449300
D. Sci. (Biol.), Director, Federal Scientific Research Institute of Viral Infections "Virome"
Russian Federation, EkaterinburgReferences
- Bleasby K., Castle J.C., Roberts C.J., et al. Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: A resource for investigations into drug disposition. Xenobiotica. 2006;36(10–11):963–88. DOI: https://doi.org/10.1080/00498250600861751
- Ene L., Duiculescu D., Ruta S.M. How much do antiretroviral drugs penetrate into the central nervous system? J. Med. Life. 2011;4(4):432–9.
- Qu Y., Weinstein A., Wang Z., et al. Legacy effect on neuropsychological function in HIV-infected men on combination antiretroviral therapy. AIDS. 2022;36(1):19–27. DOI: https://doi.org/10.1097/QAD.0000000000003071
- Heaton R.K., Ellis R.J., Tang B., et al. Twelve-year neurocognitive decline in HIV is associated with comorbidities, not age: a CHARTER study. Brain. 2023;146(3):1121–31. DOI: https://doi.org/10.1093/brain/awac465
- Ferretti F., Mora-Peris B., Underwood J., et al. Cognitive impairment in a clinical setting. J. Acquir. Immune. Defic. Syndr. 2018;77(1):e10–3. DOI: https://doi.org/10.1097/QAI.0000000000001547
- Antinori A., Arendt G., Becker J.T., et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. DOI: https://doi.org/10.1212/01.WNL.0000287431.88658.8b
- Wang Y., Liu M., Lu Q., et al. Global prevalence and burden of HIV-associated neurocognitive disorder: A meta-analysis. Neurology. 2020;95(19):e2610–21. DOI: https://doi.org/10.1212/WNL.0000000000010752
- Sacktor N. Changing clinical phenotypes of HIV-associated neurocognitive disorders. J. Neurovirol. 2018;24(2):141–5. DOI: https://doi.org/10.1007/s13365-017-0556-6
- Valcour V., Chalermchai T., Sailasuta N., et al. Central nervous system viral invasion and inflammation during acute HIV infection. J. Infect. Dis. 2012;206(2):275–82. DOI: https://doi.org/10.1093/infdis/jis326
- Spudich S., González-Scarano F. HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring. Harb. Perspect. Med. 2012;2(6):a007120. DOI: https://doi.org/10.1101/cshperspect.a007120
- Eugenin E.A., Osiecki K., Lopez L., et al. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J. Neurosci. 2006;26(4):1098–106. DOI: https://doi.org/10.1523/JNEUROSCI.3863-05.2006
- Veenstra M., León-Rivera R., Li M., et al. Mechanisms of CNS viral seeding by HIV+ CD14+ CD16+ monocytes: establishment and reseeding of viral reservoirs contributing to HIV-associated neurocognitive disorders. mBio. 2017;8(5):e01280–17. DOI: https://doi.org/10.1128/mBio.01280-17
- Yuan L., Qiao L., Wei F., et al. Cytokines in CSF correlate with HIV-associated neurocognitive disorders in the post-HAART era in China. J. Neurovirol. 2013;19(2):144–9. DOI: https://doi.org/10.1007/s13365-013-0150-5
- León-Rivera R., Veenstra M., Donoso M., et al. Central Nervous System (CNS) viral seeding by mature monocytes and potential therapies to reduce CNS viral reservoirs in the cART era. mBio. 2021;12(2):e03633–20. DOI: https://doi.org/10.1128/mBio.03633-20
- Lehmann M.H., Lehmann J.M., Erfle V. Nef-induced CCL2 expression contributes to HIV/SIV brain invasion and neuronal dysfunction. Front. Immunol. 2019;10:2447. DOI: https://doi.org/10.3389/fimmu.2019.02447
- Mediouni S., Darque A., Baillat G., et al. Antiretroviral therapy does not block the secretion of the human immunodeficiency virus Tat protein. Infect. Disord. Drug Targets. 2012;12(1):81–6. DOI: https://doi.org/10.2174/187152612798994939
- Paddick S., Holmes S., Ranasinghe S., et al. HIV‐associated neurocognitive disorders (HAND) in a cART‐treated cohort of adults aged 50 and over in Kilimanjaro, Tanzania: The first longitudinal follow‐up study of HAND in an older population in sub‐Saharan Africa: Neuropsychiatry and behavioral neurology/Dementia. Alzheimer’s & Dementia. 2020;16(S6):e041971. DOI: https://doi.org/10.1002/alz.041971
- Bergroth T., Ekici H., Gisslén M., et al. Difference in drug resistance patterns between minor HIV-1 populations in cerebrospinal fluid and plasma. HIV Med. 2009;10(2):111–5. DOI: https://doi.org/10.1111/j.1468-1293.2008.00659.x
- St. Bernard L., Abolade J., Mohri H., Markowitz M., Evering T.H. Drug resistance mutation frequency of single-genome amplification-derived HIV-1 polymerase genomes in the cerebrospinal fluid and plasma of HIV-1-infected individuals under nonsuppressive therapy. J. Virol. 2020;94(20):e01824–19. DOI: https://doi.org/10.1128/JVI.01824-19
