The effect of preventive vaccination on chickenpox incidence in Russia
- Authors: Afonina N.M.1, Mikheeva I.V.1
-
Affiliations:
- Central Research Institute for Epidemiology
- Issue: Vol 99, No 6 (2022)
- Pages: 651-660
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/1357
- DOI: https://doi.org/10.36233/0372-9311-338
- ID: 1357
Cite item
Abstract
Introduction. The significance of the chickenpox (CP) problem for public health and economy of Russia necessitated inclusion of CP vaccination in the regional immunization programs of some regions of the Russian Federation and in the vaccination schedule as an epidemic-response measure.
The purpose of the study was to assess the effectiveness of CP vaccination to provide the rationale for recommendations on expansion of the national preventive vaccination schedule.
Materials and methods. The vaccination effectiveness was assessed by comparison of the vaccination rates and CP incidence rates in 2006–2021 with the reference to the data collected from forms No. 2 and No. 5 of the National Statistical Monitoring in Russia and in its regions.
Results and discussion. Before 2019, in some regions of Russia, CP vaccination of children within regional immunization programs and vaccination of risk groups within the vaccination schedule following the epidemic-response measures had hardly any effect on the epidemiological situation. The remote-work and stay-at-home policies during the COVID-19 pandemic in 2020 resulted in a decrease in the incidence and an increased number of individuals who did not have immunity against Varicella zoster, thus subsequently leading to the increased CP incidence in the country. However, the Central, Volga, and Siberian Federal Districts were able to avoid an increase in the CP incidence due to the significantly increased vaccination coverage among children in 2020–2021. At the same time, in most of the regions, less than 2% of children aged 1–6 years were vaccinated annually. The insufficient CP vaccination coverage in the regions having extensive experience of planned immunization of children led to the shift of the incidence towards older age groups and increased risk of development of congenital infection.
Conclusion. To increase the effectiveness of CP preventive vaccination, it is recommended that the national vaccination schedule should include two-dose vaccination with the coverage of at least 90% of one-year-old children, while continuing immunization of older age individuals from the groups that are at risk of infection.
Full Text
Introduction
Currently, chickenpox (CP) is one of the most common infections in Russia [1–4].
The high intensity of the CP epidemic process is associated with significant economic losses: For more than 10 years, this disease holds a leading position among infectious diseases in terms of the economic impact, causing annual losses of more than 12 billion rubles [5, 6].
In the recent years, cases of complicated CP have been reported 3 times as often; the clinical polymorphism is expanding and the percentage of highly severe and fatal cases of infection is increasing. High risk groups for development of severe clinical forms of infection include infants [7], adults, pregnant women, and immunocompromised individuals [8, 9].
Immunization is the only effective measure for CP prevention. The live vaccine against CP, which was developed and clinically tested in Japan in the 1970s-1980s, as well as other vaccines based on it are used in many countries. CP vaccination is included in national schedules in many developed countries; the yearslong experience proved the effectiveness of preventive vaccination against this infection [10, 11].
The significance of the CP problem for public health and economy of Russia necessitated the inclusion of CP vaccination in the national healthcare practice. The Sverdlovsk Region and Moscow were the first regions where immunization against this infection was included in the regional preventive vaccination schedules in 2009, right after the foreign vaccines had been approved for CP prevention in Russia [12–15]. In 2014, CP vaccination of children and adults from risk groups1 was included in the preventive vaccination schedule as part of epidemic-response measures.
Considering the current epidemiological situation, the government specified the prospects for preventive vaccination, which were approved in 2020 as the Strategy for Development of Preventive Immunization Against Infectious Diseases for the Period till 2035.2 One of the main ways to optimize the national vaccination schedule is the extension of the list of infectious diseases controllable by vaccination, including planned CP vaccination of children.
In this connection, the study was performed to assess the effectiveness of CP vaccination to provide the rationale for recommendations on expansion of the national preventive vaccination schedule of the Russian Federation.
