Suppression of biofilm formation and survival of clinical isolates of uropathogens within epithelial cells under the effect of Fluorothiazinone in vitro
- Authors: Kapotina L.N.1, Morgunova E.Y.1, Nelyubina S.A.1, Fedina E.D.1, Koroleva E.A.1, Bondareva N.Е.1, Perepanova T.S.2, Zigangirova N.A.1
-
Affiliations:
- National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
- National Medical Research Radiological Center
- Issue: Vol 102, No 5 (2025)
- Pages: 547-559
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18974
- DOI: https://doi.org/10.36233/0372-9311-723
- EDN: https://elibrary.ru/KFAYZJ
- ID: 18974
Cite item
Abstract
The aim of this study was to investigate the effect of the innovative Russian antibacterial drug Fluorothiazinone, an inhibitor of the type III secretion system and flagellar function, on the virulence properties of urinary tract infection (UTI) pathogens.
Materials and methods. Clinical isolates of Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa species were obtained from 4 patients with recurrent UTI. Cytotoxicity analysis was performed on HeLa cells using the CytoTox 96 kit. Bacterial flagellar motility was assessed in 0.25% agar by measuring the zone of motility. Biofilms were cultivated in 96-well plates and stained with crystal violet and Congo red. Biofilm structure was assessed by microscopy, and the bacterial biofilm and exopolysaccharide matrix were quantified by measuring the optical density of the dye bound to the biofilm. Intracellular development was studied in PC-3 cells by determining the number of intracellular bacteria using a cell lysate plating method and an immunochemical method involving staining the bacteria with specific antibodies.
Results. The Fluorothiazinone antibacterial specifically inhibited the cytotoxicity and motility of E. coli and P. aeruginosa. For K. pneumoniae, susceptibility to Fluorothiazinone was observed, associated with the suppression of cytotoxicity. For all the isolates studied, inhibition of biofilm formation on an abiotic surface was demonstrated. For E. coli and K. pneumoniae, a significant decrease in intracellular replication within human prostate adenocarcinoma cells and suppression of intracellular bacterial community formation were observed.
Conclusion. Fluorothiazinone disrupts mechanisms that contribute to pathogen persistence in the formation of chronic UTI, including biofilm formation on abiotic surfaces such as catheters, stents, and drains, and the formation of intracellular bacterial communities and dormant intracellular reservoirs.
Full Text
Introduction
Recurrent urinary tract infections (UTIs) are characterized by the reappearance of an acute bacterial infection caused by the same pathogen. Recurrent UTIs (rUTIs) are typically defined as 2 or more episodes of uncomplicated UTIs in the past 6 months or 3 or more episodes in the past 12 months [1–4]. Every year, 5–10% of adult women suffer from UTIs [5], which affects treatment costs and reduces quality of life, especially considering that more than 60% of women are diagnosed with a UTI at least once in their lifetime [6, 7]. The pathogen of most UTIs is uropathogenic Escherichia coli (UPEC) [8–10]. However, Klebsiella pneumoniae is increasingly becoming the second most common etiological agent of rUTIs [11, 12]. This gram-negative bacterium has become a common pathogen responsible for both community-acquired and nosocomial UTIs, particularly in individuals with risk factors for infection, weakened immune systems, and chronic comorbidities [13–15].
The pathogens of UTIs are characterized by a rather complex mechanism of infection development in the bladder, where bacteria, after adhering to the bladder mucosa receptors, cause an inflammatory response and penetrate the urothelium, forming intracellular bacterial communities (IBC) [16, 17]. Intracellular UPEC undergo several stages in their development: invasion, IBC formation, IBC maturation and the formation of dormant intracellular reservoirs. IBC is a transient bacterial state that persists until the bacteria multiply and exit the host cells. Quiescent intracellular reservoirs are formed in the deeper layers of the urothelium. Bacteria, capable of forming biofilms and dormant intracellular reservoirs can evade phagocytosis, survive within host cells while protected from antibiotics and immune factors. They form reservoirs of persistent pathogens in the urinary tract and periodically cause UTIs.
One significant risk factor in the development of rUTIs is bladder catheterization. Catheter-associated infections account for up to 40% of all nosocomial infections [18]. The risk of infection with short-term catheterization (up to 7 days) is up to 10% and increases by 3–7% each day the catheterization continues [19]. With prolonged catheterization (over 30 days), bacteriuria develops in almost 100% of patients [18]. In patients with catheter-associated UTIs, the formation of biofilms on the surfaces of catheters, stents, drains, etc. is a factor in the development of urosepsis, leading to significant complications in the course of the infection and treatment inefficacy [20].
UTIs are also largely associated with the rise of antibiotic resistance in uropathogens. Antibiotics remain the cornerstone of treatment and prevention of UTIs— approximately 25% of antibiotic prescriptions are issued for UTIs [21]. Worldwide spread of antibiotics, prolonged antibiotics prophylaxis or frequent use of different antibiotic combinations in patients with rUTIs can contribute to the development of further resistance. The accumulation of resistant forms of bacteria can develop within a few weeks. For example, E. coli resistant to at least 4 antibiotics was identified in fecal isolates from over 90% of patients who received prophylactic treatment with low-dose antibiotics for 2 weeks [22].
Identifying the characteristics of the complex interaction between uropathogens and the host organism is necessary for both a deeper understanding of the pathogenesis of UTIs and for selecting new treatment approaches for these widespread bacterial infections.
The aim of the study was to investigate the effect of the innovative Russian antibacterial drug Fluorothiazinone (FT; N.F. Gamaleya Research Institute of Epidemiology and Microbiology, RU LP-No. (005077)-(RG-RU)) on the virulence properties of isolates obtained from the urine of patients with chronic UTI.