- Nightingale S., Geretti A.M., Beloukas A., et al. Discordant CSF/plasma HIV-1 RNA in patients with unexplained low-level viraemia. J. Neurovirol. 2016;22(6):852–60. DOI: https://doi.org/10.1007/s13365-016-0448-1
- Churchill M., Nath A. Where does HIV hide? A focus on the central nervous system. Curr. Opin. HIV AIDS. 2013;8(3): 165–9. DOI: https://doi.org/10.1097/COH.0b013e32835fc601
- Bavaro D.F., Calamo A., Lepore L., et al. Cerebrospinal fluid compartmentalization of HIV-1 and correlation with plasma viral load and blood–brain barrier damage. Infection. 2019;47(3):441–6. DOI: https://doi.org/10.1007/s15010-019-01268-8
- Bednar M.M., Sturdevant C.B., Tompkins L.A., et al. Compartmentalization, viral evolution, and viral latency of HIV in the CNS.: 2. Curr HIV/AIDS Rep. 2015;12(2):262–71. DOI: https://doi.org/10.1007/s11904-015-0265-9
- Olivieri K.C., Agopian K.A., Mukerji J., Gabuzda D. Evidence for adaptive evolution at the divergence between lymphoid and brain HIV-1 nef genes. AIDS Res. Hum. Retroviruses. 2010;26(4):495–500. DOI: https://doi.org/10.1089/aid.2009.0257
- Gega A., Kozal M.J., Chiarella J., et al. Deep sequencing of HIV-1 variants from paired plasma and cerebrospinal fluid during primary HIV infection. J. Virus Erad. 2015;1(4): 264–8.
- Shahid A., MacLennan S., Jones B.R., et al. The replication-competent HIV reservoir is a genetically restricted, younger subset of the overall pool of HIV proviruses persisting during therapy, which is highly genetically stable over time. J Virol. 2024;98(2):e0165523. doi: 10.1128/jvi.01655-23
- Hu W.S., Hughes S.H. HIV-1 reverse transcription. Cold Spring Harb. Perspect. Med. 2012;2(10):a006882. DOI: https://doi.org/10.1101/cshperspect.a006882
- Singh A.K., Das K. Insights into HIV-1 Reverse Transcriptase (RT) inhibition and drug resistance from thirty years of structural studies. Viruses. 2022;14(5):1027. DOI: https://doi.org/10.3390/v14051027
- Madden T. Chapter 16. The BLAST sequence analysis tool. In: The NCBI Handbook. National Center for Biotechnology Information (US); 2003.
- Madeira F., Pearce M., Tivey A.R.N., et al. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022;50(W1):W276–9. DOI: https://doi.org/10.1093/nar/gkac240
- Liu T.F., Shafer R.W. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin. Infect. Dis. 2006;42(11): 1608–18. DOI: https://doi.org/10.1086/503914
- Posada D., Crandall K.A. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14(9):817–8. DOI: https://doi.org/10.1093/bioinformatics/14.9.817
- Kumar S., Stecher G., Li M., et al. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018;35(6):1547–9. DOI: https://doi.org/10.1093/molbev/msy096
- Apetrei C., Hahn B., Rambaut A., et al. HIV Sequence Compendium 2021. Los Alamos,New Mexico; 2021. URL: https://www.hiv.lanl.gov/content/sequence/HIV/COMPENDIUM/2021/sequence2021.pdf
- Wilson E.B. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Association. 1927;22(158):209–12. DOI: https://doi.org/10.2307/2276774
- Guex N., Peitsch M.C., Schwede T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis. 2009;30(Suppl. 1):S162–73. DOI: https://doi.org/10.1002/elps.200900140
- Waterhouse A., Bertoni M., Bienert S., et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Research. 2018;46(W1):W296–303. DOI: https://doi.org/10.1093/nar/gky427
- Daliparty V.M., Balasubramanya R. HIV Encephalitis. Treasure Island (FL): StatPearls Publishing; 2023.
- Jagtap S.B., Patil P.M. Clinical profile of neurological manifestations and CD4 counts in patient living with HIV cases and its outcome with treatment with ART at a tertiary hospital. MedPulse International Journal of Medicine. 2021;21(3):78–84. DOI: https://doi.org/10.26611/10212033
- Ulfhammer G., Edén A., Antinori A., et al. Cerebrospinal fluid viral load across the spectrum of untreated human immunodeficiency virus type 1 (HIV-1) infection: a cross-sectional multicenter study. Clin. Infect. Dis. 2022;75(3):493–502. DOI: https://doi.org/10.1093/cid/ciab943
- London R.E. HIV-1 Reverse transcriptase: a metamorphic protein with three stable states. Structure. 2019;27(3):420–6. DOI: https://doi.org/10.1016/j.str.2018.11.011
- Sarafianos S.G., Marchand B., Das K., et al. Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J. Mol. Biol. 2009;385(3): 693–713. DOI: https://doi.org/10.1016/j.jmb.2008.10.071
Supplementary files