Study of humoral and cellular immunity following the immunization of C57Bl/6 mice with a prototype of the inactivated Chikungunya vaccine
- Authors: Otrashevskaia E.V.1, Kaa K.V.2, Oksanich A.S.1, Murashko N.V.1, Kusliy A.G.3, Krasko A.G.4, Zverev V.V.1, Ignatyev G.M.1
-
Affiliations:
- I.I. Mechnikov Research Institute for Vaccines and Sera
- Chumakov Federal Scientific Center for Research and Development of Immune-and- Biological Products
- Vector-Bialgam
- Republican Scientific-Practical Center of Epidemiology and Microbiology
- Issue: Vol 101, No 2 (2024)
- Pages: 193-207
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/16587
- DOI: https://doi.org/10.36233/0372-9311-436
- EDN: https://elibrary.ru/qlerjf
- ID: 16587
Cite item
Abstract
Introduction. Chikungunya virus infection is a problem for the health care system of affected regions due to the lack of specific prevention, as well as effective antiviral drugs. The critical role of cellular immunity in viral control and clearance for the Chikungunya fever has been demonstrated in many studies. Therefore, effective stimulation of not only humoral but also cellular immunity is of undeniable importance when assessing the effectiveness of a potential vaccine for the prevention of this infection.
The aim of the present study was to investigate the formation of protective immunity after administration of a drug containing inactivated Chikungunya virus (CHIKV) to C57Bl/6 mice.
Materials and methods. Inactivated CHIKV (concentrations of 10 μg and 40 μg) was administered intramuscularly to C57Bl/6 mice twice with an interval of 14 days. Indicators of humoral immunity were assessed by ELISA and neutralization test (NT), cellular immunity — by the production of IFN-γ and splenocyte proliferation in vitro. The concentration of cytokines IL-1, IL-2, IL-6, IL-10, IL-12p70 and TNF was determined by ELISA. When assessing the protective immunity in animals, CHIKV was injected into the dorsal surface of the foot of the right hind paw at a dose of 2.89 ± 0.10 lg TCD50 in a volume of 20 μl.
Results. The most pronounced immune response was noted to the administration of 40 μg of inactivated CHIKV, which was manifested in the balanced production of the studied cytokines, the formation of specific humoral (according to the results of ELISA and NT) and cellular — specific proliferation of splenocytes in vitro and production of IFN-γ. When assessing efficacy, the development of foot edema in immunized animals was significantly lower than in animals in the control group.
Discussion. CHIKV, inactivated by beta-propiolactone, had pronounced immunogenic properties. The balance of production of pro- and anti-inflammatory cytokines, as well as the Th1/Th2 immune response, characterized the formation of adaptive immunity in mice without a pronounced inflammatory response. The formation of a specific humoral and cellular immune response has been demonstrated. A study of protection in a non-lethal animal model confirmed the efficacy of the inactivated vaccine.
Conclusion. Double administration of the inactivated CHIKV vaccine at a dose of 40 μg to C57Bl/6 mice demonstrated pronounced immunogenicity, which allows us to evaluate it as a promising prophylactic vaccine.
Full Text
Introduction
Research on Chikungunya virus (CHIKV) has received global attention in the recent decades because of its wide geographical distribution, affecting countries in Asia, Oceania, Africa, North, Central and South America. Chikungunya virus infection is a public health problem in endemic regions due to the lack of specific prophylaxis as well as effective antiviral drugs. Between 2004 and 2009, a sudden epidemic of Chikungunya fever affected 31 million people in the Indian peninsula [1, 2]. In 2013, CHIKV from several Caribbean countries spread rapidly to 45 countries in North, Central, and South America [3]. The lethality of Chikungunya fever is low, mainly among newborns and patients with chronic diseases of the cardiovascular, respiratory, and nervous systems [1, 4]. However, lesions of the musculoskeletal system (arthritis and arthralgia) caused by Chikungunya fever often lead to long-term disability. The cause of arthritic joint damage is immune-mediated mechanisms triggered by the pronounced production of pro-inflammatory mediators. CHIKV infection is believed to provide lifelong immunity, and recurrent infections are practically unreported [5, 6]. This factor, along with the lack of specific therapy for both the infection itself and its consequences, makes vaccination the most promising way to prevent Chikungunya fever. It should also be kept in mind that, in addition to the population of Chikungunya endemic countries, there is a population of travelers and professionals visiting or working in endemic countries, but there is also a risk of Chikungunya-endemic regions in developed countries due to climate change and other unforeseen factors that may contribute to the emergence and spread of infection. The fact that the U.S. Food and Drug Administration (FDA) and the European Medicines Agency have granted Fast Track/Priority Medicine status to several vaccine candidates for the prevention of Chikungunya disease confirms the urgency of the problem and the challenge of bringing effective medical products to market to prevent Chikungunya fever.1
In 2023, Valneva's Ixchiq vaccine (VLA1553),2 developed from an attenuated strain of CHIKV with a deletion of nonstructural protein genes, was registered in the FDA [4]. A number of potential candidate vaccines have been tested on animal models in preclinical trials and on humans in clinical trials [4, 7]. At this stage, phase I clinical trials of an inactivated vaccine3 developed by Indian scientists have been completed, which has shown sufficient immunogenicity in laboratory animals [8]. However, no inactivated vaccine for the prevention of Chikungunya fever has been registered on the market, which makes its development an objective of high priority. Inactivated vaccine technology is traditional and successful for a large number of viral vaccines. This technological platform is considered to be one of the safest [4, 8–11], and drug development with it does not require genetic manipulation of the virus. In small comparative studies on BALB/c mice, the advantage of a beta-propiolactone inactivated CHIKV antigen preparation over a formalin-inactivated preparation in the formation of specific humoral and cellular immunity was demonstrated [8, 9]. Previously, a dose-dependent effect of humoral and cellular immunity formation in BALB/c mice on the administration of beta-propiolactone inactivated CHIKV antigen was demonstrated [11].
Heteroploid cells of cell line 4647 were used for the preparation of an experimental inactivated vaccine for the prevention of Chikungunya fever at the stage of virus propagation. This cell line was obtained in 1974 from the kidneys of an adult green monkey. The cell bank of cell line 4647, established in 1983, has passed national licensing and at this stage fully meets the recommendations of the World Health Organization. This cell line has been authorized for use in the production of inactivated vaccines [12]. Previously, the possibility of culturing 4647 cells on microcarriers in bioreactors was demonstrated [13]. In our country, the vaccine for hepatitis A prophylaxis Algavak M, which uses this cell line (Vector-BiAlgam), is registered and produced. High sensitivity of cell line 4647, along with Vero cell line, to CHIKV has also been shown [14].