Materials and methods
To assess the epidemiological situation, we analyzed the data from Federal Statistical Monitoring Form No. 2 "Information about Infectious and Parasitic Diseases" with reference to CP incidence in 2006-2021 in Russia and in its regions, as well as Forms No. 23-17 "Information about Outbreaks of Infectious Diseases" in 2017–2021. The assessment of CP vaccination coverage was based on the data from Federal Statistical Monitoring Form No. 5 "Information about Preventive Vaccination" in Russia and its regions in 2013–2021.
The descriptive epidemiological study included the retrospective analysis of incidence distribution by year, age, and region (across federal districts (FDs) and regions of Russia), including vaccination coverage among specific groups of population. As the statistical data on the number of individuals vaccinated against CP were absent, the one-dose CP vaccination coverage of the child population was estimated with the reference to the proportion (shown as percentage) of the number of vaccinated people in the total population of the 1-6-year-old age group during the studied year. The statistical analysis of the relationship between the CP incidence in the population and the number of individuals vaccinated against this infection was performed using the linear correlation method and the Pearson correlation coefficient (r).
Results
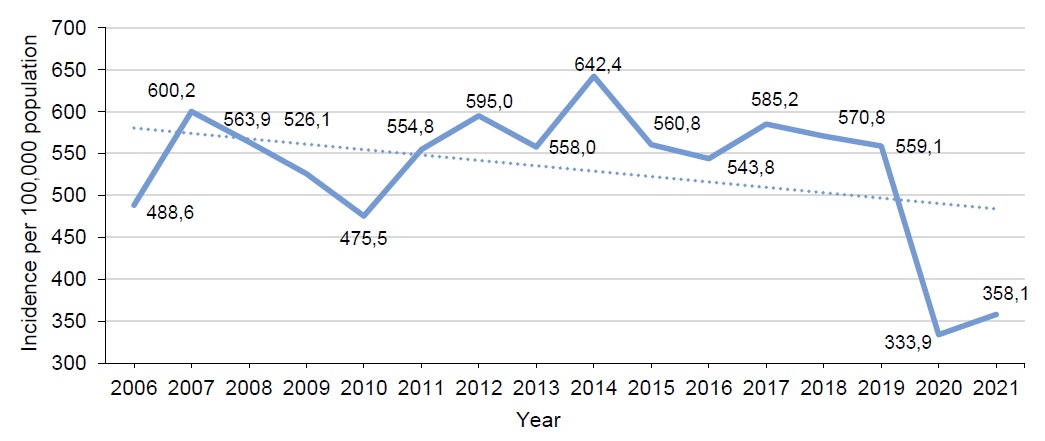
Before 2019, CP affected 800,000-900,000 people annually in Russia; the incidence rate was around 600 cases per 100,000 population, and no decreasing trend in the incidence was observed (Fig. 1).
Fig. 1. Dynamics of CP incidence in Russia in 2006–2021.
In 2020, when the COVID-19 pandemic started, Russia for the first time reported a 40% decrease in the CP incidence: a total of 490,000 cases were reported compared to 821,000 cases in 2019; the incidence rate was 333.9 per 100,000 population. In 2021, the incidence rate remained low – 358.1 per 100,000 population; however, the upward trend was observed (Fig. 1).
The highest CP incidence rates were usually recorded among preschool children: In the age group of 3-6-year-olds, the annual rates reached 6,000–8,000 per 100,000 children in the above age group; among children aged 1-2 years, the rates were 2.5-3.0 thousand per 100,000 children of the above age. In 2020, the incidence rates decreased in all age groups of the population, while in 2021, the incidence increased by 15% in all age groups (Fig. 2).
Fig. 2. Dynamics of CP incidence in Russia in 2006–2021, by age groups.