Materials and methods
Bacterial isolates
Clinical isolates of K. pneumoniae 5811, 5672, P. aeruginosa 5595, and E. coli 5424/1 were isolated from the urine of 4 adult patients of both sexes from different age groups with clinical signs of UTI who were hospitalized at the N.A. Lopatkin Research Institute of Urology and Interventional Radiology, a branch of the National Medical Research Center of Radiology of the Ministry of Health of Russia. The study was conducted with the informed consent of the patients. Urine isolates were collected as part of a clinical trial according to Protocol 07-FT-2024, RCT No. 513 dated 10/30/2024.
Bacteria culturing
The bacterial species were confirmed by streak plate method on solid media: LB agar (BD Difco), cetrimide agar (“Himedia”), followed by analysis of the protein profile of single colonies using the Bruker MALDI Biotyper instrument (Beckman Colter Diagnostics).
Clinical uropathogenic isolates were grown for 18 hours at 37°C in Luria–Bertani broth (LB broth) with continuous aeration on a shaker (240 rpm) to a concentration of 109 microbial cells/mL, which was assessed using a McFarland standard on a Densi-La-Meter II densitometer (ERBA Lachema). The resulting bacterial suspension was diluted to the desired concentrations in fresh LB broth.
Study drug
The active pharmaceutical substance FT was used in the study.
To study the effect of FT on motility and biofilm formation by clinical isolates, a stock solution with a concentration of 2 mg/mL in 0.3 M CH3COONa buffer, pH 7.0 ± 0.2, was used. The solution was prepared as follows: 0.021 g of the substance was added to a solution of 0.120 g of sodium hydroxide in 10 mL of distilled water. The mixture was then stirred on a magnetic stirrer until the FT crystals were completely dissolved, and subsequently acidified with glacial acetic acid to a pH of 7–8. The resulting solution was filtered through a 0.22 μm syringe filter into a sterile tube. In the tests, the antibacterial drug was used at a final concentration of 100 μg/mL.
To study the cytotoxicity and intracellular survival of the bacteria, a solution of FT in dimethyl sulfoxide at a concentration of 10 mg/mL was used, with a final concentration of FT in the medium of 20 μg/mL.
Cytoxicity analysis
Cytotoxicity analysis was performed according to the method [23] with some modifications. HeLa cells (cervical carcinoma cells, ATCC CCL-2™) grown in IMDM culture medium (BioloT) with 10% fetal bovine serum (HyClone) without the addition of antibiotics were infected with a bacterial culture at a multiplicity of infection (MOI) of 10 and 50, with 1 × 105 cells per well. FT at a concentration of 20 μg/mL was added immediately after the bacterial culture was introduced and incubated at 37°C and 5% CO2 for 20 hours. The plates were centrifuged for 20 minutes at 1500 rpm.
The activity of released lactate dehydrogenase (LDH) was determined in the supernatants using the CytoTox 96 non-radioactive cytotoxicity assay kit (Promega). LDH is a cytosolic enzyme present in nucleated cells that is released into the extracellular environment after the cell membrane is damaged. The percentage of LDH release was calculated relative to an uninfected control (0% LDH release) and HeLa cells lysed with Triton X-100 (100% LDH release). LDH in bacterial cultures in the medium without a cell monolayer was not detected.
Swimming motility
To assess swimming motility, cultures were grown for 18 hours at 37°C with aeration on a shaker (240 rpm), diluted 100-fold in LB broth, and cultured for 3 hours under the conditions described above. 2 μL were inoculated into the depth of 0.25% agar containing 1% tryptone and 0.5% NaCl in 90 mm diameter Petri dishes. They were observed for 48 hours. Bacterial motility was assessed by measuring the diameter of the bacterial migration zone from the center of the plate. These values served as positive controls when assessing bacterial motility.
To assess the effect of FT on the motility of clinical isolates, an FT solution in acetate buffer at a concentration of 100 μg/mL was used. For this purpose, the solution of FT was added to the prepared and cooled to 45–50°C 0.25% agar, mixed thoroughly, and poured into Petri dishes. After the agar solidified, the bacterial culture was introduced into the depth of the agar in a volume of 2 μL. The samples were cultured at 30°C in a humid chamber for 48 hours. The effect of FT was evaluated by measuring the diameter of the bacterial movement zone. Isolates with a zone of motility less than 10 mm after 48 hours were classified as non-motile, while those with a zone greater than 10 mm were classified as motile. Acetate buffer without FT was added to the control cups.
Biofilm formation
The ability of the isolates to form biofilms was studied using a modified method by G.A. O'Toole et al. [24]. Biofilm cultivation was performed in TC-treated 96-well polystyrene microplates (Corning 3599). Bacterial cultures were grown in LB broth at 37°C overnight on a shaker at 240 rpm. Overnight cultures were grown to ≈ 107 CFU/mL in sterile LB broth supplemented with an FT solution in acetate buffer at a final concentration of 100 μg/mL, and 200 μL were added to each well of the plate. The drug was not added to the wells selected as a positive control. Sterile LB broth was added to the wells as a negative control.
The plates were incubated statically at 37°C for 48 hours. After incubation, the contents of the wells were aspirated and washed three times with 200 μL of sterile phosphate-buffered saline, air-dried, and fixed with 96% ethanol for 15 minutes. The ethanol was then removed and the wells were dried.
Staining was performed using two methods: for 15 minutes with 0.1% crystal violet (Merck) or for 30 minutes with 0.1% Congo red (Merck). After the specified time, the dye was aspirated, the wells were washed with distilled water and air-dried. For quantitative analysis, the dyes bound to the biofilm were extracted with 200 μL of 96% ethanol, and the extract was transferred to the wells of a clean plate.