The immunogenicity of candidate vaccines against Chikungunya is evaluated by developers not only from the angle of humoral immunity formation, as a gold standard of neutralizing activity of specific post-vaccine antibodies, but also from the angle of cellular immunity formation, which is extremely important for virus clearance in Chikungunya fever [4, 8, 10]. For the experimental study of Chikungunya fever, lower primates are the main models used, since they are natural hosts of CHIKV in nature and the pathogenesis of the disease has a similar pattern to humans. White mice of different lines are used as laboratory animals in the stages of pre-clinical trials. Adult immunodeficient mice, such as AG129 mice, are used to model lethal infection, and immunocompetent mice of the C57Bl/6, Swiss albino or BALB/c lines are used for the non-lethal model. Previously, J. Gardner et al. described a non-lethal model of CHIKV infection in which C57Bl/6 mice developed symptoms similar to human joint lesions and developed viremia for 4–5 days. When CHIKV was inoculated into the dorsal side of the foot, C57Bl/6 mice developed perimetadorsal swelling with maximum effect on the 3rd–7th day, and the development of arthritis, tendonitis, and fasciitis was also noted, as confirmed by histologic evidence of acute and persistent inflammation [15, 16]. This model is often used to evaluate the efficacy of vaccine preparations being developed against CHIKV [17–20]. We also infected mice in the foot to evaluate the efficacy of the inactivated vaccine for the prevention of Chikungunya fever. To evaluate the immunogenicity of the candidate inactivated vaccine, we studied humoral and cellular immunity in C57Bl/6 line mice after double immunization with different doses of the drug, as well as the in vivo efficacy of the drug. Since the normal functioning of the immune system is based on the balance of Th1 and Th2 based on their production of regulatory cytokines, we additionally studied the concentration of individual cytokines in the serum of immunized animals in dynamics.
The aim of the study was to investigate the formation of protective immunity after administration of a preparation containing CHIKV to C57Bl/6 mice.
Materials and methods
The strain Nika2021, obtained from the virus collection of the Nicaragua branch of SPbSRIVS of Russian FMBA, was used in the experiments. The technique of isolation and passivation of the CHIKV strain has been described previously [21]. The nucleotide sequence of CHIKV strain Nika2021 is presented in GenBank, acc.no OQ320495.
Cell line 4647 (Vector-Bialgam Production Bank) was cultured in Igla MEM medium (BioloT) with 5% fetal calf serum (BioloT).
C57Bl/6 mice (haplotype H-2b) of both sexes weighing 12-14 g were used in the study. The animals were obtained from the Stolbovaya cattery of the Scientific Center for Biomedical Technologies of the Federal Medical and Biological Agency of the Russian Federation. The study protocol was approved by the Ethics Committee of the I.I. Mechnikov Research Institute for Vaccines and Sera (protocol No. 6, April 4, 2023).
Vaccine
Propagation of the Nika2021 CHIKV strain was carried out in cell line 4647 by roller cultivation using a multiplicity of 0.0001 TCD50 infection per cell. Inactivation was carried out with beta-propiolactone (at a ratio of 1 : 1000) under constant agitation for 48 h at 5ºC. The inactivated virus-containing fluid was concentrated 50-fold by ultrafiltration (Vivaflow 100 concentrator (Sartorius)). The inactivated virus concentrate was purified by exclusion chromatography (Sepharose-6FF sorbent (GE Healthcare)). Control of the obtained CHIKV preparation was carried out according to the following parameters: sterility, pH, absence of endotoxins, content of residual DNA of 4647 cells according to the methods set forth in the State Pharmacopoeia4. The preparation was sterile, endotoxins were absent, residual DNA content of 4647 cells < 5 ng/mL, pH 7.4. The concentration of inactivated CHIKV was determined by enzyme-linked immunosorbent assay (ELISA) using the BioScreen Chikungunya (Ag) kit (Bioservice). The resulting inactivated preparation at concentrations of 10 and 40 μg of antigen was sorbed on aluminum hydroxide (Al(OH)3, Brenntag SE). The Al(OH)3 content of the final drug dose was 0.5 mg per dose (0.5 mL). The doses of 10 and 40 μg were used because previously these doses were used to immunize BALB/c mice (haplotype H-2d) and to evaluate the content of specific antibodies in serum in dynamics [14]. In these experiments, the dose of the preparation containing 10 µg of CHIKV antigen, when administered twice, caused an insignificant rise in specific antibodies in the group of animals after the second immunization with a drop by the 35th day of observation. In the serum of animals immunized twice with a dose of 40 µg of CHIKV antigen, a rise in specific antibodies was observed 7 days after the first immunization and persisted until day 35 [14].
Due to the available data, the same antigen concentrations were chosen for the current study. Al(OH)3 at a concentration of 0.5 mg per injection volume (0.5 ml) was used as a control preparation.
Methods
ELISA analysis of antibodies (IgG) titer to CHIKV was performed as previously described [14]. Test serum samples were diluted 1 : 200 and further in step "2" to 1 : 12,800 in phosphate-buffered saline (PBS). Each serum dilution was evaluated in 3 repetitions. A serum dilution that was more than 2 times the background value was considered positive. Serum obtained from mice before immunization was used as a negative control.
The neutralization test (NT) was performed as described previously [11] in 96-well plates using Vero cell culture and Nica2021 strain at a dose of 100 TCD50. The result obtained was converted to log2 for statistical processing.
The proliferative activity of splenocytes was studied by photometric method according to the previously described method [14, 25]. The following antigens were used to stimulate the proliferative activity of splenocytes: CHIKV antigen inactivated by beta-propiolactone (5 μg/mL); hepatitis A virus (HAV) antigen used for the production of Algavac-M vaccine (5 μg/mL), as well as the following mitogens: concanavalin A (ICN; 5 μg/mL) and Salmonella typhimurium lipopolysaccharide (Sigma; 5 μg/mL). Each antigen and mitogen was used to stimulate animal splenocytes in vitro in 4 repeats. FSB was used as a control.
Determination of interferon-γ (IFN-γ) production by mouse splenocytes in vitro. Mouse splenocytes were placed in wells of 24-well plates at a concentration of 2 × 106 cells/mL. CHIKV and HAV antigens at concentrations of 5 μg/mL were added to the cells to stimulate IFN-γ production. Concanavalin A (ICN; 5 μg/mL) was used as a positive control for stimulation of IFN-γ production, and PBS was used as a negative control. Cells were cultured for 48 h in 5% CO2 at 37ºC. At the end of cultivation, the culture fluid of activated and control cells was collected and the concentration of IFN-γ was determined using a mouse kit (R&D Systems).