Childcare organizations were most frequent locations of large foci of infection. Annually, childcare centers reported 2.1-2.8 thousand epidemic CP outbreaks. Schools ranked second by the number of large outbreaks (662-826 multiple foci of infection annually), thus demonstrating the presence of a significant proportion of children without immunity against the pathogen in older age groups. In 2017-2019, in Russia there were 9,785 epidemic CP outbreaks involving 153,313 people. The highest number of large foci (3,663) was recorded in 2019. The outbreak-related CP incidence cannot be assessed for 2020 and 2021, as in statistical monitoring Form No. 23-17, multiple foci of CP and novel coronavirus infection were recorded together.
Based on the official statistical data, from 2013 to 2021 in Russia, more than 850,000 people were vaccinated against CP. A total of 32.0–199.8 thousand people were vaccinated annually (Fig. 3).
Fig. 3. Dynamics of people vaccinated against CP in Russia in 2013–2021.
CP vaccination rates did not show any stable upward trend. On the contrary, the immunization coverage decreased during some years. In 2014, the number of the vaccinated decreased two-fold compared to 2013 (85,000 and 41,000 vaccinated individuals, respectively), while in 2015, it was by 25% smaller than in 2014. Having slightly increased in 2016, the immunization coverage resumed its downward trend during the next 2 years. Since 2019 the CP immunization coverage has demonstrated a steady increase; in 2020, the number of the vaccinated increased by 30% compared to 2019, and in 2021, the number of the vaccinated increased by 40% compared to 2020 (Fig. 3).
Thus, during the COVID-19 pandemic, the CP preventive vaccination rates did not decrease; on the contrary, preventive measures against this infection were intensified.
The correlation analysis of the relationship between the CP incidence rates and the number of individuals vaccinated against this infection in Russia showed the inverse relationship of a moderate degree in the age groups of children aged 3-6 and 7-14 years (correlation coefficients r = –0.54 ± 0.37 and r = –0.53 ± 0.37, respectively; p < 0.01) and the inverse relationship of a low degree among adults (correlation coefficient r = –0.37 ± 0.41; p < 0.01) and children aged under 1 year (r = –0.34 ± 0.42; p< 0.01). The statistical analysis of the data produced high values of correlation coefficient errors due to the low-degree relationship and the insufficiently long monitoring. The further collection of data and the vaccination breakdown by age groups are required for measurement of the strength of correlations.
The assessment of vaccination distribution by age groups did not demonstrate any increase in the vaccination coverage of adults representing risk groups in 2019–2021: 45,000–49,000 adults were vaccinated in the country annually. The similar situation was observed in all FDs.
On the other hand, the vaccination coverage of children increased significantly — from 62 thousand in 2019 to 154.5 thousand in 2021. At the same time, in 2021, the number of the vaccinated increased in all FDs, except Ural FD (Fig. 4).
Fig. 4. The number of children vaccinated against CP in Russia and its FDs in 2019–2021.
Considering that regions independently implement regional immunization programs, we analyzed CP preventive vaccination rates in individual regions of Russia during the recent years. The analysis of the 2020–2021 period showed an increase in the number of regions that expand their preventive vaccination schedules by including planned CP vaccination of children. In the meantime, in 2020, most of FDs had total vaccination coverage of less than 1% of children aged 1–6 years, except for Central and Siberian FDs, where the coverage was 1.69% and 1.1%, respectively. In 2021, the vaccination coverage of children increased in all FDs, except for Ural FD (Table).
Percentage of children aged 1–6 years, vaccinated against CP in 2020–2021, by FDs
Federal District | 2020 | 2021 | ||
number of vaccinations | vaccination coverage of children from 1 to 6 years old, % | number of vaccinations | vaccination coverage of children from 1 to 6 years old, % | |
Central | 43 902 | 1,69 | 65 602 | 2,53 |
Siberian | 14 687 | 1,10 | 28 476 | 2,14 |
Northwestern | 8250 | 0,85 | 14 405 | 1,48 |
Far Eastern | 4725 | 0,72 | 8494 | 1,30 |
Urals | 12 187 | 1,22 | 11 122 | 1,11 |
Volga | 6981 | 0,33 | 20 707 | 0,97 |
Southern | 2592 | 0,22 | 4736 | 0,41 |
North Caucasian | 581 | 0,06 | 810 | 0,09 |
Russian Federation | 93 905 | 0,87 | 154 352 | 1,44 |
Central FD increased the vaccination coverage by 50% in 2021 compared to 2020; the vaccination coverage of preschool children in 2021 was estimated at 2.53%; Siberian FD reported a two-fold increase in the number of the vaccinated, reaching the coverage of 2.1%; in Northwestern FD, the number of the vaccinated increased by 75% and the coverage was 1.25%; in Far-Eastern FD, the number of the vaccinated increased by 80%, the coverage reached 1.3%; Volga FD reported the 3-fold increase in the number of the vaccinated and the coverage of 0.97%; in Southern FD, the number of the vaccinated increased by 83% and the coverage was 0.41%, (Fig. 4, Table 2).
