Molecular genetic determinants of virulence of Streptococcus agalactiae isolated from pregnant women and newborns in St. Petersburg and the Leningrad region in 2010–2023
- Authors: Shalepo K.S.1, Khusnutdinova T.A.1, Budilovskaya O.V.1, Krysanova A.A.1, Sapozhnikov K.V.1, Savicheva A.M.1, Kogan I.Y.1
-
Affiliations:
- D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology
- Issue: Vol 101, No 2 (2024)
- Pages: 217-226
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18582
- DOI: https://doi.org/10.36233/0372-9311-501
- EDN: https://elibrary.ru/qnunfe
- ID: 18582
Cite item
Abstract
Introduction. Colonization of the reproductive organs of pregnant women with group B streptococci (GBS; Streptococcus agalactiae) can lead to severe perinatal and neonatal pathology. In modern conditions, aside from the prevention of antenatal infection of the fetus during childbirth using antibacterial drugs, vaccination is also necessary. In this regard, surveillance of GBS genotypes is an important task.
Objective. To determine the molecular genetic determinants of virulence of Streptococcus agalactiae isolated from pregnant women and newborns, and to monitor the distribution of capsular polysaccharides types and pili profiles in GBS clinical isolates.
Materials and methods. The study used clinical isolates of GBS (n = 420) isolated from pregnant women and newborns in 2010–2023.The bacteriological method was used for isolation of S. agalactiae. PCR method was used to determine the types of capsular polysaccharides, pili, and whether the strains belonged to the hypervirulent sequence type ST-17.
Results. During 13 years of observation, the predominance of Ia, III and V genotypes of GBS capsular polysaccharides was noted both in pregnant women and in newborns. The frequency of occurrence of genotype Ib increased from 0.7 to 6.7%, genotype V from 12.1 to 24.4%, and the prevalence of genotype III decreased significantly from 41.1 to 21.1%. Hypervirulent sequence type ST-17 was detected in 6 pregnant women and 2 newborns. However, there were no signs of neonatal infection in these children. More than half of all clinical isolates of S. agalactiae had pili genotypes PI-1 + PI-2a, as well as pili genotypes PI-2a and PI-1 + PI-2b. The distribution of pili types did not change over 13 years of the surveillance period.
Conclusion. Surveillance of the GBS capsular polysaccharides and pili genotypes is necessary for the development of effective preventive vaccines.
Full Text
Introduction
Group B streptococci (GBS), of which Streptococcus agalactiae is the only representative, are short-chain Gram-positive cocci, facultative anaerobes with β-hemolytic activity. They are usually commensal organisms and colonize the gastrointestinal tract and lower reproductive tract in about 20-30% of women [1]. In Russia, this figure reaches 16.3% [2].
When colonized with GBS, symptoms are usually absent. However, GBS in adults can provoke pyelonephritis, arthritis, development of abscesses, mastitis, endocarditis, septicemia, and others. S. agalactiae are a common cause of inflammatory diseases of the urogenital tract of women and the main etiologic agent of early neonatal period infections. They are most relevant in the etiology of perinatal infections, as they can cause genitourinary tract infections, preterm labor, stillbirth, chorioamnionitis, postpartum sepsis and a number of unfavorable outcomes for the mother and child, such as neonatal sepsis, meningitis or pneumonia. Fetal infection most often occurs intrauterine, when GBS ascend from the vagina into the amniotic fluid, but infection may occur during the fetus' passage through the mother's birth canal (intrapartum).
Neonatal infections caused by GBS are clinically subdivided into early infections, which develop before the 6th day of life, and late infections, which develop 7-89 days after birth [3].
The complex pathogenesis of the disease in the mother and child associated with S. agalactiae is associated with a large arsenal of virulence factors of this microorganism, which can vary widely from an asymptomatic carrier to the development of an invasive infection.
More than 20 different virulence factors of the microorganism contribute to the pathogenesis of GBS infection. These include adhesins, enzymes, and other proteins that can vary widely depending on the strain of the microbe. Cell adhesion molecules play an important role in the attachment of the microorganism to the host cell. Specific adhesins mediate colonization of S. agalactiae to various receptors present on the surface of the mucosal epithelium, ensuring its transmission to newborns during their passage through the maternal birth canal. These proteins are essential for the microorganism at the initial stage of colonization and promote invasion into the host epithelial and endothelial cells, as well as participate in the formation of biofilms. Pili are an important factor of virulence and pathogenicity of GBS and are long filamentous protein structures consisting of covalently bound protein subunits attached to the cell wall, protruding beyond the capsule that covers the bacterial surface [4].
Many GBS virulence factors influence the ability of each strain to colonize a particular biotope or cause severe disease. One of the key virulence factors is the GBS capsular polysaccharide (CPS), first identified by Dr. Rebecca Lunsfield in the 1930s. To date, 10 unique CPS serotypes (Ia, Ib, II-IX) are known [1, 5], with 6 of them (Ia, Ib, II-V) accounting for 98% worldwide [1].