The concentration of cytokines in the mice sera — interleukins IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12p70, tumor necrosis factor-α (TNF-α), IFN-γ — was determined using mouse kits (R&D Systems) according to the manufacturer’s instructions.
To assess the efficacy, the test was performed as described previously [15]. Animals were injected with CHIKV at a dose of 2.89 ± 0.10 lg TCD50 in a volume of 20 μl into the dorsal surface of the foot of the right hind paw. To control the specificity of the swelling, 20 μL of 0.9% NaCl infusion solution was injected into the foot of the left hind paw. The degree of perimetadorsal edema was assessed on day 6. After measuring the height and width with an electronic caliper at the site of maximum swelling of both feet of the hind paws, the swelling index (width × height of the infected foot/width × height of the opposite foot) was calculated. To assess the viremia after infection, the biological activity of CHIKV was determined in the sera of mice by titration of virus in Vero cell line [14]. The result of titration was considered by the expressed cytopathic effect and the tissue cytopathic dose causing 50% cell death (TCD50) was calculated by Kerber's method modified by Ashmarin and expressed in log10 TCD50/mL [23].
Statistical analysis of the obtained data was performed using the standard software package Microsoft Office Excel 2016. Data are presented as mean (M) and standard deviation of the mean (SD), where acceptable. The validity of differences between the compared values was assessed using Student's t-test of unpaired, two-tailed distribution (t-test). Differences were considered statistically significant at a significance level of p < 0.05.
Study design
The animals were divided into the following groups:
- Group A — 100 mice immunized with the drug at a dose of 40 µg/0.5 ml on the initial and 14th days of the experiment;
- Group B — 100 mice immunized with the drug at a dose of 10 µg/0.5 ml on the initial and 14th days of the experiment;
- Group C — 100 mice that were intramuscularly injected with Al(OH)3 on the initial and 14th days of the experiment.
The drug was injected into the thigh muscle intramuscularly, dividing 1 dose into 2 injections, 0.25 ml of the drug in each limb.
Animals were observed up to 35 days (21st day after the 2nd immunization). Five animals from each group on the 0th day (before immunization), 14th day (before the 2nd immunization), 21st, 28th and 35th days after the start of immunization were used to collect blood samples, which were pooled for further determination of antibody content by two methods. The spleen was isolated from the same animals for further obtaining splenocyte suspension. Splenocytes were used in studies of proliferative activity and IFN-γ production according to the methods described above. In another 5 animals from each group on the initial, 1, 3, 5, 7, 9, 14, 15, 17, 19, 21, 28 and 35th days of observation blood was collected from the retro-orbital sinus of the eye and individual blood samples were collected for further determination of cytokine profile. All serum samples after centrifugation were transferred into tubes in a volume of 200 μl and stored at –70ºC for subsequent one-stage study. Also on the 35th day, 10 remaining mice from each group were used to evaluate the efficacy of the candidate drug in the non-lethal model described previously [15].
All procedures on individual mice were performed outside visual, auditory or olfactory contact with other animals. Work with animals was carried out in accordance with the International Principles of the "European Convention for the Protection of Vertebrate Animals Used for Experiments and Other Scientific Purposes" ETS No. 123 (Strasbourg, 1986) and Decision of the Council of the Eurasian Economic Commission from 03.11.2016 No. 81 "On Approval of the Rules of Good Laboratory Practice of the Eurasian Economic Union in the field of circulation of medicines".
Results
Humoral immunity
The dose of the preparation containing 10 µg of CHIKV antigen caused an insignificant and short-term rise in specific antibodies in ELISA after the 2nd immunization of animals of group B (Table 1). In this group of animals, the maximum level of specific antibodies was 4 times lower than in the group of animals of group A. Administration of the preparation containing 40 µg of CHIKV antigen to animals of group A resulted in the formation of a sufficiently high level of specific antibodies on the 14th day after the 1st immunization, which increased 4-fold after the 2nd vaccination by the end of the observation period (Table 1).
Table 1. Specific humoral immunity indices in C57Bl/6 mice immunized with the preparation contained different CHIKV antigen dose
Group | NT, log2 | ELISA IgG, titer | NT, log2 | ELISA IgG, titer | NT, log2 | ELISA IgG, titer | NT, log2 | ELISA IgG, titer | NT, log2 | ELISA IgG, titer |
0 day (1st immunization) | 14th day (2nd immunization) | 21st day | 28th day | 35th day | ||||||
А | Н. о. N. d. | Н. о. N. d. | 4,80 ± 0,14* | 1 : 1600** | 5,45 ± 0,15* | 1 : 3200** | 6,84 ± 0,18* | 1 : 6400** | 6,80 ± 0,38* | 1 : 6400** |
В | Н. о. N. d. | Н. о. N. d. | 2,16 ± 0,18 | 1 : 200 | 2,20 ± 0,18 | 1 : 400 | 3,12 ± 0.10 | 1 : 400 | 2,20 ± 0,10 | 1 : 200 |
С | Н. о. N. d. | Н. о. N. d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Note. *р < 0.01, **р < 0.001 in comparison with group В. N. d. — not detected. Animals were bled on days 0 and 14 for the specific immunity testing before antigen administration.
In both groups, viral neutralizing antibodies were registered on day 14 after the 1st immunization and reached maximum values after the 2nd immunization (Table 1). In animals of group B, virus-neutralizing antibodies were significantly lower (p < 0.01) than in group A. In the serum of animals of group C, no antibodies were detected in any of the methods.