Optical density was measured using a Multiskan EX spectrophotometer (Thermo Scientific) at a wavelength of 540 nm for crystal violet dye and 492 nm for Congo red. To study the biofilm structure, staining was performed similarly; the wells were washed and thoroughly air-dried, then the plates were examined under a light microscope (Nikon Eclipse 50i, Nikon) at 200× magnification. For each isolate, 3 parallel experiments were performed and each were repeated thrice.
Internalization and survival of bacteria in PC-3 cells
The study used the PC-3 cell line (human prostate adenocarcinoma cells, ATCC CRL-1435). The cells were incubated in RPMI-1640 cell culture medium (BioLot) supplemented with 10% fetal bovine serum (HyClone) without the addition of antibiotics, at 37°C with 5% CO2 for 24 hours. Cell counts were determined using a Scepter 2.0 cell counter (Millipore).
Clinical isolates were grown for 18 hours at 37°C in LB broth with continuous aeration on a shaker (240 rpm) to a concentration of 109 microbial cells/mL.
A monolayer of PC-3 cells, grown in a 24-well culture plate with sterile 12 mm diameter round coverslips, was infected with a bacterial culture. Infection doses for the intracellular model were selected based on the cytotoxicity results obtained for the clinical isolates.
To assess cell invasion, cells were incubated for 1 hour, then washed three times with medium to remove unattached bacteria, and incubated for 1 hour at 37°C and 0.5% CO2 in medium containing gentamicin (Gibco) to kill extracellular bacteria. The concentration of gentamicin was determined by the minimum inhibitory concentration (MIC) for each isolate. The cells were washed three times to remove gentamicin. To determine the number of bacteria, PC-3 cells infected with bacterial cultures were lysed with 0.25% Triton X-100 (Sigma) for 10 minutes at room temperature. Uninfected PC-3 cells were used as a negative control. Next, the lysates were plated on cetrimide or LB agar (depending on the species of the isolate being studied) using the serial dilution method. The number of intracellular bacteria was expressed in CFU/mL. The average value was calculated from the bacterial population of 2 wells in 3 experiments.
To assess the onset of intracellular reproduction, the cell monolayer was cultured for 4 hours, and all procedures for removing extracellular bacteria and reseeding were performed as described above.
Intracellular survival of bacteria in PC-3 cells was assessed 24 hours after infection with the bacterial culture. After this, 4 hours after infection, the cells were incubated with gentamicin for 1 hour to eliminate extracellular bacteria. The plate was then washed three times to remove the gentamicin, and fresh nutrient medium with a maintenance concentration of gentamicin was added. All procedures for removing extracellular bacteria and culturing were performed similarly to those described above.
To study the effect of FT, a solution of FT in dimethyl sulfoxide was added to the culture medium at a concentration of 20 μg/mL at different stages of the experiment: 1 hour before infection, at the moment of cell infection, 2 and 5 hours after infection and washing off gentamicin, followed by incubation in the presence of FT for 24 hours from the moment of infection.
For microscopic analysis, cells were fixed with acetone and stained with mouse polyclonal antibodies (antibodies were obtained for each isolate) for 30 minutes at 37°C and 5% CO2 and with anti-mouse IgG-FITC conjugate (“Merck”) for 30 minutes at 37°C and 5% CO2. Samples were analyzed using a Nikon Eclipse 50i fluorescence microscope (Nikon) at 1000× magnification.
Statistical analysis
Statistical processing and visualization of the analysis results for in vitro bacterial studies were performed using GraphPad Prism v. 8.4.3 (GraphPad Software). The data from the experimental and control groups were compared using the Student's t-test. The criterion for statistical significance of the difference in the data obtained was considered to be p<0.05. All experiments were conducted in triplicate.
Results
Characteristics of clinical isolates
Antibiotic susceptibility analysis of the studied clinical isolates was performed in the clinical diagnostic laboratory of the N.A. Lopatkin Research Institute of Urology and Interventional Radiology, a branch of the National Medical Research Center of Radiology of the Ministry of Health of Russia, and the results are presented in the Table.
MIC values of antibiotics in relation to the tested clinical isolates
Antibiotic susceptibility testing for isolated microorganisms | E. coli 5424/1 | P. aeruginosa 5595 | K. pneumoniae 5811 | K. pneumoniae 5672 | ||||
quality result | MIC, mg/L | quality result | MIC, mg/L | quality result | MIC, mg/L | quality result | MIC, mg/L | |
Amikacin | S | ≤ 8 | S | S | ≤ 8 | S | ≤ 8 | |
Amoxicillin/clavulanic acid | R | R | R | |||||
Ampicillin | R | > 16 | R | R | > 16 | R | > 16 | |
Ampicillin/sulbactam | R | |||||||
Gentamicin | R | 128 | R | 16 | R | 512 | S | ≤ 2 |
Imipenem | S | ≤ 0.25 | S | R | > 8 | S | ≤ 0.5 | |
Colistin | R | |||||||
Levofloxacin | S | R | > 2 | R | > 4 | |||
Meropenem | S | ≤ 0.125 | S | R | > 8 | I | 8 | |
Nitrofurantoin | R | |||||||
Piperacillin/tazobactam | S | ≤ 4/4 | S | R | S | ≤ 4/4 | ||
Tigecycline | S | ≤ 1 | R | |||||
Tobramycin | S | ≤ 2 | S | S | ≤ 2 | |||
Trimethoprim/sulfamethoxazole | S | ≤ 2/38 | I | S | ≤ 2/38 | |||
Fosfomycin with glucose-6-phosphate | S | ≤ 16 | S | ≤ 16 | ||||
Cefazolin | R | > 32 | R | R | > 32 | R | > 32 | |
Cefepime | S | R | > 8 | |||||
Ceftazidime | R | R | > 8 | R | > 16 | |||
Ceftriaxone | S | ≤ 1 | R | R | > 4 | R | > 4 | |
Cefuroxime | R | > 16 | ||||||
Ciprofloxacin | S | ≤ 0.25 | R | > 1 | R | > 1 | ||
Ertapenem | S | ≤ 0.25 | R | R | > 1 | R | > 2 | |
Note: S — susceptible; R — resistant; I — intermediate.