There are several approaches to determine the serotypes of S. agalactiae. The Lancefield precipitation test (latex-agglutination method) is considered the standard method for the determination of GBS serotypes. Molecular biological methods based on DNA analysis include real-time PCR-based GBS genotyping, multiplex PCR analysis for direct identification of S. agalactiae capsule type (Ia to IX), and full-genome sequencing. Serotyping of GBS isolates is important for understanding local epidemiology and monitoring CPS genotype change.
In addition to serotype distinction, GBS can also be clustered genetically by multi-locus sequence typing. This is accomplished by sequencing 7 housekeeping genes for any strain of GBS, and the combination of these 7 genomic loci creates a sequence type (ST) [6]. Among a global and ecologically diverse sample of strains, 4 major sequence types were found: ST-1, ST-17, ST19, and ST23 [6]. Of these, ST-1 and ST-19 were mainly associated with asymptomatic carriers of GBS, ST-23 was common among both asymptomatic carriers and cases of invasive disease, and ST-17 serotype III strains were associated with neonatal invasive infections [6].
There are several approaches to antenatal screening for GBS worldwide. First, it is universal screening for S. agalactiae late in pregnancy (35-37 weeks) using a culture-based diagnostic method. Another approach is based on the presence of obstetric risk factors for infection (delivery at < 37 weeks' gestation, anhydrous interval of more than 18 h, body temperature above 38ºC). Universal antenatal screening for GBS is used in the United States and Canada [7]. Screening based on risk factors used in Sweden and the UK is more cautious, as women are tested only if they show symptoms of a disease associated with GBS, such as preterm labor, or if they have a history of adverse pregnancy outcomes [8].
In Russia, since 2021, the Order of the Ministry of Health No. 1130n of 20.10.2020 "On Approval of the Procedure for the Provision of Medical Care in the Profile "Obstetrics and Gynecology" has been in effect, according to which screening of pregnant women for GBS is required at 35-37 weeks of pregnancy. According to this order, exclusively vaginal or both vaginal and rectal secretions are examined and bacteriologic examination is performed to isolate S. agalactiae. If GBS is isolated, antibacterial drugs are prescribed in labor to prevent antibiotic prophylaxis of antenatal infection of the fetus.
Currently, measures have been developed to prevent early GBS infections based on parenteral administration of antibiotics during labor to women with a high risk of transmission of newborn microorganism. While the efficacy of this method is not in doubt, the choice of women for whom antibiotic prophylaxis is indicated is a matter of debate.
Although antibiotic prophylaxis is a good method of preventing vertical transmission of infection caused by S. agalactiae and reducing the incidence of infection in newborns, there is a risk of S. agalactiae resistance to antibiotics.
Vaccination of both pregnant women and women planning pregnancy is currently considered as a prophylaxis against the development of invasive forms of GBS infection in newborns. This strategy is based on the ability of maternal IgG to penetrate the placenta and provide immune protection not only against early-onset disease, but also against late-onset disease and postpartum complications associated with GBS. GBS occupies environmental niches (e.g., lower gastrointestinal tract and vaginal mucosa) that are relatively immunologically inert. In fact, a significant proportion of women colonized with GBS have low serum IgG concentrations to CPS, and these women pose a high risk of early infection with their newborns [9]. The development of a safe and effective vaccine that can be administered during or before pregnancy remains a major goal, as current antibiotic-based therapeutic prophylaxis strategies do not eliminate all cases of invasive infections caused by GBS.
Vaccine development is aimed at determinants of S. agalactiae that colonize the vaginal biotope and are capable of invasion. CPS was recognized as the most promising of them, on the basis of which scientists from the USA and South Africa developed a trivalent vaccine in 2013, which is successfully undergoing clinical trials [10].
In 2017, clinical trials of a polyvalent vaccine targeting serotypes Ia, Ib, II, III, and V of GBS were initiated. At the same time, an increase in the number of infections caused by serotype IV of GBS was noted, so this serotype was added to the hexavalent vaccine. This vaccine achieved coverage of 98% of neonatal infections caused by GBS.
The new hexavalent conjugated GBS vaccine (GBS6) targets serotypes Ia, Ib, II, III, IV, and V of GBS [11], which are prevalent worldwide. Monovalent, bivalent, and trivalent vaccines against serotypes Ia, Ib, II, III, and V of GBS have been previously studied in non-pregnant and pregnant women. These vaccines have been shown to be safe and to cause a post-vaccine increase in antibodies to GBS in infants and vaccinated individuals. However, there are no licensed vaccines against GBS infection yet [11]. The task is also complicated by the proportion of capsule-free GBS strains, which is as high as 10% in some populations.
Another promising target is the surface proteins and pili proteins of GBS.
A large systematic review of all existing GBS vaccine candidates was conducted in 2024. All GBS vaccine candidates demonstrated good immunogenicity and safety data for non-pregnant and pregnant women. The most promising candidate vaccines are a CPS-based polyvalent vaccine or a broad-spectrum surface subunit protein vaccine. Overall, the discovery of these molecular virulence factors of GBS identifies several promising targets for prophylactic vaccine development. This systematic review also emphasizes that there are still significant uncertainties in the determinants of antibody response, especially in individuals with low baseline levels of antibodies to GBS [12].
To reduce perinatal morbidity and mortality, more attention should be paid to clinical practice, screening, widespread detection and treatment of possible maternal and child infection with GBS, and prevention of antenatal fetal and neonatal infection. Monitoring of circulating strains of GBS in the region will allow to evaluate the strategy of prevention of invasive neonatal infections.