Cellular immunity
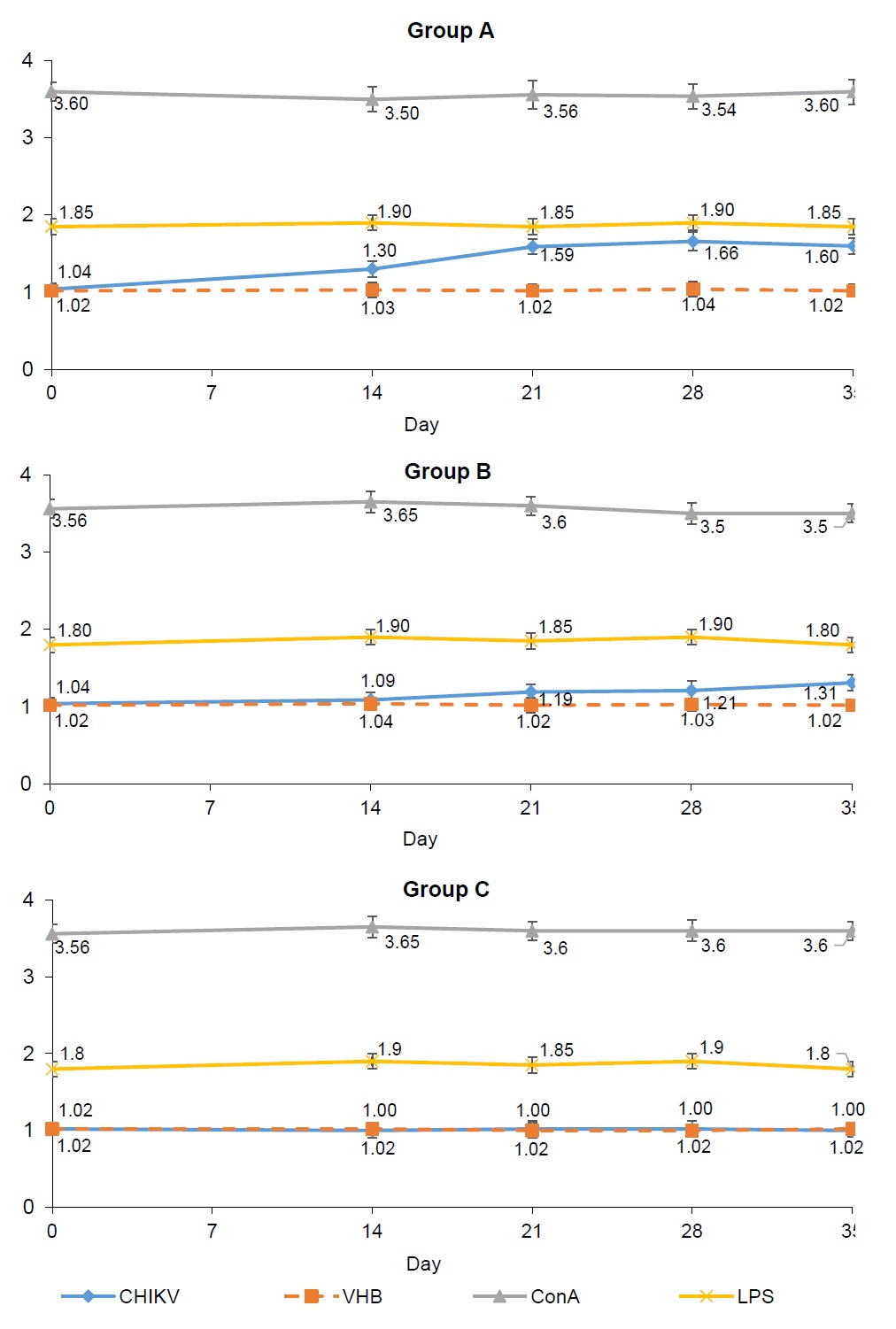
The effect of immunization with an inactivated preparation of CHIKV antigen on the formation of a specific cellular response was evaluated in the splenocyte proliferation reaction and IFN-γ production after antigen stimulation. The used viral antigens (specific CHIKV and heterologous HAV) did not inhibit splenocyte proliferation in vitro on day "0" (Fig. 1).
In response to stimulation with mitogens (concanavalin A and lipopolysaccharide), splenocytes from animals of all groups showed pronounced proliferation, which was significantly higher than that with viral antigens at all periods of observation (p < 0.05). The index of stimulation (IS) of splenocytes of animals in group A was statistically higher, starting from the 7th day after the 2nd immunization and up to the end of observation, (p < 0.005 on the 21st and 28th days; p < 0.05 on the 35th day) relative to the similar indicators in animals in group B. The statistically significant difference between the IS of splenocytes by CHIKV antigen in animals of groups A and B at all observation periods after the 2nd immunization indicates not only the specificity of the proliferative response of splenocytes in immune animals, but also a dose-dependent effect. Splenocytes of non-immune animals of group C responded with a pronounced proliferation to stimulation with only T- and B-cell mitogens (concanavalin A and lipopolysaccharide), while IS did not change upon stimulation with viral antigens at all observation periods (Fig. 1).
The increase in IFN-γ production by splenocytes of animals during their stimulation with specific antigen (Table 2) indicates the formation of a specific cellular immune response in immunized animals.
Table 2. Production of IFN-γ in response to splenocytes in vitro stimulation of C57Bl/6 mice, immunized with the preparation contained different CHIKV antigen dose
Group | Antigen | INF-γ concentration, pg/ml | ||||
0 day (1st immunization) | 14th day (2nd immunization) | 21st day | 28th day | 35th day | ||
A | CHIKV | 5,0 ± 0,4 | 12,8 ± 1,3*+# | 49,0 ± 4,2*+# | 57,0 ± 5,7*+# | 47,0 ± 2,7*+# |
VHB | 5,4 ± 0,9 | 4,8 ± 0,8 | 5,4 ± 0,9 | 5,0 ± 0,7 | 5,8 ± 0,4 | |
Conkanavalin A | 1066,0 ± 84,0 | 1110,0 ± 22,4 | 1269,0 ± 74,7 | 1093,0 ± 55,5 | 1125,0 ± 125,0 | |
PBS | 5,8 ± 0,4 | 5,4 ± 0,9 | 5,4 ± 0,9 | 5,0 ± 0,7 | 4,4 ± 0,5 | |
B | CHIKV | 4,4 ± 0,5 | 6,8 ± 0,8 | 19,6 ± 3,6*# | 16,0 ± 1,6*# | 13,6 ± 2,7*# |
VHB | 5,0 ± 0,7 | 5,0 ± 0,7 | 5,4 ± 0,9 | 5,0 ± 0,7 | 5,4 ± 0,9 | |
Conkanavalin A | 1133,0 ± 90,9 | 1108,0 ± 36,8 | 1249,0 ± 57,3 | 1229,0 ± 27,0 | 1089,0 ± 73,7 | |
PBS | 5,9 ± 0,8 | 5,9 ± 0,8 | 5,0 ± 0,7 | 5,0 ± 0,7 | 4,4 ± 0,5 | |
C | CHIKV | 5,0 ± 0,7 | 5,0 ± 0,7 | 5,4 ± 0,9 | 4,8 ± 0,8 | 4,8 ± 0,8 |
VHB | 5,0 ± 0,7 | 5,4 ± 0,9 | 4,8 ± 0,8 | 5,8 ± 0,4 | 5,4 ± 0,9 | |
Conkanavalin A | 1153,0 ± 29,5 | 1145,0 ± 94,2 | 1249,0 ± 35,8 | 1128,0 ± 56,6 | 1113,0 ± 46,6 | |
PBS | 5,0 ± 0,7 | 5,8 ± 0,4 | 5,4 ± 0,9 | 5,0 ± 0,7 | 5,8 ± 0,4 | |
Note. *р < 0.05 in comparison with day 0; +р < 0.005 in comparison to those in group В; #р < 0.05 in comparison to those in group C. Animals were bled on days 0 and 14 for the specific immunity testing before antigen administration.