FT does not affect the viability of clinical isolates
The effect of FT on bacterial viability was assessed by culturing in nutrient LB broth in the presence of FT at concentrations of 60, 80 and 100 μg/mL for 24 hours with continuous aeration on a shaker (240 rpm) and determining CFU by the streak/spread plate method. According to the mechanism of action, and as previously shown for bacteria of different species, FT did not affect the viability of the studied clinical isolates.
FT suppresses the cytotoxicity of clinical isolates
Both K. pneumoniae isolates were characterized by significant cytotoxicity. For K. pneumoniae 5811 isolate, cytotoxicity at MOI10 was 65%, and at MOI50, it was 78%. For K. pneumoniae 5672 isolate, cytotoxicity at an MOI of 10 was 70%, and at an MOI of 50, it was 76%.
The cytotoxicity of P. aeruginosa 5595 and E. coli 5424/1 on the HeLa cell line was less pronounced. At MOI10, the cytotoxicity of the P. aeruginosa 5595 isolate was 50%, and at MOI50, it was 61%. For E. coli isolate 5424/1, 38 and 60% at MOI10 and MOI50, respectively.
In the presence of FT at a concentration of 20 μg/mL, cytotoxicity after 20 hours of culturing was significantly reduced, by 1.5–2.0 times at MOI10 and MOI50 for all 4 clinical isolates.
FT suppresses swimming motility
In a semi-liquid medium, bacterial cells are capable of swimming motility, moving by means of flagella. K. pneumoniae belongs to bacteria that do not have swimming motility via to flagella. In light of this, the effect of FT on swimming activity was evaluated for 2 uropathogenic isolates: P. aeruginosa 5595 and E. coli 5424/1.
It has been established that both clinical isolates exhibit swimming motility. In the presence of FT at a concentration of 100 μg/mL in the culture medium, a pronounced suppression of motility associated with flagellar activity was observed (Fig. 1).
Fig. 1. Suppression of swimming motility of isolates after 48 hours of incubation in the presence of FT.
Representative images of swimming motility on Petri dishes and assessment of the change in the diameter of the zone of motility of P. aeruginosa 5595 (a) and E. coli 5424/1 (b) in the presence of FT at a concentration of 100 μg/mL. Error bars represent the SD of 2 replicates.
The diameter of the bacterial growth zone in semi-solid agar for P. aeruginosa 5595 after 48 hours was 65–67 mm, and in the presence of FT, it was 25–27 mm. For E. coli 5424/1 isolate, the diameter of the zone of motility after 48 hours was 35–37 mm; in the presence of FT, the zone decreased to 12–14 mm. In control dishes with acetate buffer without the addition of FT, motility was not inhibited. This demonstrated the suppression of flagellar motility by FT.
FT suppresses biofilm formation
The ability to form biofilms and the effect of FT on this process were evaluated for 4 isolates. The biofilm density was quantified by staining the bacteria with crystal violet. After 48 hours, P. aeruginosa 5595 formed an intense multi-layered mature biofilm, K. pneumoniae 5672 and E. coli 5424/1 isolates formed a dense monolayer with areas of multi-layered structure, and K. pneumoniae 5811 isolate showed the slowest biofilm formation rate: by 48 hours, a monolayer with the formation of microcolonies was observed.
Adding FT simultaneously with the introduction of the bacterial culture led to a significant reduction in biofilm density and disruption of its structure. All isolates formed a sparse monolayer with a cellular structure on an abiotic surface under the influence of FT, but microcolony formation and biofilm maturation did not occur (Fig. 2).
Fig. 2. FT alters the structure and biomass of biofilms formed by isolates K. pneumoniae 5811 (a), K. pneumoniae 5672 (b), P. aeruginosa 5595 (c) and E. coli 5424/1 (d).
Microphotographs of formed biofilm fragments from control and experimental samples with the addition of FT at a concentration of 100 μg/mL after 48 hours of incubation are presented. Biofilm biomass accumulation was assessed by staining with crystal violet at an optical density of 540 nm.
When quantifying bacterial density in biofilms under the influence of phototherapy, a significant reduction in the number of bacteria within the biofilm was observed: the maximum suppression (by 85%) was for K. pneumoniae 5672, 79% for E. coli 5424/1, 75% for P. aeruginosa 5595, and 71% for K. pneumoniae 5811 (Fig. 2).
The formation of the biofilm exopolysaccharide matrix (EPM) was assessed by Congo Red staining. It was found that all the isolates studied are capable of forming EPM, but the K. pneumoniae 5811 biofilm was characterized by the lowest EPM intensity. Under the effect of FT, all isolates lost their ability to synthesize EPM (Fig. 3).
Fig. 3. FT inhibits the production of EPM biofilm by K. pneumoniae 5811 (a), K. pneumoniae 5672 (b), P. aeruginosa 5595 (c), and E. coli 5424/1 (d).
Microphotographs of biofilm fragments from control and experimental samples with the addition of FT at a concentration of 100 μg/mL are presented after 48 hours of incubation. EPM biofilm products were evaluated by the degree of Congo Red staining of the matrix at an optical density of 492 nm.
Quantitative assessment showed a significant decrease in biofilm formation for all isolates: K. pneumoniae 5811 — 76%, K. pneumoniae 5672 — 74%, P. aeruginosa 5595 — 70%, E. coli 5424/1 — 67% (Fig. 3). The solvent without FT had no effect on biofilm formation.