The aim of this study was to determine the molecular genetic determinants of virulence of GBS isolated from pregnant women and newborns, and to monitor the distribution of CPS types and pili profiles of GBS strains in St. Petersburg and the Leningrad region.
Materials and methods
Clinical isolates of GBS isolated from pregnant women in the third trimester of gestation and neonatal infections in 2010–2023 at the D.O. Ott Research Institute of Gestational Diseases were used in the study. Clinical materials in women included the middle portion of freely released urine, vaginal and/or cervical canal secretions and rectal mucosa; in newborns – secretions of the posterior pharyngeal stalk, nasal cavity, surface of the external auditory canal, axillary skin, and meconium.
The study was conducted with the voluntary informed consent of the patients and legal representatives of newborns; the study protocol was approved by the local ethical committee of the D.O. Ott Research Institute of AG&R (protocol No. 114 of 14.12.2021).
Clinical materials were examined by bacteriological method using selective nutrient broth (Todd-Hewitt broth) containing colistin sulfate, nalidixic acid and oxolinic acid, which inhibit associated microbiota, and on Mueller-Hinton agar with addition of 5% defibrinated blood. The isolated streptococci were identified using the SAMR test and by MALDI-TOF protein profiling (Bruker). For further studies, the strains were stored at –70ºC in trypticase-soy broth supplemented with 30% glycerol.
Bacterial DNA extraction was performed using AmpliPrime DNA-Sorb-AM DNA extraction reagent kit (Central Research Institute of Epidemiology of Rospotrebnadzor) according to the manufacturer's instructions.
Serotype and pili variant were determined by polymerase chain reaction (PCR), for which Tersus plus PCR kit (Eurogen) was used. Thermocycling was performed using the Terzik amplifier (DNA-Technology). To determine the types of CPS, the method proposed by K. Yao et al. [13].
The pili type was detected using the method developed by S. Teatero et al. [14]. The method includes two PCR reactions. The first reaction is multiplex; it contains a mixture of 4 primer pairs to the genes of PI loci (Pilus islands) — PI-1, PI-1b, PI-2a, PI-2b, the amplicons of which differ in size. The second reaction contains a pair of primers corresponding to the start and end of 2 genes flanking the PI-1 prophage locus. If the prophage is absent, these primers amplify only the genes flanking the prophage, corresponding to 648 nucleotide pairs. This reaction is used as a control for PI-1-negative isolates.
PCR with primers to the gbs2018-ST-17 gene was used to determine hypervirulent ST-17. If the GBS strain was classified as hypervirulent ST-17, a 210 bp amplicon was obtained.
DNA electrophoresis in 2% agarose gel was used to separate the amplicons obtained. The DNA length marker 100+ bp DNA Ladder (Eurogen) was used to establish the molecular size of the amplicons. The results were visualized using the Infinity gel documentation system (VilberLourmat).
Statistical processing of data
For statistical processing of data, we used the R v. 4.3.0 software. The study is observational, which included 100% of available study cases for 2010–2011, 2016–2020 and 2021–2023, so power was not checked. The proportions of patients with a given feature are presented both in absolute values (n/N, where n is the number of patients with the feature, N is the group size) and as relative units (%) with confidence interval (CI) calculated using the Klopper–Pearson method. The difference between the fractions was evaluated using Fisher's exact test. In the case of multiple comparisons, the Hill correction was applied. The critical level after which the null hypothesis was rejected was accepted at p = 0.05.
Results
To monitor the distribution of CPS genotypes and pili profiles in GBS isolated from pregnant women and newborns in St. Petersburg and Leningrad region in 2010–2023, we examined 420 clinical isolates of GBS. We divided these clinical isolates into the periods 2010–2011, 2016–2020, and 2021–2023. The observation periods were chosen because a 10-year comparative evaluation of GBS virulence factors and antibiotic resistance was performed in the above periods. In 2021–2023, new data began to accumulate as the monitoring of GBS continued, and this period was also taken for analysis.