IFN-γ production by group A animal splenocytes was significantly higher than in group B at all observation periods (p < 0.05–0.005). In animals of group A, the increased production of IFN-γ by splenocytes persisted up to 35 days of observation. The dose-dependent effect of IFN-γ production by splenocytes of immune animals was demonstrated.
Cytokine concentrations in the serum of mice
Administration of Al(OH)3 did not lead to a significant increase in the concentration of any of the studied cytokines in the serum of mice. On the contrary, administration of inactivated CHIKV antigen led to an increase in the concentration of the cytokines studied both after the 1st and 2nd immunization with maximum elevations after the 1st immunization. The exception was the production of IFN-γ. By the 35th day after the 1st immunization, the production of all cytokines studied returned to the initial values (Fig. 2–4).
Fig. 2. Dynamic of cytokines IL-6, IL-10, IL-12 in C57Bl/6 mice’ sera immunized with the different doses of inactivated CHIKV. Here and on Figs. 3, 4: arrow — day of immunization; animals were bled on days 0 and 14 for the specific immunity testing before antigen administration.
Fig. 3. Dynamic of cytokines TNF-α and IL-1β in C57Bl/6 mice’ sera immunized with the different doses of inactivated CHIKV.
Fig. 4. Dynamic of cytokines IL-2, IL-4 and IFN-γ in C57Bl/6 mice’ sera immunized with the different doses of inactivated CHIKV.
The maximum rise in the concentration of IL-6, IL-10 and IL-12 in the blood serum of mice was observed on the 1st day after the 1st immunization (Fig. 2). IL-10 concentration increased almost 4-fold relative to the initial level (p < 0.0001), i.e. to a greater extent than IL-6 and IL-12 concentrations, which increased almost 2-fold (p < 0.001). The concentration of IL-10, IL-12 and IL-6 in group A animals vaccinated with 40 µg dose was significantly higher than in group B animals vaccinated with 10 µg dose both after the 1st and after the 2nd immunization (p < 0.001 for IL-10, IL-12 and p < 0.005 for IL-6).
The maximum rise in the concentration of IL-1β and TNF-α in the serum of mice was detected on the 3rd day after the 1st immunization; p < 0.001 relative to the initial level (Fig. 3). At the same time, the concentration of IL-1β and TNF-α in animals of group A, immunized with a dose of 40 μg, was significantly higher than in animals of group B, immunized with a dose of 10 μg after the 1st (p < 0.001) and 2nd immunization (p < 0.005).
The maximum rise in IL-2 and IL-4 concentration was observed on the 7th day after the 1st immunization (Fig. 4). In group A IL-4 showed almost 4-fold increase (p < 0.0001). IL-4 and IL-2 concentrations in group A animals immunized with a 40 µg dose were significantly higher than those in group B animals immunized with a 10 µg dose, both after the 1st and 2nd immunizations (p < 0.001).
The dynamics of IFN-γ in both groups of immunized animals was different from all other cytokines. A very moderate rise in IFN-γ level (p < 0.05) after the 1st immunization on days 3–7 and a pronounced rise (5-fold) after the 2nd immunization (p < 0.0001) were observed in group A. In group B, there was practically no rise in IFN-γ after the 1st immunization, but after the 2nd immunization the rise was statistically significant (p < 0.01). The maximum concentration of IFN-γ in group A animals vaccinated with 40 µg dose was significantly higher than in group B animals vaccinated with 10 µg dose after the 2nd immunization (p < 0.0001). The dynamics of IFN-γ production in animal splenocytes, described above, also demonstrated a more significant rise in IFN-γ levels after the 2nd immunization of animals in both groups.
The results of evaluating the efficacy of the candidate vaccine are presented in Table 3. It should be noted that injection of 0.9% NaCl solution into the foot of the animal was not accompanied by an increase in edema. The foot measurements were used to calculate the edema index, which differed statistically significantly between vaccinated and unvaccinated animals; group A animals had a statistically lower edema index relative to that of group B animals.
Table 3. Feet swelling indices and CHIKV titres’ in the mice’ sera twice immunized with the different doses of inactivated CHIKV antigen measured after the CHIKV injection in a dose of 2,89 ± 0,10 lg ТCID50/ml into the dorsal part of foot
Group | Swelling index | CHIKV titer, lg ТCID50/ml | |
6th day | 3th day | 6th day | |
А | 1,2 ± 0,21+# | Н.о. | N.d. | Н.о. | N.d. |
В | 2,1 ± 0,24 | 1,4 ± 0,13# | Н.о. | N.d. |
С | 3,04 ± 0,21+ | 4,3 ± 0,18 | 1,8 ± 0,18 |
Note. +р < 0.05 in comparison with group В; #р < 0.005 in comparison with group C. N.d. — not detectable (lower threshold).
Viremia was observed at both observation points only in the group of non-immunized animals of group C. In contrast, no viremia was detected in group A animals immunized with the maximum dose of CHIKV antigen.
Discussion
Lifelong immunity and the virtual absence of re-infection, cross-protection between different strains of CHIKV [2, 4–6] and the lack of specific therapy for infection make vaccination the most promising way to prevent Chikungunya fever.
Recognizing the urgent need for an effective vaccine and the lack of its availability on the market, the World Health Organization supports countries in surveillance and control of arboviruses, including CHIKV5. Various strategies and technological platforms have been used to develop an effective vaccine [4, 7]. It should be noted that the safety of inactivated vaccines is achievable solely due to the cost of organizing production, compliance with the necessary biosafety conditions, and constant monitoring of the completeness of virus inactivation. Nevertheless, the advantages of inactivated vaccines, such as the absence of the risk of virulence reversion of the vaccine strain, as well as the stability of the preparation itself, which makes storage and transportation much easier and cheaper, explain the widespread use of this technology platform for vaccine development. In the present study, we demonstrated the immunogenic properties of a beta-propiolactone inactivated preparation for the prevention of Chikungunya fever after double administration into C57Bl/6 mice. The immunogenicity of the candidate drug was confirmed by the formation of specific humoral and cellular immunity in laboratory animals, as well as by a balanced response of T-helper clones. The results obtained in this study are comparable to those previously described in a study by M. Tiwari et al. [8]. The maximum increase in specific antibodies in ELISA and NT reactions, as well as in splenocyte IS in vitro, was observed in animals inoculated with both doses of the preparation after double immunization. However, high titers of specific antibodies and IS of splenocytes were maintained until the end of the observation period only in the group of animals immunized with the preparation containing 40 µg of CHIKV antigen. The dose-dependent effect of the preparation was observed both when analyzing the titers of specific antibodies and IS of splenocytes in vitro and when analyzing the concentrations of cytokines in the serum of mice. The production of all cytokines studied both in the serum of mice and during in vitro stimulation of animal splenocytes was maximal in the group of animals inoculated with the preparation containing 40 µg of CHIKV antigen. Studies of cytokine production after administration of the inactivated vaccine for the prevention of Chikungunya fever are not available in open scientific sources, to the best of our knowledge. However, it is extremely important to make sure that the formed post-vaccine immunity will be sufficiently balanced between T-helper clones, since the normal functioning of the immune system is based on the balance of Th1 and Th2, based on their production of regulatory cytokines. Unbalanced activation of T-helper clones can lead to the development of immunopathological conditions, which in the case of a preparation containing CHIKV antigen is of particular importance.