The highest vaccination rates among preschool children in 2021 were demonstrated by the following regions of Russia: Sakhalin Region — 11.6% (Far-Eastern FD), Yamal-Nenets Autonomous District — 10.1% (Ural FD), Novosibirsk Region — 9.6% (Siberian FD), Penza Region — 9.3% (Volga FD). In Moscow, the vaccination rate among preschool children was 5.3% in 2021 and in the Sverdlovsk Region, the rate was less than 1%.
The comparative analysis of the CP incidence across federal districts during the past three years showed that the CP incidence varied across regions of Russia: As usual, in Northwestern and Ural FDs, the incidence rates were significantly higher than the average rate in Russia, while in Southern and North Caucasus FDs, the reported incidence rate was relatively low (1.5-3.0 times as low as the average rate in Russia; Fig. 5).
Fig. 5. CP incidence in Russia and its FDs in 2019–2021.
In 2020, the CP incidence decreased by 40% in all regions of Russia, regardless of the implemented regional child immunization programs, thus leading to the conclusion that the restrictive measures taken in response to COVID-19 had an impact on the incidence.
At the same time, the analysis of the incidence with a breakdown into FDs and regions of Russia showed that in 2021, after the restrictions had been removed, some FDs reported a significant increase in the CP incidence (Northwestern, Southern, Ural, and Far-Eastern FDs), while some districts remained epidemiologically stable in terms of CP (Central, Volga, Siberian, North Caucasus FDs; Fig. 5). Note that the incidence did not increase in the districts that actively promoted child immunization programs for CP prevention in 2020–2021.
In Central FD, the increase in immunization coverage was reported by most of the regions; Moscow, the Kursk, Yaroslavl, Bryansk, and Tver Regions ranked among the regions having the highest vaccination rates (more than 2%). In Volga FD, the child vaccination was included in regional schedules in the Penza, Nizhny Novgorod, Orenburg Regions, in the Udmurt Republic, the Republic of Tatarstan, and the Perm Territory in 2020–2021. The immunization coverage of preschool children increased in the largest regions of Siberian FD — Novosibirsk, Omsk, and Irkutsk Regions, where the number of vaccinated children totaled around 40,000 children over the last two years.
The experience of some regions of Russia demonstrates the significance of maintaining steadily high rates of planned preventive immunization of children to eliminate the risk of any future worsening of the epidemiological situation and the shift of incidence towards older age groups.
For example, during the first years of CP vaccination of children (2009–2013) within their regional vaccination schedules, the Sverdlovsk Region and Moscow reported a decreasing trend in the incidence. However, in later years, the epidemiological situation worsened in both regions: In the Sverdlovsk Region, the incidence increased in 2015 due to sharp reduction in the vaccination coverage; in Moscow, the incidence started increasing in 2019 due to the yearslong vaccination with low vaccination rates (Fig. 6).
Fig. 6. CP incidence and preventive immunization coverage in Moscow and the Sverdlovsk Region in 2006–2021. Note. *non-highlighted columns show data for 2009–2012 from unofficial reports and extrapolated data from incomplete published information.
The performed analysis of CP incidence by age groups in Moscow [14] across years showed the increase in the incidence among adults since 2016, the increase in the incidence among infants and the increase in the CP incidence among newborns under 1 month old in 2020–2021.