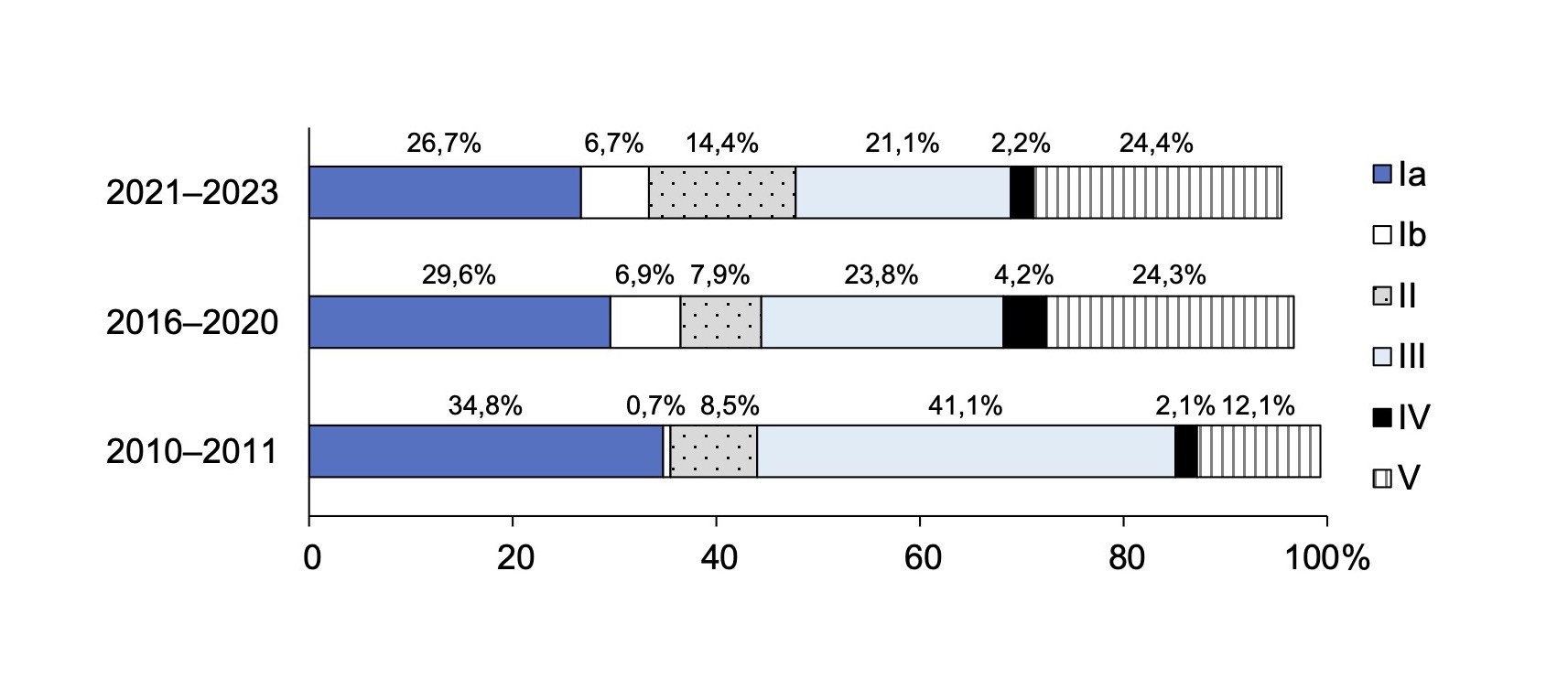
Table 1 presents data on the distribution of CPS in clinical isolates of GBS isolated from pregnant women and newborns in St. Petersburg and Leningrad Region in different periods. In the region, genotypes Ia, III, and V CPS of GBS were dominant during all periods of observation, while genotypes VI, VII, and IX were rare. Genotype VIII was absent throughout the entire observation period. During the 13 years of follow-up, the frequency of detection of different GBS genotypes varies. Thus, from 2010-2011 to 2021-2023, the frequency of genotype Ib (from 0.7% to 6.7%; p = 0.015) and genotype V (from 12.1% to 24.4%; p = 0.02) increased, while the prevalence of genotype III decreased significantly (from 41.1% to 21.1%; p = 0.003). It should be noted a negative dynamic in the frequency of detection of genotypes Ia, Ib, III, which are included in the trivalent vaccine, from 76.6% to 54.4% (Table 1). The detection of 6 genotypes (Ia, Ib, II, II, II, IV, V) remained above 95.0%. This distribution of genotypes indicates that a hexavalent CPS-based vaccine would be effective in our region. These changes require close attention and continuous monitoring, especially with the prospect of creating prophylactic vaccines.
Table 1. Distribution of capsular polysaccharides in clinical isolates of S. agalactiae isolated from pregnant women and newborns together in St. Petersburg and the Leningrad region, n/N; % (95% CI)
Genotype | 2010–2011 (N = 141) | 2016–2020 (N = 189) | 2021–2023 (N = 90) | Total |
Ia | 49/141; 34,8% (26,9–43,2%) | 56/189; 29,6% (23,2–36,7%) | 24/90; 26,7% (17,9–37%) | 129/420; 30,7% (26,3–35,4%) |
Ib | 1/141; 0,7% (0–3,9%) | 13/189*; 6,9% (3,7–11,5%) | 6/90*; 6,7% (2,5–13,9%) | 20/420; 4,8% (2,9–7,3%) |
II | 12/141; 8,5% (4,5–14,4%) | 15/189; 7,9% (4,5–12,8%) | 13/90; 14,4% (7,9–23,4%) | 40/420; 9,5% (6,9–12,7%) |
III | 58/141; 41,1% (32,9–49,7%) | 45/189**; 23,8% (17,9–30,5%) | 19/90**; 21,1% (13,2–31%) | 122/420; 29% (24,7–33,6%) |
IV | 3/141; 2,1% (0,4–6,1%) | 8/189; 4,2% (1,8–8,2%) | 2/90; 2,2% (0,3–7,8%) | 13/420; 3,1% (1,7–5,2%) |
V | 17/141; 12,1% (7,2–18,6%) | 46/189*; 24,3% (18,4–31,1%) | 22/90*; 24,4% (16–34,6%) | 85/420; 20,2% (16,5–24,4%) |
VI | 0/141; 0% (0–2,6%) | 2/189; 1,1% (0,1–3,8%) | 2/90; 2,2% (0,3–7,8%) | 4/420; 1% (0,3–2,4%) |
VII | 1/141; 0,7% (0–3,9%) | 2/189; 1,1% (0,1–3,8%) | 2/90; 2,2% (0,3–7,8%) | 5/420; 1,2% (0,4–2,8%) |
VIII | 0/141; 0% (0–2,6%) | 0/189; 0% (0–1,9%) | 0/90; 0% (0–4%) | 0/420; 0% (0–0,9%) |
IX | 0/141; 0% (0–2,6%) | 2/189; 1,1% (0,1–3,8%) | 0/90; 0% (0–4%) | 2/420; 0,5% (0,1–1,7%) |
Ia, Ib, III | 108/141; 76,6% (68,7–83,3%) | 114/189**; 60,3% (53–67,3%) | 49/90**; 54,4% (43,6–65%) | 271/420; 64,5% (59,7–69,1%) |
Ia, Ib, II, III, IV, V | 140/141; 99,3% (96,1–100%) | 183/189; 96,8% (93,2–98,8%) | 86/90; 95,6% (89–98,8%) | 409/420; 97,4% (95,4–98,7%) |
Note. Data for 2010–2020 were obtained and published by us earlier [14, 15]. *p < 0.05, **p < 0.05 compared to the data for 2010–2011.