Of the proinflammatory cytokines, IL-6 was the first to respond to vaccine administration. TNF-α and IL-1β showed a moderate rise on the 3rd day, and the maximum rise of IL-2 was registered on the 7th day after the drug administration. The release of pro-inflammatory cytokines was balanced by a significant rise in anti-inflammatory IL-10 and IL-4. IL-10 is known to play a role not only in controlling inflammatory reactions and limiting immune overreaction, and its appearance in the first days also indicates stimulation of CHIKV-specific antibody production [24, 25]. There have been interesting studies on the role of IL-10 on the administration of Al(OH)3 to IL-10-deficient mice. Experimentally, we found that the absence of IL-10 signaling did not compromise Al(OH)3-induced cellular infiltration at the injection site, but did result in an enhanced antigen-specific Th1 response after injection. Al(OH)3 enhanced IL-10 transcription and secretion by macrophages and dendritic cells. Collectively, these results indicated that Al(OH)3 injection promotes the production of IL-10, which can block Th1-immune response [25]. IL-4 also serves as a marker of Th2 lymphocyte subpopulation activation. In our study, along with IL-10, there was a significant increase in IL-4 on the 7th day after vaccination. The sufficiently pronounced Th-2-cell immune response in our experiment can be explained not only by the efficacy of the inactivated CHIKV antigen itself, but also by the presence of the adjuvant Al(OH)3 in the preparation. Until recently, it was known that Al(OH)3-based adjuvants preferentially stimulate the Th2-cell immune response. It has been repeatedly demonstrated that aluminum salts induce Th2-cell response specifically in mice [8, 25]. Aluminum salts have been the most widely used adjuvants for almost a century. Aluminum was originally thought to provide a depot effect by which antigen is slowly released from the surface of microparticles at the site of administration, allowing antigen-presenting cells to gradually utilize the antigen. In recent years, it has been shown that the mechanism of action of aluminum salts as an adjuvant in humans is not as unambiguous and is much more complex than originally thought. Factors that strongly influence the immune response induced by the application of Al(OH)3 include adsorption rate, adsorption strength, size and homogeneity of Al(OH)3 particles, adjuvant dosage, and the nature of the antigens used [26, 27]. The current understanding of the mechanism of action of Al(OH)3-based adjuvants includes, in addition to the repository effect, a prophagocytic effect and activation of the proinflammatory pathway NLRP3. All this together stimulates both innate and adaptive immunity, as well as activates the complement system [26]. The secretion of IL-1β and IL-6 in vitro was demonstrated to increase significantly under the action of Al(OH)3 in human monocytes [28]. Aluminum salts induce uric acid production in vivo, which is a necessary factor for the infiltration of inflammatory cells [29]. In turn, elevated uric acid levels lead to NLRP3 activation of the inflammasome and thus IL-1β secretion [29]. Moreover, two new immunologically relevant cellular pathways of monocyte stimulation by Al(OH)3 have been identified: the first is type I IFN secretion, potentially induced by TLR and/or NOD-like signaling; the second is IFN-γ-induced presentation of HLA class I and II antigens [30]. The functional state of the Th1-subpopulation is usually judged by the production of IFN-γ by immunocompetent cells. The formation of specific cellular immunity in mice after double injection of inactivated CHIKV antigen at a dose of 40 μg in our study is confirmed by the in vitro splenocyte IS data, which corresponds to the published data [8, 11]. A 5-fold increase in the concentration of IFN-γ in the blood serum of mice after the 2nd immunization also testifies to the stimulation of specific cellular immunity. The powerful release of IL-10 on the 1st day after the drug administration to mice was followed by an increase in the production of not only IFN-γ, but also TNF-α and IL-2, which are also mediators of Th1-cell response. It can be argued that in our experiment the route of administration and the ratio of the dose of antigen and Al(OH)3 and, more likely, the combination of CHIKV antigen and Al(OH)3, led to a balanced response of pro- and anti-inflammatory cytokines, as well as stimulation of both humoral and cellular immunity.
To study the efficacy of the inactivated vaccine, we used a non-lethal model of CHIKV infection in C57Bl/6 mice, which had not been previously used by domestic developers of similar preparations. This model demonstrated the specificity of paw edema and transient virosemia in non-immune animals. In immunized animals, paw edema was statistically less significant, and viremia was recorded once only in the group of animals immunized with the minimum dose. This non-lethal model of CHIKV infection in mice allowed us to confirm the efficacy of the developed candidate vaccine against CHIKV, which is explained, among other things, by the balance between T-helper, Th1 and Th2 clones, which was confirmed by the concentration of regulatory cytokines in the serum of immunized animals.
The results of the presented study of immunogenicity of the preparation containing inactivated CHIKV, as well as the results of the study of efficacy of this preparation allow us to evaluate it as promising for further studies. The drug dose of 40 µg/0.5 ml and the scheme of twice-daily immunization can also be assessed as adequate. Our chosen technological platform allows us to produce the vaccine for Chikungunya fever prophylaxis on cell line 4647 rather quickly.
Conclusion
Two-fold administration of CHIKV virus (inactivated by beta-propiolactone and purified, 40 µg/0.5 ml) into C57Bl/6 mice provided the development of specific Th1/Th2-immune response – humoral (antibodies with viral neutralizing effect), cellular (expressed proliferation of splenocytes in vitro and IFN-γ production), as well as balanced production of pro- and anti-inflammatory cytokines. Efficacy studies in a non-lethal animal model confirmed the efficacy of the inactivated vaccine. The results obtained allow us to evaluate the vaccine as promising for the prevention of Chikungunya fever.