Thus, all the isolates studied are capable of formation of a biofilm on an abiotic surface, and FT inhibited the formation of a mature biofilm.
FT suppresses the invasion and intracellular replication of uropathogens
The ability of the studied uropathogenic isolates to form biofilms was assessed using the PC-3 cell line model (human prostate adenocarcinoma cells) by infecting the cells with the isolates and observing them during the invasion and intracellular replication stages at 4 and 24 hours. To assess the effect of FT on these processes, the drug was introduced 1 hour before infection, simultaneously with infection, and also after the medium was changed (at 2 and 5 hours).
The number of viable intracellular bacteria was assessed by culturing dilutions of the cell lysate after removing extracellular bacteria by gentamicin treatment for 1 hour. Simultaneously, the cell monolayer was analyzed by microscopy after staining with specific antibacterial antibodies.
For the E. coli 5424/1 isolate, an inoculum of 1.5 × 106 CFU/mL was used for infection, which resulted in MOI55. After 1 hour, the invasion rate was 0.47%, while under the effect of FT, it was 0.053%. After 4 hours, the number of intracellular bacteria in the control group was 2.1 × 105 CFU/mL, while in the presence of FT, it was an order of magnitude lower, at 1.1 × 104 CFU/mL. After 24 hours, an increase in the number of E. coli 5424/1 was observed to 2.4 × 106 CFU/mL in the control samples, while bacterial growth was significantly reduced in the presence of FT to 7.5 × 102 CFU/mL.
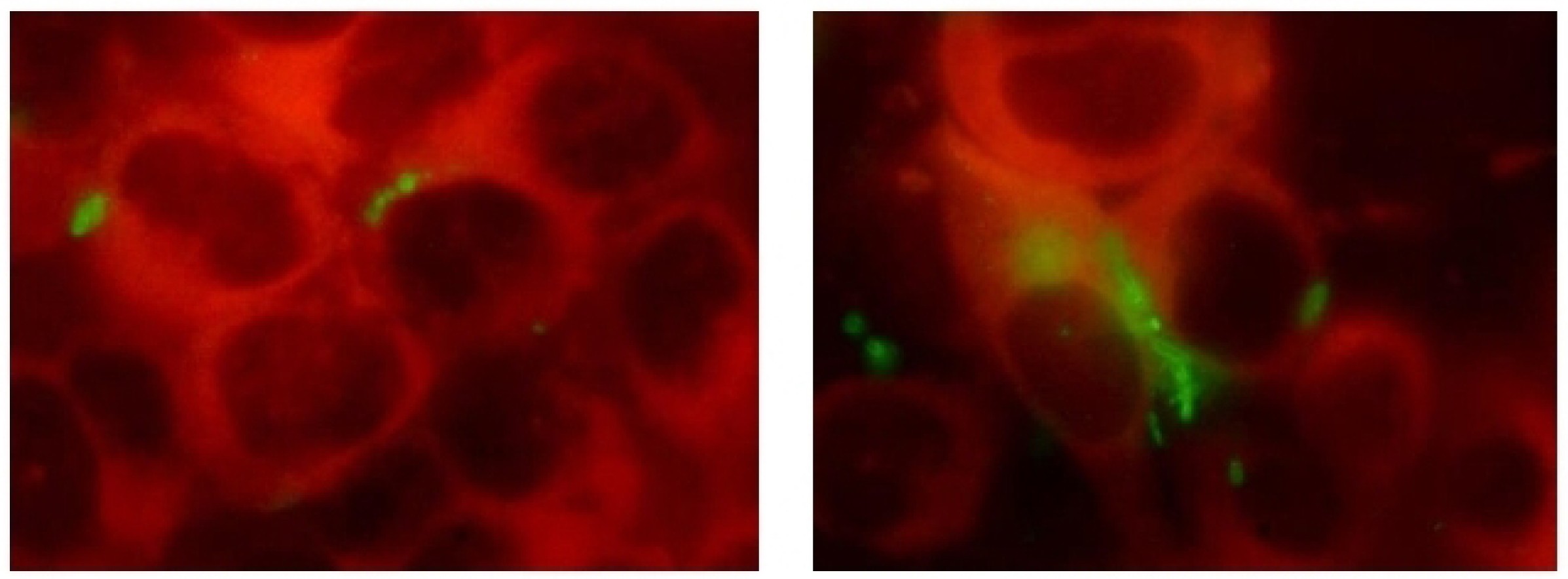
In the micrographs of the infected cell monolayer in the control samples, intracellular cell conglomerates were detected as early as 4 hours, which formed typical IBC by 24 hours. In the presence of FT, IBC were practically not identified (Fig. 4).
Fig. 4. The effect of FT on the invasion and intracellular reproduction of E. coli 5424/1 in PC-3 cells over 24 hours.
a — micrographs of PC cells infected with E. coli 5424/1 at 1, 4 and 24 hours; b — micrographs of PC-3 cells infected with E. coli 5424/1 at 1, 4 and 24 hours under the effect of FT. TRITC/Cy3/TagRFP/Alexa Fluor 546 filter; c — the number of intracellular E. coli 5424/1 bacteria in PC-3 cells. Data are presented as M ± SEM of 3 independent experiments (p < 0.05).
For the K. pneumoniae 5811 isolate, IBC formation was also observed, and it was suppressed in the presence of FT. This isolate showed lower rate of invasion, which was 0.005% for the control, while almost similar rate of invasion was observed under FT treatment. This isolate showed lower rate of invasion, which was 0.005% for the control and similar for the FT treatment group. (бы я исправила) This isolate showed to have effective intracellular replication: after 4 hours, the number of intracellular bacteria was 5 × 105 CFU/mL, and after 24 hours, it was 2.0 × 106 CFU/mL. Under the effect of FT, bacterial replication? was suppressed — 5.9 × 102 CFU/mL after 24 hours. The inhibition of IBC formation in the presence of FT is clearly demonstrated in the microphotographs (Fig. 5).