These data are more clearly presented in the figure, which shows the dynamics of the increase in the frequency of detection of genotypes Ib, V and a decrease in the frequency of detection of genotype III of GBS.
Detection of CPS in a pregnant woman before delivery is a prognostic factor for newborn colonization by CPS (with vertical transmission of the pathogen). It was important to evaluate the frequency of detection of CPS genotypes in mothers and their newborns. In our study, 199 mother-newborn pairs were identified. CPS was detected in all mothers and neonatal infection colonization was 52.76% (45.84-59.58%).
Genotypes III (17.0%), Ia (16.1%) and V (10.5%) were most frequently identified in the mother-newborn pair. Genotypes IV, VII and IX were found in 0.5% of cases. Genotypes VI and VII were rare in our population and were not isolated in the mother-newborn pair. All presented genotypes isolated in the mother matched the genotypes isolated in newborns (Table 2).
Dynamics of the frequency of detection of the main capsular polysaccharides in S. agalactiae isolated from pregnant women and newborns in St. Petersburg and the Leningrad region during different periods.
Table 2. Frequency of detection of genotypes of capsular polysaccharides in the mother-newborn pairs in St. Petersburg and the Leningrad region in 2010–2023
Genotype | Mother neonate pairs, N = 105 | |
n | % (95% CI) | |
Ia | 32 | 16,1 (11,27–21,94) |
Ib | 3 | 1,5 (0,31–4,54) |
II | 13 | 6,53 (3,76–10,95) |
III | 34 | 17,0 (12,46–27,96) |
IV | 1 | 0,5 (0,01–3,08) |
V | 20 | 10,05 (6,53–15,08) |
VI | 0 | 0 |
VII | 1 | 0,5 (0,01–3,08) |
VIII | 0 | 0 |
IX | 1 | 0,5 (0,01–3,08) |
GBS isolates isolated from 2021-2023 were analyzed for belonging to hypervirulent ST-17. Of the 90 GBS isolates isolated from pregnant women and newborns, hypervirulent ST-17 was detected in 6 (6.7%) GBS isolates belonging to genotype III. 2 children born to these 6 women also had GBS isolated with this sequencing type, but they did not show signs of GBS infection.
Table 3 presents data on the distribution of pili genotypes of S. agalactiae isolated from pregnant women and newborns in St. Petersburg during different periods of observation.
Table 3. Distribution of pili genotypes in S. agalactiae strains isolated from pregnant women and newborns in St. Petersburg and the Leningrad region, n/N; % (95% CI)
Pilus | 2010–2011 | 2016–2020 | 2021–2023 | Total |
PI-1 + PI-2a | 76/141; 53,9% (45,7–61,9%) | 110/189; 58,2% (50,8–65,3%) | 44/90; 48,9% (38,2–59,7%) | 230/420; 54,8% (49,9–59,6%) |
PI-1 + PI-2b | 20/141; 14,2% (8,9–21,1%) | 19/189; 10,1% (6,2–15,3%) | 12/90; 13,3% (7,1–22,1%) | 51/420; 12,1% (9,2–15,7%) |
PI-1b + PI-2a | 1/141; 0,7% (0–3,9%) | 4/189; 2,1% (0,6–5,3%) | 0/90; 0% (0–4%) | 5/420; 1,2% (0,4–2,8%) |
PI-1b + PI-2b | 0/141; 0% (0–2,6%) | 3/189; 1,6% (0,3–4,6%) | 0/90; 0% (0–4%) | 3/420; 0,7% (0,1–2,1%) |
PI-2a | 43/141; 30,5% (23–38,8%) | 47/189; 24,9% (18,9–31,7%) | 31/90; 34,4% (24,7–45,2%) | 90/420; 21,4% (17,6–25,7%) |
PI-2b | 1/141; 0,7% (0–3,9%) | 6/189; 3,2% (1,2–6,8%) | 3/90; 3,3% (0,7–9,4%) | 10/420; 2,4% (1,1–4,3%) |
More than half of all S. agalactiae clinical isolates distributed in our region had pili of genotype PI-1 + PI-2a, which was 53.9% in 2010–2011, 58.2% in 2016–2020, and 48.9% in 2021–2023, a difference statistically insignificant. PI-2a (30.5% in 2010–2011, 24.9% in 2016–2020, and 21.4% in 2021–2023 (p > 0.05) and PI-1 + PI-2b pili genotypes were the next most common (frequency of detection was 12.1% over the entire follow-up period). The PI-2b GBS genotype was one of the rarest, which is also consistent with studies conducted in Europe. The GBS genotype including the recently described PI-1b locus was, as expected, extremely rare.