1 Code of Federal Regulations. Title 21. Section 601.91. Approval based on evidence of effectiveness from studies in animals. Washington: FDA; 2020.
2 FDA approves first vaccine to prevent disease caused by Chikungunya virus. U.S. Food and Drug Administration. Published November 9, 2023. Accessed November 12, 2023. URL: https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-prevent -disease-caused-chikungunya-virus
3 Phase-I open label, dose-escalation clinical trial to evaluate the safety, tolerability and immunogenicity of chikungunya vaccine in healthy adults of 18 to 50 years age. 2017. Clinical Trials Registry—India, CTRI, Hyderabad.
URL: https://clinicaltrials.gov/study/NCT04603131
4 F.S.3.3.1.0029.15. Vaccine for the prevention of hepatitis A, cultured, purified, concentrated, adsorbed, inactivated liquid. State Pharmacopoeia of the Russian Federation. Moscow; 2018. Vol. 4.
5 Chikungunya. URL: https://www.who.int/news-room/fact-sheets/detail/chikungunya
About the authors
Elena V. Otrashevskaia
I.I. Mechnikov Research Institute for Vaccines and Sera
Email: e.v.otrashevskaja@mail.ru
ORCID iD: 0000-0002-2491-4072
leading researcher, Laboratory of molecular biotechnology
Russian Federation, MoscowKonstantin V. Kaa
Chumakov Federal Scientific Center for Research and Development of Immune-and- Biological Products
Email: e.v.otrashevskaja@mail.ru
ORCID iD: 0000-0002-8446-1853
researcher, Laboratory of molecular biology of viruses
Russian Federation, MoscowAlexey S. Oksanich
I.I. Mechnikov Research Institute for Vaccines and Sera
Email: e.v.otrashevskaja@mail.ru
ORCID iD: 0000-0002-8600-7347
Cand. Sci. (Med.), senior researcher, Laboratory of molecular biotechnology
Russian Federation, MoscowNikita V. Murashko
I.I. Mechnikov Research Institute for Vaccines and Sera
Email: e.v.otrashevskaja@mail.ru
ORCID iD: 0009-0002-9161-064X
junior researcher, Laboratory of molecular biotechnology
Russian Federation, MoscowAlexander G. Kusliy
Vector-Bialgam
Author for correspondence.
Email: e.v.otrashevskaja@mail.ru
ORCID iD: 0000-0002-0732-9314
Cand. Sci. (Med.), quality director
Russian Federation, KoltsovoAnatoliy G. Krasko
Republican Scientific-Practical Center of Epidemiology and Microbiology
Email: e.v.otrashevskaja@mail.ru
ORCID iD: 0000-0002-2765-3525
Cand. Sci. (Med.), leading researcher, Republican collection of pathogenic biological agents
Belarus, MinskVitaly V. Zverev
I.I. Mechnikov Research Institute for Vaccines and Sera
Email: e.v.otrashevskaja@mail.ru
ORCID iD: 0000-0001-5808-2246
D. Sci. (Med.), Professor, RAS Full Member, scientifical director
Russian Federation, MoscowGeorge M. Ignatyev
I.I. Mechnikov Research Institute for Vaccines and Sera
Email: e.v.otrashevskaja@mail.ru
ORCID iD: 0000-0002-9731-3681
D. Sci. (Med.), Professor, main expert, Laboratory of molecular biotechnology
Russian Federation, MoscowReferences
- Weaver S.C., Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015;372(13):1231–9. doi: https://doi.org/10.1056/nejmra1406035
- Deeba D., Islam A., Kazim S. N., et al. Chikungunya virus: Recent advances in epidemiology, host pathogen interaction & vaccine strategies. Pathog. Dis. 2016;74(3):ftv119. doi: https://doi.org/10.1093/femspd/ftv119
- Staples J.E., Breiman R.F., Powers A.M. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin. Infect. Dis. 2009;49(6):942–8. doi: https://doi.org/10.1086/605496
- Отрашевская Е.В., Трухин В.П., Меркулов В.А., Игнатьев Г.М. Прогресс в разработке вакцин для профилактики лихорадки Чикунгунья и перспективы появления на рынке. БИОпрепараты. Профилактика, диагностика, лечение. 2023;23(1):42–64. Otrashevskaya E.V., Trukhin V.P., Merkulov V.A., Ignat'ev G.M. Chikungunya vaccines: advances in the development and prospects for marketing approval. Biological Products. Prevention, Diagnosis, Treatment. 2023;23(1):42–64. doi: https://doi.org/10.30895/2221-996X-2023-23-1-42-64 EDN: https://elibrary.ru/uoykrm
- Galatas B., Ly S., Duong V., et al. Long-lasting immune protection and other epidemiological findings after Chikungunya emergence in a Cambodian Rural Community, April (2012). PLoS Negl. Trop. Dis. 2016;10(1):e0004281. doi: https://doi.org/10.1371/journal.pntd.0004281
- Pierro A., Rossini G., Gaibani P., et al. Persistence of anti-chikungunya virus-specific antibodies in a cohort of patients followed from the acute phase of infection after the 2007 outbreak in Italy. New Microbes New Infect. 2015;7:23–5. doi: https://doi.org/10.1016/j.nmni.2015.04.002
- Reyes-Sandoval A. 51 years in of Chikungunya clinical vaccine development: A historical perspective. Hum. Vaccin. Immunother. 2019;15(10):2351–8. doi: https://doi.org/10.1080/21645515.2019.1574149
- Tiwari M., Parida M., Santhosh S.R., et al. Assessment of immunogenic potential of Vero adapted formalin inactivated vaccine derived from novel ECSA genotype of Chikungunya virus. Vaccine. 2009;27(18):2513–22. doi: https://doi.org/10.1016/j.vaccine.2009.02.062
- Kumar M., Sudeep A.B., Arankalle V.A. Evaluation of recombinant E2 protein-based and whole-virus inactivated candidate vaccines against Chikungunya virus. Vaccine. 2012;30(43): 6142–9. DOI: https://doi.org/10.1016/j.vaccine.2012.07.072
- Rudd P.A., Raphael A.P., Yamada M., et al. Effective cutaneous vaccination using an inactivated Chikungunya virus vaccine delivered by Foroderm. Vaccine. 2015;33(39):5172–80. doi: https://doi.org/10.1016/j.vaccine.2015.07.099