Fig. 5. The effect of FT on the invasion and intracellular reproduction of K. pneumoniae 5811 in PC-3 cells over 24 hours.
a — micrographs of PC-3 cells infected with K. pneumoniae 5811 at 1, 4 and 24 hours; b — micrographs of PC-3 cells infected with K. pneumoniae 5811 at 1, 4 and 24 hours under the influence of FT. TRITC/Cy3/TagRFP/Alexa Fluor 546 filter. c — number of intracellular K. pneumoniae 5811 bacteria in PC-3 cells. Data are presented as M ± SEM of 3 independent experiments (p < 0.05).
Discussion
The diagnosis of UTIs, which can mean both infectious disease and asymptomatic bacteriuria, is sometimes difficult to establish, especially in recurrent cases, due to complex mechanism of interaction between uropathogens and tissues of the urinary tract and the body's immune system. International guidelines recommend relying solely on clinical symptoms of the disease [1]. Although UTIs are caused by pathogens from various taxonomic groups, and most of these bacteria are extracellular, they nevertheless utilize a common strategy for developing an infectious process: effective adhesion, invasion of epithelial cells, and the formation of pathogen reservoirs in the form of biofilms, dormant intracellular reservoirs, or surface biofilms (on catheters, foreign bodies). This strategy allows them to evade the action of antibiotics and host immunity factors, which determines clinical and epidemiological relevance of recurrent UTIs.
New approaches in treating such chronic infections should be based on understanding the pathogenesis mechanisms and on blocking of specific links of these mechanisms. In the current study, we evaluated the effect of the active pharmaceutical substance, antibacterial drug FT, on the expression of virulent properties of clinical isolates of 3 different species: K. pneumoniae, P. aeruginosa and E. coli under in vitro conditions.
FT is an original domestic antibacterial drug that differs from antibiotics in its mechanism of action, as it does not kill the bacteria but suppresses their virulence. The specific inhibition of the type III secretion system (T3SS), which is responsible for the production of toxins that destroy immune cells and promote intracellular bacterial survival, has been demonstrated for this antibacterial drug. FT also inhibits bacterial flagellar motility and biofilm formation. Suppression of the virulence factors leads to the halting of infectious process in the host organism, as demonstrated in animal experiments [25–27]. FT therapy is effective against bacteria resistant to different classes of antibiotics because the target of action is present regardless of the presence of resistance determinants [28].
In clinical trials on patients with acute UTI, it was found that FT reduced the development of recurrences by 10 times at 2 and 3 months after the end of therapy compared to cefepime, which can be explained by the effective eradication of uropathogens from their reservoirs, including the intracellular ones [29].
It is known that P. aeruginosa and E. coli secrete cytotoxins directly into eukaryotic cells via the T3SS. In this regard, we evaluated the effect of FT on the cytotoxicity of these isolates. The presence of the T3SS is not demonstrated for K. pneumoniae, but a cytotoxic effect associated with the presence of the capsular polysaccharide has been described [30].
For all analyzed isolates that were characterized by significant toxicity toward epithelial cells, a statistically significant suppression of cell death was demonstrated in the presence of FT.
Along with the flagellum, the target of FT is the cilium, which has a similar structure at the base of the organelle. For motile isolates of P. aeruginosa and E. coli, almost complete blockage of flagellar function was observed, which was expressed by the absence of a spreading zone in semi-solid agar. It should be noted that bacterial growth was not inhibited in the presence of FT, as shown in preliminary experiments.
The presence of surface structures in uropathogens, such as pili, fimbriae and flagella, facilitates adhesion to urothelial receptors as the first stage of bladder colonization, and thus they are an important target for suppression of infection development. One example of confirmation of the efficacy of this adhesion-blocking approach is the use of D-mannose in a clinical study, which showed a statistically significant reduction in the likelihood of recurrent UTIs [31].
Thus, the suppression of bacterial motility observed under the effect of FT, which is related to the function of flagella, is aimed at blocking of the first stage of infection development — the stage of urothelial colonization.
The key link in the development of a chronic infectious process with a recurrent course, caused by the long-term persistence of pathogens, is the bacteria's ability to actively invade urothelial cells and form intracellular biofilm-like communities.
The effect of FT on the 24-hour development cycle of K. pneumoniae and E. coli isolates in human prostate adenocarcinoma cells was evaluated. It was previously established that FT has high tissue availability due to its pharmacological properties, it penetrates cells, and retains activity in eukaryotic cells [32]. The introduction of FT into the culture medium significantly reduced the number of bacteria forming intracellular bacterial communities, as demonstrated by determining viable microorganisms and identifying intracellular structures under microscopy 24 hours after the pathogens came into contact with the cells.
Currently, the mechanisms by which FT suppresses intracellular bacterial replication are being studied. Given that FT does not have a direct antibacterial effect, the proposed mechanism involves blocking bacterial virulence factors responsible for interaction with the host cell during bacterial coexistence and persistence.
The ability of FT to suppress biofilms has been previously demonstrated for various pathogens, including carbapenem-resistant hospital-acquired infection agents. Studying the dynamics of biofilm formation in vitro and the effect of FT on this process showed that the FT blocks biofilm formation at the stage of microcolony aggregation and multilayer structure formation. Cultivation in the presence of FT has an inhibitory effect on the ability of isolates to form biofilms and, consequently, reduces the production of exopolysaccharides [26, 27]. The obtained results are of significant practical importance, as the EPM is a key factor in biofilm resistance to antibiotics and protection against the immune response.