Discussion
In this study, we tracked the changes in CPS S. agalactiae genotypes among pregnant women and newborns in St. Petersburg and Leningrad region. In our region, CPS genotype Ia was dominant in 2010–2011 and was detected in 34.8% of cases, in 2016–2020 it was found in 29.6% and currently in 26.7%. During the entire follow-up period, one third (30.7%) of all clinical isolates of GBS belonged to genotype Ia. Genotype III was the second most common genotype. However, there was a change in the frequency of detection of genotype III of GBS over the 13 years, namely a significant decrease from 41.1% in 2010–2011 to 21.1% in 2021–2023 (p = 0.003). Thus, the dominant genotypes over the entire surveillance period remain Ia, III and V. These data are consistent with those obtained in other countries. Genotypes III, Ia, and V were the most common genotypes of GBS in several regions (Europe and North America, East Asia, South, East/Central Africa, and Australia/New Zealand) [17]. The most common GBS genotype colonizing pregnant women worldwide was genotype III, occurring at an incidence of 25% (95% CI 23–27%), and genotype Ia, detected in 19% (95% CI 17–21%) of women [17].
Genotype IV was found in Europe and North America at a frequency of 4%, and in South Africa at 3%. Genotypes VI–IX were rarely detected in Europe (1%) [17]. In St. Petersburg and the Leningrad Region, genotypes VI, VII, and IX were rare (1; 1.2; 0.5%, respectively). Genotype VIII was never detected throughout the entire observation period.
It should be noted that according to the results of our study, the frequency of occurrence of genotype Ib, which is one of the vaccine components, increased from 0.7% in 2010–2011 to 6.7% in 2021–2023 (p = 0.015).
Globally, Ia, Ib, II-V genotypes cover more than 99% of all cases of GBS infections, including early and late neonatal infection [18]. In our region, the coverage of these genotypes is (99% to 95.5% at different periods of follow-up). These changes require close monitoring of the represented genotypes for vaccine development.
The vertical transmission rate of GBS in mother-newborn pair in our study was 52.76% and overlap of molecular genetic characteristics among GBS-positive mother-newborn pairs was shown. The same CPS genotypes were dominant in the mother-newborn pair. According to different authors, the frequency of vertical transmission from GBS colonized mothers ranges from 54.2% in Turkey to 14.1% in China [19].
According to the literature, hypervirulent ST-17 GBS strains are associated with the risk of neonatal invasive infections. We detected this sequencing-type of GBS in 6 women and 2 newborns. When hypervirulent ST-17 strains of S. agalactiae in pregnant women, their newborn children should be monitored, as they fall into the risk group for early and late infection and have an increased risk of meningitis [20].
The main limitation of CPS-based vaccines is the need to provide protection against multiple serotypes, so multivalent vaccine strategies are of great interest. The Novartis/GSK trivalent vaccine (Novartis/GSK GBS3) containing a CPS Ia/Ib/III conjugate is considered safe for pregnant women, induces the production of high maternal antibody titers, and the antibodies are transmitted transplacentally to the fetus [21]. Unfortunately, the trivalent vaccine still does not include all clinically relevant GBS genotypes and may lead to selection of non-vaccine strains by capsule replacement, a phenomenon that has been observed in many high-risk populations and among ST-17 strains [20].
According to our data, the efficacy of the trivalent vaccine in pregnant women in 2010–2011 would be 76.6%, in 2016–2020 — 60.3%; currently, its efficacy is only 54.4%. The task is also complicated by the proportion of capsule-free GBS strains, which is as high as 10% in some populations. A new hexavalent (serotypes Ia, Ib, II, III, IV, and V) conjugated GBS vaccine (GBS6) [11], which accounts for approximately 98% of the pathogenic GBS genotypes that are prevalent worldwide, has been investigated. Monovalent, bivalent and trivalent vaccines against genotypes Ia, Ib, II, III and V of GBS have been previously studied in non-pregnant and pregnant women. These vaccines have been shown to be safe and to cause a post-vaccine increase in antibodies to GBS in newborns and vaccinated women. There are no licensed vaccines against diseases caused by GBS [11]. However, the presence of new genotypes and atypical GBS isolates, as well as the possibility of genotype switching, support the need for continuous genotype surveillance to contribute to the development of a universal vaccine [10, 12].