Discussion
Although starting from 2009, CP vaccination was performed among preschool children in some regions of Russia (Moscow, the Sverdlovsk Region) [15, 16] and the vaccination in the risk groups was performed within the vaccination schedule as part of epidemic-response measures starting from 2014, the incidence of this infection did not demonstrate any downward trend in Russia until 2019, while different organizations reported epidemic outbreaks of infection (in 2017–2019, there were more than 3,000 large foci annually, demonstrating no decreasing trend).
The occurrence of multiple CP foci at childcare centers and schools, healthcare organizations, social service centers and other organizations is explained by extremely high contagiousness of the pathogen. Russian researchers noted that epidemic control measures were often inefficient in combating CP outbreaks without post-exposure immunization in foci of infection [2].
Starting from 2014, urgent preventive vaccination in the focus of CP has been regulated by the vaccination schedule based on epidemic-response measures; therefore, the high outbreak-related incidence in 2017–2019 could be caused by its insufficient coverage.
Considering that Russia has no home-produced vaccine for CP prevention and the purchasing of foreign vaccines for regions entails difficulties, the CP vaccination rates did not have any stable upward trend in the country until 2019.
The CP vaccination rates cannot be assessed in regions of Russia due to the absence of the respective data in statistical form No. 6 "Information about children and adults vaccinated against infectious diseases".
Being aware of the high CP incidence, regions of Russia are including CP vaccination of children in their preventive vaccination schedules. Since 2019, despite the heavy workload of healthcare facilities due to the spread of COVID-19, a significant number of regions have been increasing CP preventive vaccination rates. The vaccination rates have increased through planned vaccination of child population following regional immunization programs rather than through vaccination of adult individuals representing high-risk groups and implemented under the vaccination schedule as an epidemic-response measure.
The results of the comparative analysis of the CP incidence and preventive immunization rates broken down by FDs showed that in 2020, the epidemiological situation for CP improved mostly due to the restrictive measures necessitated by the spread of COVID-19 rather than because of the increased vaccination rates.
Considering the increased numbers of children who did not have CP due to the wide-scale restrictive measures at childcare centers and schools in 2020, another cyclic epidemic surge in the CP incidence was projected for 2021, especially among children who had experienced COVID-19. Meanwhile, the expected increase in the incidence was not observed in FDs where most of the regions included CP vaccination of children in their regional immunization programs and where the immunization coverage of preschool children was higher than the national average coverage levels.
However, the fact that before 2021, in most of the regions, less than 2% of children aged 1–6 years had been vaccinated against CP annually demonstrates low vaccination rates, due to which it will take longer time to reach the 85-90% vaccination coverage recommended by WHO [10]. The WHO documents clearly state that planned immunization of children at the coverage levels lower than the recommended level can change the epidemiological characteristics of the infection and result in the increased number of infection cases among children of the older age and among adults [10]. This situation has been observed in Moscow since 2016 [14]. The ageing trend in CP increases the risk of infection affecting pregnant women and, consequently, the risk of intrauterine infection of newborns who develop congenital or neonatal CP [16].
Such adverse trends and problems were observed in many countries during initial stages of the implementation of CP preventive vaccination more than 15 years ago [17, 18].
For this reason, in 2006, the Advisory Committee on Immunization Practices of the United States Centers for Disease Control and Prevention issued recommendations on two-dose vaccination of children, catch-up vaccination of individuals who received only one-dose of the vaccine, one-dose vaccination of all healthy people older than 13 years old, having no history of the infection and not vaccinated, mandatory vaccination of newly enrolled students attending a school or college [19].
Taking into account the experience of other countries and WHO recommendations [10], special attention should be given to planning regional immunization programs, drawing on the results of the thorough epidemiological analysis, to minimize the risk of adverse consequences that may occur during the initial stage of CP preventive vaccination. The vaccination coverage should be not less than 90%, two-dose CP vaccination should be implemented, and the proper epidemiological surveillance should be performed for all infections caused by the Varicella zoster virus (CP, congenital infection, shingles) as well as for preventive vaccination against the above infection3.