Conclusion
In experiments conducted to study the effect of FT, the inhibitor of the type III secretion system and flagella, on the virulence properties of clinical isolates of three species of uropathogens under in vitro conditions, it was shown that FT suppresses key stages of bacterial persistence development in chronic infections, such as invasion of epithelial cells and the formation of pathogen reservoirs in the form of intracellular bacterial communities and surface biofilms. Properties of FT, such as biofilm suppression and intracellular replication inhibition, are a significant advantage of the innovative Russian antibacterial drug over currently used antibiotics.
About the authors
Lydiya N. Kapotina
National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: lidiya.kapotina57@mail.ru
ORCID iD: 0000-0003-0159-5053
Cand. Sci. (Technics), senior researcher, Chlamydia department
Russian Federation, MoscowElena Yu. Morgunova
National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: lena.morgunova.1968@mail.ru
ORCID iD: 0000-0003-3142-0983
researcher, Chlamydia department
Russian Federation, MoscowStanislava A. Nelyubina
National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: 19ctasy94@mail.ru
ORCID iD: 0000-0002-5157-1415
junior researcher, Chlamydia department
Russian Federation, MoscowElena D. Fedina
National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: e.fedina@mail.ru
ORCID iD: 0000-0002-7205-4310
Cand. Sci. (Med.), senior researcher, Chlamydia department
Russian Federation, MoscowEkaterina A. Koroleva
National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: korolevakate@yandex.ru
ORCID iD: 0000-0001-9702-2940
Cand. Sci. (Biol.), senior researcher, Chlamydia department
Russian Federation, MoscowNataliya Е. Bondareva
National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: nataliia.d@mail.ru
ORCID iD: 0000-0002-5244-2965
Cand. Sci. (Med.), senior researcher, Chlamydia department
Russian Federation, MoscowTamara S. Perepanova
National Medical Research Radiological Center
Email: perepanova2003@mail.ru
ORCID iD: 0000-0002-2877-0029
Dr. Sci. (Med.), Professor, Head, Infectious and inflammatory diseases and clinical pharmacology group, N. Lopatkin Scientific Research Institute of Urology and Interventional Radiology
Russian Federation, MoscowNaylia A. Zigangirova
National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Author for correspondence.
Email: zigangirova@mail.ru
ORCID iD: 0000-0003-3188-1608
Dr. Sci. (Biol.), Professor, RAS Corresponding member, Head, Department of medical microbiology
Russian Federation, MoscowReferences
- Bonkat G., Wagenlehner F., Kranz J. Keep it simple: a proposal for a new definition of uncomplicated and complicated urinary tract infections from the EAU urological infections guidelines panel. Eur. Urol. 2024;86(3):195–7. DOI: https://doi.org/10.1016/j.eururo.2024.05.007
- Bell B.G., Schellevis F., Stobberingh E., et al. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014;14:13. DOI: https://doi.org/10.1186/1471-2334-14-13
- Pigrau C., Escolà-Vergé L. Recurrent urinary tract infections: from pathogenesis to prevention. Med. Clin. (Barc.). 2020;155(4):171–7. DOI: https://doi.org/10.1016/j.medcli.2020.04.026
- WHO. Antimicrobial resistance: global report on surveillance;2014. https://who.int/publications/i/item/9789241564748
- Flores-Mireles A.L., Walker J.N., Caparon M., Hultgren S.J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015;13(5):269–84. DOI: https://doi.org/10.1038/nrmicro3432
- Sabih A., Leslie S.W. Complicated Urinary Tract Infections. Treasure Island;2025.
- Tandogdu Z., Wagenlehner F. Global epidemiology of urinary tract infections. Curr. Opin. Infect. Dis. 2016;29(1):73–9. DOI: https://doi.org/10.1097/QCO.0000000000000228
- Klein R.D., Hultgren S.J. Urinary tract infections: microbial pathogenesis, host–pathogen interactions and new treatment strategies. Nat. Rev. Microbiol. 2020;18(4):211–26. DOI: https://doi.org/10.1038/s41579-020-0324-0
- Палагин И.С., Сухорукова М.В., Дехнич А.В. и др. Антибиотикорезистентность возбудителей внебольничных инфекций мочевых путей в России: результаты многоцентрового исследования «ДАРМИС-2018». Клиническая микробиология и антимикробная химиотерапия. 2019;21(2):134–46. Palagin I.S., Sukhorukova M.V., Dekhnich A.V., et al. Antimicrobial resistance of pathogens causing community-acquired urinary tract infections in russia: results of multicenter study «DARMIS-2018». Clinical Microbiology and Antimicrobial Chemotherapy. 2019;21(2):134–46. DOI: https://doi.org/10.36488/cmac.2019.2.134-146 EDN: https://elibrary.ru/vdyqtb
- Mittal S., Sharma M., Chaudhary U. Biofilm and multidrug resistance in uropathogenic Escherichia coli. Pathog. Glob. Health. 2015;109(1):26–9. DOI: https://doi.org/10.1179/2047773215Y.0000000001
- Paczosa M.K., Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. 2016;80(3):629–61. DOI: https://doi.org/10.1128/MMBR.00078-15
- Jalil M.B., Al Atbee M.Y.N. The prevalence of multiple drug resistance Escherichia coli and Klebsiella pneumoniae isolated from patients with urinary tract infections. J. Clin. Lab. Anal. 2022;36(9):e24619. DOI: https://doi.org/10.1002/jcla.24619
- Ashurst J.V., Dawson A. Klebsiella Pneumonia. Treasure Island;2023.