Not only the capsule but also the pili is an important factor in increasing the virulence of GBS. Most GBS contain PI-1 + PI-2a, and the presence of these pili stabilizes colonization [22]. The combination of PI-1 and PI-2a was predominant among GBS isolates colonizing 38% of pregnant women, PI-2a in 32%. The largest proportion of genotypes with pili islet protein genes was genotype Ia — 69% (PI-2a only) and genotype III — 85% of strains (PI-1 and PI-2b) [23].
I. Margarit et al. demonstrated in an extensive study of GBS pili on a large collection of clinical isolates that all GBS isolates contain at least one or a combination of two PIs, and a pili-based vaccine would provide sufficient widespread protection [24]. More than half of all S. agalactiae clinical isolates distributed in our region had pili of genotype PI-1 + PI-2a. The next most common genotypes were PI-2a (24.9 to 34.4%) and PI-1 + PI-2b (12.1%) pili genotypes. As our studies show, the distribution of pili genotypes did not change during the observed period. The data we obtained coincide with those obtained in the USA and European countries [7]. It is important to note that a vaccine based on pili components would have been effective throughout the entire period.
Conclusion
Despite fruitful research in recent years, our understanding of the dual role of GBS as a commensal and pathogen is still far from complete. More fundamental studies that unravel the molecular basis of virulence factors and host response to GBS are necessary to develop new prophylaxis methods that may be effective in preventing GBS infections in newborns.
About the authors
Kira Shalepo Shalepo
D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology
Author for correspondence.
Email: 2474151@mail.ru
ORCID iD: 0000-0002-3002-3874
Cand. Sci. (Med.), senior researcher, Department of medical microbiology
Russian Federation, St. PetersburgTatiana A. Khusnutdinova
D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology
Email: 2474151@mail.ru
ORCID iD: 0000-0002-2742-2655
Cand. Sci. (Med.), senior researcher, Department of medical microbiology
Russian Federation, St. PetersburgOlga V. Budilovskaya
D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology
Email: 2474151@mail.ru
ORCID iD: 0000-0001-7673-6274
Cand. Sci. (Med.), senior researcher, Department of medical microbiology
Russian Federation, St. PetersburgAnna A. Krysanova
D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology
Email: 2474151@mail.ru
ORCID iD: 0000-0003-4798-1881
Cand. Sci. (Med.), senior researcher, Department of medical microbiology
Russian Federation, St. PetersburgKirill V. Sapozhnikov
D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology
Email: 2474151@mail.ru
ORCID iD: 0000-0002-2476-7666
Cand. Sci. (Med.), specialist, Department of reproductology
Russian Federation, St. PetersburgAlevtina M. Savicheva
D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology
Email: 2474151@mail.ru
ORCID iD: 0000-0003-3870-5930
D. Sci. (Med.), Professor, Head, Department of medical microbiology
Russian Federation, St. PetersburgIgor Yu. Kogan
D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology
Email: 2474151@mail.ru
ORCID iD: 0000-0002-7351-6900
D. Sci. (Med.), Professor, RAS Corresponding Member, Director
Russian Federation, St. PetersburgReferences
- Russell N.J., Seale A.C., O’Driscoll M., et al. Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin. Infect. Dis. 2017;65(Suppl. 2):S100–11. doi: https://doi.org/10.1093/cid/cix658
- Хван В.О., Шипицина Е.В., Зациорская С.Л. и др. Частота и факторы риска колонизации беременных женщин стрептококками группы В. Журнал акушерства и женских болезней. 2017;66(6):44–58. Khvan V.O., Shipitsyna E.V., Zatsiorskaya S.L., et al. Frequency and risk factors of colonization of pregnant women with group B streptococci. Journal of Obstetrics and Woman Disease. 2017;66(6):44–58. doi: https://doi.org/10.17816/JOWD66644-58 EDN: https://elibrary.ru/knjtfe
- Verani J.R., McGee L., Schrag S.J. Prevention of perinatal group B streptococcal disease — revised guidelines from CDC, 2010. MMWR Recomm. Rep. 2010;59(RR-10):1–36.