Conclusions
In Russia, the CP vaccination included in the vaccination schedule based on epidemic-response measures had hardly any effect on the CP incidence, while long-lasting closure of organizations and lockdown restrictions during the spread of COVID-19 in 2020 significantly improved the epidemiological situation for CP. In the meantime, the CP immunization coverage of children is still extremely low; the vaccination rate among children aged 1 to 6 years is less than 2%.
Central, Volga, and Siberian FDs did not have an increase in the CP incidence, which was projected for 2021, due to the childhood vaccination against this infection, which was included in their regional immunization programs.
Based on the regional experience, two-dose CP vaccination should be included in the national preventive vaccination schedule, reaching at least 90% of one-year-old children.
Implementation of preventive vaccination is intrinsically linked with improvement of the epidemiological surveillance of the infection caused by the Varicella zoster virus: Improvement of the record-keeping of outbreak-related incidence, namely, record-keeping of all CP outbreaks, using Form 23-17, record-keeping of congenital infection cases, and collecting of information about CP vaccination of children and adults to be included in Form 6.
1 Decree No. 1122n of the Ministry of Health of the Russian Federation of 6/1/2021 "On Approval of the National Preventive Vaccination Schedule, the Preventive Vaccination Schedule Based on Epidemic-Response Measures, and the Procedure for Preventive Vaccination".
2 Executive Order No. 2390-r of the Government of the Russian Federation of 18/9/2020, "On Approval of the Strategy for Development of Preventive Immunization Against Infectious Diseases for the Period till 2035".
3 MR 3.1.0224-20. 3.1. Prevention of Infectious Diseases. Epidemiological surveillance of the infection caused by the Varicella Zoster virus. Guidelines (approved by the Chief Public Health Officer of the Russian Federation on 14.12.2020)
About the authors
Nataliya M. Afonina
Central Research Institute for Epidemiology
Email: irina_mikheeva@mail.ru
ORCID iD: 0000-0002-3205-4025
Cand. Sci. (Med.), researcher, Laboratory of immunization
Russian Federation, MoscowIrina V. Mikheeva
Central Research Institute for Epidemiology
Author for correspondence.
Email: irina_mikheeva@mail.ru
ORCID iD: 0000-0001-8736-4007
D. Sci. (Med.), Professor, Head, Laboratory of immunization
Russian Federation, MoscowReferences
- Kaira A.N., Lavrov V.F., Svitich O.A., Solomay T.V., Volosnikova A.V. Features of the epidemiology of chickenpox in a single territory. Epidemiologiya i vaktsinoprofilaktika. 2020; 19(2): 63–9. https://doi.org/10.31631/2073-3046-2020-19-2-63-69 (in Russian)
- Sitnik T.N., Shteynke L.V., Gabbasova N.V. Chicken-pox: «growing» up infection. Epidemiologiya i vaktsinoprofilaktika. 2018; 17(5): 54–9. https://doi.org/10.31631/2073-3046-2018-17-5-54-59 (in Russian)
- Kharchenko G.A., Kimirilova O.G. Chickenpox: clinic, treatment, prevention. Epidemiologiya i infektsionnye bolezni. 2017; (2): 72–5. (in Russian)
- Afonina N.M., Mikheeva I.V. Modern epidemiological characteristic of chickenpox in Russia. One Health & Risk Management. 2020; (1): 12–21. https://doi.org/10.38045/ohrm.2020.1.03 (in Russian)
- State Report «On the state of sanitary and epidemiological welfare of the population in the Russian Federation in 2019». Moscow; 2020. (in Russian)