- Clegg S., Murphy C.N. Epidemiology and virulence of Klebsiella pneumoniae. Microbiol. Spectr. 2016;4(1). DOI: https://doi.org/10.1128/microbiolspec.UTI-0005-2012
- Filev R., Lyubomirova M., Bogov B., et al. Urinary tract infections caused by Klebsiella pneumoniae and prolonged treatment with trimethoprim/sulfamethoxazole. Microorganisms. 2025;13(2):422. DOI: https://doi.org/10.3390/microorganisms13020422
- Andersen T.E., Khandige S., Madelung M., et al. Escherichia coli uropathogenesis in vitro: invasion, cellular escape, and secondary infection analyzed in a human bladder cell infection model. Infect. Immun. 2012;80(5):1858–67. DOI: https://doi.org/10.1128/iai.06075-11
- Anderson G.G., Palermo J.J., Schilling J.D., et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301(5629):105–7. DOI: https://doi.org/10.1126/science.1084550
- Spaulding C.N., Hultgren S.J. Adhesive pili in UTI pathogenesis and drug development. Pathogens. 2016;5(1):30. DOI: https://doi.org/10.3390/pathogens5010030
- Oh W.S., Hur J.A., Kim E.S., et al. Factors associated with specific uropathogens in catheter-associated urinary tract infection: developing a clinical prediction model. J. Int. Med. Res. 2014;42(6):1335–47. DOI: https://doi.org/10.1177/0300060514543035
- Jiménez-Alcaide E., Medina-Polo J., García-González L., et al. Healthcare-associated urinary tract infections in patients with a urinary catheter: Risk factors, microbiological characteristics and patterns of antibiotic resistance. Arch. Esp. Urol. 2015;68(6):541–50. (in Spanish)
- Akkerman A.E., Kuyvenhoven M.M., Verheij T.J.M., van Dijk L. Antibiotics in Dutch general practice: nationwide electronic GP database and national reimbursement rates. Pharmacoepidemiol. Drug Saf. 2008;17(4):378–83. DOI: https://doi.org/10.1002/pds.1501
- Maharjan G., Khadka P., Siddhi S.G., et al. Catheter-associated urinary tract infection and obstinate biofilm producers. Can. J. Infect. Dis. Med. Microbiol. 2018;2018:7624857. DOI: https://doi.org/10.1155/2018/7624857
- Specian A.F.L., Serpeloni J.M., Tuttis K. LDH, proliferation curves and cell cycle analysis are the most suitable assays to identify and characterize new phytotherapeutic compounds. Cytotechnology. 2016;68(6):2729–44. DOI: https://doi.org/10.1007/s10616-016-9998-6
- O'Toole G., Kaplan H.B., Kolter R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000:54:49–79. DOI: https://doi.org/10.1146/annurev.micro.54.1.49
- Zigangirova N.A., Nesterenko L.N., Sheremet A.B., et al. Fluorothiazinone, a small-molecular inhibitor of T3SS, suppresses salmonella oral infection in mice. J. Antibiot. 2021;74(4):244–54. DOI: https://doi.org/10.1038/s41429-020-00396-w
- Koroleva E.A., Soloveva AV., Morgunova E.Y., et al. Fluorothiazinone inhibits the virulence factors of uropathogenic Escherichia coli involved in the development of urinary tract infection. J. Antibiot. (Tokyo). 2023;76(5):279–90. DOI: https://doi.org/10.1038/s41429-023-00602-5
- Bondareva N.E., Soloveva A.V., Sheremet A.B., et al. Preventative treatment with Fluorothiazinone suppressed Acinetobacter baumannii-associated septicemia in mice. J. Antibiot. (Tokyo). 2022;75(3):155–63. DOI: https://doi.org/10.1038/s41429-022-00504-y
- Savitskii M.V., Moskaleva N.E., Brito A., et al. Pharmacokinetics, tissue distribution, bioavailability and excretion of the anti-virulence drug Fluorothiazinone in rats and rabbits. J. Antibiot. (Tokyo). 2024;77(6):382–8. DOI: https://doi.org/10.1038/s41429-024-00719-1
- Белобородов В.Б., Зигангирова Н.А., Лубенец Н.Л. и др. Влияние цефепима/фтортиазинона в сравнении с цефепимом/плацебо на клиническое излечение и микробиологическую эрадикацию у пациентов с осложнёнными инфекциями мочевыводящих путей: проспективное рандомизированное клиническое исследование. Антибиотики и Химиотерапия. 2024;(9-10):31–9. Beloborodov V.B., Zigangirova N.A., Lubenec N.L., et al. The effect of cefepime/fluorothiazinone compared with cefepime/placebo on clinical cure and microbiological eradication in patients with complicated urinary tract infections: a prospective randomized clinical trial. Antibiotics and Chemotherapy. 2024;(9-10):31–9. DOI: https://doi.org/10.37489/0235-2990-2024-69-9-10-31-39 EDN: https://elibrary.ru/AAHDQM
- Cano V., Moranta D., Llobet-Brossa E., et al. Klebsiella pneumoniae triggers a cytotoxic effect on airway epithelial cells. BMC Microbiol. 2009;9:156. DOI: https://doi.org/10.1186/1471-2180-9-156
- Parazzini F., Ricci E., Fedele F., et al. Systematic review of the effect of D-mannose with or without other drugs in the treatment of symptoms of urinary tract infections/cystitis (review). Biomed. Rep. 2022;17(2):69. DOI: https://doi.org/10.3892/br.2022.1552
- Zigangirova N.A., Lubenec N.L., Beloborodov V.B., et al. A new «non-traditional» antibacterial drug fluorothiazinone – clinical research in patients with complicated urinary tract infections. Antibiotics (Basel). 2024;13(6):476. DOI: https://doi.org/10.3390/antibiotics13060476
Supplementary files