- Rosini R., Margarit I. Biofilm formation by Streptococcus agalactiae: influence of environmental conditions and implicated virulence factors. Front. Cell. Infect. Microbiol. 2015;5:6. DOI: https://doi.org/10.3389/fcimb.2015.00006
- Le Doare K., O’Driscoll M., Turner K., et al. Intrapartum antibiotic chemoprophylaxis policies for the prevention of group B streptococcal disease worldwide: systematic review. Clin. Infect. Dis. 2017;65(Suppl. 2):S143–51. doi: https://doi.org/10.1093/cid/cix654
- Jones N., Bohnsack J.F., Takahashi S., et al. Multilocus sequence typing system for group B streptococcus. J. Clin. Microbiol. 2003;41(6):2530–6. doi: https://doi.org/10.1128/jcm.41.6.2530-2536.2003
- Russell N.J., Seale A.C., O’Sullivan C., et al. Risk of early-onset neonatal group B streptococcal disease with maternal colonization worldwide: systematic review and meta-analyses. Clin. Infect. Dis. 2017;65(Suppl. 2):S152–9. doi: https://doi.org/10.1093/cid/cix655
- Margarit I., Rinaudo C.D., Galeotti C.L., et al. Preventing bacterial infections with pilus-based vaccines: the group B streptococcus paradigm. J. Infect. Dis. 2009;199(1):108–15. doi: https://doi.org/10.1086/595564
- Madhi S.A., Dangor Z., Heath P.T., et al. Considerations for a phase-III trial to evaluate a group B Streptococcus polysaccharide-protein conjugate vaccine in pregnant women for the prevention of early- and late-onset invasive disease in young-infants. Vaccine. 2013;31(Suppl. 4):D52–7. doi: https://doi.org/10.1016/j.vaccine.2013.02.029
- Absalon J., Segall N., Block S.L., et al. Safety and immunogenicity of a novel hexavalent group B streptococcus conjugate vaccine in healthy, non-pregnant adults: a phase 1/2, randomised, placebo-controlled, observer-blinded, dose-escalation trial. Lancet Infect. Dis. 2021;21(2):263–74. doi: https://doi.org/10.1016/s1473-3099(20)30478-3
- Bjerkhaug A.U., Ramalingham S., Mboizi R., et al. The immunogenicity and safety of Group B Streptococcal maternal vaccines: a systematic review. Vaccine. 2024;42(2):84–98. doi: https://doi.org/10.1016/j.vaccine.2023.11.056
- Yao K., Poulsen K., Maione D., et al. Capsular gene typing of Streptococcus agalactiae compared to serotyping by latex agglutination. J. Clin. Microbiol. 2013;51(2):503–7. doi: https://doi.org/10.1128/jcm.02417-12
- Teatero S., Neemuchwala A., Yang K., et al. Genetic evidence for a novel variant of the pilus Island 1 backbone protein in group B Streptococcus. J. Med. Microbiol. 2017;66(10):1409–15. DOI: https://doi.org/10.1099/jmm.0.000588
- Колоусова К.А., Шипицына Е.В., Шалепо К.В., Савичева А.М. Факторы вирулентности и патогенности штаммов Streptococcus agalactiae, выделенных у беременных и новорождённых. Журнал акушерства и женских болезней. 2021;70(5):15–22. Kolousova K.A., Shipitsyna E.V., Shalepo K.V., Savicheva A.M. Virulence and pathogenicity factors of S. agalactiae strains isolated from pregnant women and newborns. Journal of Obstetrics and Woman Disease. 2021;70(5):15–22. doi: https://doi.org/10.17816/JOWD75671 EDN: https://elibrary.ru/oateln
- Shipitsyna E., Shalepo K., Zatsiorskaya S., Krysanova A., et al. Significant shifts in the distribution of vaccine capsular polysaccharide types and rates of antimicrobial resistance of perinatal group B streptococci within the last decade in St. Petersburg, Russia. European Journal of Clinical Microbiology & Infectious Diseases. 2020;39(8):1487–93. doi: https://doi.org/10.1007/s10096-020-03864-1 EDN: https://elibrary.ru/xrupas
- Bianchi-Jassir F., Paul P., To K.N., et al. Systematic review of Group B Streptococcal capsular types, sequence types and surface proteins as potential vaccine candidates. Vaccine. 2020;38(43):6682–94. doi: https://doi.org/10.1016/j.vaccine.2020.08.052
- Nanduri S.A., Petit S., Smelser C., et al. Epidemiology of invasive early-onset and late-onset group B Streptococcal disease in the United States, 2006 to 2015: multistate Laboratory and Population-Based Surveillance. JAMA Pediatr. 2019; 173(3):224–33. doi: https://doi.org/10.1001/jamapediatrics.2018.4826
- Li S., Wen G., Cao X., et al. Molecular characteristics of Streptococcus agalactiae in a mother-baby prospective cohort study: Implication for vaccine development and insights into vertical transmission. Vaccine. 2018;36(15):1941–8. doi: https://doi.org/10.1016/j.vaccine.2018.02.109
- Brokaw A., Furuta A., Dacanay M., et al. Waldorf bacterial and host determinants of group B streptococcal vaginal colonization and ascending infection in pregnancy. Front. Cell. Infect. Microbiol. 2021;11:720789. doi: https://doi.org/10.3389/fcimb.2021.720789
- Swamy G.K., Metz T.D., Edwards K.M., et al. Safety and immunogenicity of an investigational maternal trivalent group B streptococcus vaccine in pregnant women and their infants: results from a randomized placebo-controlled phase II trial. Vaccine. 2020;38(44):6930–40. doi: https://doi.org/10.1016/j.vaccine.2020.08.056
- Liu Y., Liu J. Group B streptococcus: virulence factors and pathogenic mechanism. Microorganisms. 2022;10(12):2483. doi: https://doi.org/10.3390/microorganisms10122483
- Rinaudo C.D., Rosini R., Galeotti C.L., et al. Specific involvement of pilus type 2a in biofilm formation in group B Streptococcus. PLoS One. 2010;5(2):e9216. doi: https://doi.org/10.1371/journal.pone.0009216
- Margarit I., Rinaudo C.D., Galeotti C.L., et al. Preventing bacterial infections with pilus-based vaccines: the group B streptococcus paradigm. J. Infect. Dis. 2009;199(1):108–15. doi: https://doi.org/10.1086/595564
Supplementary files