- Mikheeva M.A., Mikheeva I.V. Ranking dynamics of economic burden of infectious diseases as a criterion of effectiveness of epidemiologic control. Zhurnal mikrobiologii, epidemiologii i immunobiologii. 2020; 97(2): 174–81. https://doi.org/10.36233/0372-9311-2020-97-2-174-181 (in Russian)
- Mikhaylova E.V. Chickenpox in newborns: symptoms, complications, modern methods of therapy and prevention. In: Materials of the XIII Congress of Children's Infectious Diseases of Russia «Actual Issues of Infectious Pathology and Vaccine Prevention» [Materialy XIII Kongressa detskikh infektsionistov Rossii «Aktual'nye voprosy infektsionnoy patologii i vaktsinoprofilaktiki».]. Moscow; 2014: 48–9. (in Russian)
- Balikin V.F., Filosofova M.S. The expansion of clinical polymorphism and the increase in the severity of Varicella zoster infection in children. In: Materials of the XIII Congress of Children's Infectious Diseases of Russia «Actual Issues of Infectious Pathology and Vaccine Prevention» [Materialy XIII Kongressa detskikh infektsionistov Rossii «Aktual'nye voprosy infektsionnoy patologii i vaktsinoprofilaktiki».]. Moscow; 2014: 8. (in Russian)
- Zryachkin N.I., Buchkova T.N., Chebotareva G.I. Complications of chickenpox (literature review). Zhurnal infektologii. 2017; 9(3): 117–28. https://doi.org/10.22625/2072-6732-2017-9-3-117-128 (in Russian)
- WHO. Varicella and herpes zoster vaccine: WHO position paper. Weekly Epidemiological Record. 2014; 89(25). Available at:
- https://www.who.int/publications/i/item/who-wer-8925-265-288
- Epidemiology and Prevention of Vaccine-Preventable Diseases. In: The Pink Book: Course Textbook. 2021. Available at:
- https://www.cdc.gov/vaccines/pubs/pinkbook/index.html
- Kovtun O., Romanenko V., Kazakevich N., Savvina N. Regional vaccination programme: ways to establish, results and prospects. Pediatricheskaya farmakologiya. 2010; 7(4): 19–23. (in Russian)
- Yakovleva T.V., Akimkin V.G., Lytkina I.N. The prospect of the national immunizations schedule. Epidemiologiya i vaktsinoprofilaktika. 2011; (1): 44–50. (in Russian)
- Afonina N.M., Mikheeva I.V. Effectiveness of regional varicella vaccination programmes. Infektsionnye bolezni: novosti, mneniya, obuchenie. 2022; 11(3): 95–103. https://doi.org/10.33029/2305-3496-2022-11-3-95-103 (in Russian)
- Filippov O.V., Bol'shakova L.N., Elagina T.N. Regional Schedule of Vaccination in Moscow: History, Development, Prospects. Epidemiologiya i vaktsinoprofilaktika. 2020; 19(4): 63–75. https://doi.org/10.31631/2073-3046-2020-19-4-63-75 (in Russian)
- Ksenofontova O., Rozhkova L., Savvinova T., Kharitonov A. Experience of vaccine prevention for varicella in Yekaterinburg. Pediatricheskaya farmakologiya. 2010; 7(4): 34–7. (in Russian)
- Lamont R.F., Sobel J.D., Carrington D., Mazaki-Tovi S., Kusanovic J.P., Vaisbuch E., et al. Varicella-zoster virus (chickenpox) infection in pregnancy. BJOG. 2011; 118(10): 1155–62. https://doi.org/10.1111/j.1471-0528.2011.02983.x
- Asano Y. Varicella vaccine: the Japanese experience. J. Infect. Dis. 1996; 174(Suppl. 3): S310–3. https://doi.org/10.1093/infdis/174.supplement_3.s310
- Chaves S.S., Gargiullo P., Zhang J.X., Civen R., Guris D., Mascola L., et al. Loss of vaccine-induced immunity to varicella over time. N. Engl. J. Med. 2007; 356(11): 1121–9. https://doi.org/10.1056/nejmoa064040
- Marin M., Güris D., Chaves S.S., Schmid S., Seward J.F. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2007; 56(RR-4): 1–40.
Supplementary files