- Игнатьев Г.М., Каа К.В., Антонова Л.П. и др. Иммуногенные свойства препарата, содержащего инактивированный β-пропиолактоном антиген вируса Чикунгунья. Журнал микробиологии, эпидемиологии и иммунобиологии. 2021;98(5):519–27. Ignatyev G.M., Atrasheuskaya A.V., Antonova L.P., et al. Immunogenic properties of the preparation containing the Chikungunya virus antigen inactivated by β-propiolactone. Journal of Microbiology, Epidemiology and Immunobiology. 2021;98(5):519–27. DOI: https://doi.org/10.36233/0372-9311-159 EDN: https://elibrary.ru/kezrsh
- Миронова Л.Л., Конюшко О.И., Попова В.Д., Грачев В.П. К вопросу об использовании различных различных видов культур клеток в производстве противовирусных препаратов. Успехи современного естествознания. 2011;(12):43–5. Mironova L.L., Konushko O.I., Popova V.D., Grachev V.P. To the question about using various different types of cell cultures in the antiviral preparations’ production. Advances in Current Natural Sciences. 2011;(12):43–5. EDN: https://elibrary.ru/oiplor
- Радаева И.Ф., Думченко Н.Б., Нечаева Е.А. Культивирование клеток на микроносителях в биореакторах. Вестник ПНИПУ. Химическая технология и биотехнология. 2019;(2):22–32. Radaeva I.F., Dumchenko N.B., Nechaeva E.A. The cultivation of cells on microcarriers in bioreactors. PNRPU Bulletin. Chemical Technology and Biotechnology. 2019;(2):22–32. DOI: https://doi.org/10.15593/2224-9400/2019.2.02 EDN: https://elibrary.ru/vkxbiy
- Каа К.В., Игнатьев Г.М., Синюгина А.А., Ишмухаметов А.А. Чувствительность клеточных линий к вирусу Чикунгунья и подбор метода наработки вирусного материала в промышленных объемах. БИОпрепараты. Профилактика, диагностика, лечение. 2023;23(1):111–20. Kaa K.V., Ignatyev G.M., Sinyugina A.A., Ishmukhametov A.A. Susceptibility of various cell lines to the Chikungunya virus and method selection for commercial-scale production of viral material. Biological Products. Prevention, Diagnosis, Treatment. 2023;23(1):111–20. doi: https://doi.org/10.30895/2221-996X-2023-23-1-111-120 EDN: https://elibrary.ru/kcmppz
- Gardner J., Anraku I., Le T.T., et al. Chikungunya virus arthritis in adult wild-type mice. J. Virol. 2010;84(16):8021–32. doi: https://doi.org/10.1128/jvi.02603-09
- Morrison T.E., Oko L., Montgomery S.A., et al. A mouse model of chikungunya virus-induced musculoskeletal inflammatory disease: evidence of arthritis, tenosynovitis, myositis, and persistence. Am. J. Pathol. 2011;178(1):32–40. doi: https://doi.org/10.1016/j.ajpath.2010.11.018
- Hallengärd D., Kakoulidou M., Lulla A., et al. Novel attenuated Chikungunya vaccine candidates elicit protective immunity in C57Bl/6 mice. J. Virol. 2014;88(5):2858–66. doi: https://doi.org/10.1128/jvi.03453-13
- Arevalo M.T., Huang Y., Jones C.A., Ross T.M. Vaccination with a chikungunya virus-like particle vaccine exacerbates disease in aged mice. PLoS Negl. Trop. Dis. 2019;13(4):e0007316. doi: https://doi.org/10.1371/journal.pntd.0007316
- Slifka D.K., Raue H.P., Weber W.C., et al. Development of a next-generation chikungunya virus vaccine based on the HydroVax platform. PLoS Pathog. 2022;18(7):e1010695. doi: https://doi.org/10.1371/journal.ppat.1010695
- Broeckel R.M., Haese N., Ando T., et al. Vaccine-induced skewing of T cell responses protects against Chikungunya virus disease. Front. Immunol. 2019;10:2563. doi: https://doi.org/10.3389/fimmu.2019.02563
- Игнатьев Г.М., Каа К.В., Оксанич А.С. и др. Индикация и идентификация вирусов денге и Чикунгунья в комарах рода Aedes spp., отловленных в Центральной Америке. Журнал микробиологии, эпидемиологии и иммунобиологии. 2020; 97(3):227–32. Ignatyev G.M., Kaa K.V., Oksanich A.S., et al. Indication and identification of Dengue and Chikungunya viru- ses in Aedes spp. mosquitoes captured in Central America. Journal of Microbiology, Epidemiology and Immunobiology. 2020;97(3): 227–32. DOI: https://doi.org/10.36233/0372-9311-2020-97-3-4 EDN: https://elibrary.ru/ufhtab
- Mayer U.B., Haller C., Haidinger W., et al. Bacterial ghosts as an oral vaccine: a single dose of Escherichia coli O157:H7 bacterial ghosts protects mice against lethal challenge. Infect. Immun. 2005;73(8):4810–7. doi: https://doi.org/10.1128/iai.73.8.4810-4817.2005
- Ашмарин И.П., Воробьев А.А. Статистические методы в микробиологических исследованиях. Ленинград;1962. Ashmarin I.P., Vorobyov A.A. Statistical Methods in Microbiological Research. Leningrad;1962.
- Rojas J.M., Avia M., Martín V., Sevilla N. IL-10: A multifunctional cytokine in viral infections. J. Immunol. Res. 2017;2017:6104054. DOI: https://doi.org/10.1155/2017/6104054
- Oleszycka E., McCluskey S., Sharp F.A., et al. The vaccine adjuvant alum promotes IL-10 production that suppresses Th1 responses. Eur. J. Immunol. 2018;48(4):705–15. doi: https://doi.org/10.1002/eji.201747150
- He P., Zou Y., Hu Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccin. Immunother. 2015;11(2):477–88. doi: https://doi.org/10.1080/21645515.2014.1004026
- Badran G., Angrand L., Masson J.D., et al. Physico-chemical properties of aluminum adjuvants in vaccines: Implications for toxicological evaluation. Vaccine. 2022;40(33):4881–8. doi: https://doi.org/10.1016/j.vaccine.2022.06.064
- Vrieling H., Kooijman S., de Ridder J.W., et al. Activation of human monocytes by colloidal aluminum salts. J. Pharm. Sci. 2020; 109(1):750–60. DOI: https://doi.org/10.1016/j.xphs.2019.08.014
- Eisenbarth S.C., Colegio O.R., O’Connor W., et al. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminum adjuvants. Nature. 2008;453(7198):1122–6. doi: https://doi.org/10.1038/nature06939
- Kooijman S., Brummelman J., van Els C.A.C.M., et al. Novel identified aluminum hydroxide-induced pathways prove monocyte activation and pro-inflammatory preparedness. J. Proteomics. 2018;175:144–55. doi: https://doi.org/10.1016/j.jprot.2017.12.021
Supplementary files