Bioconjugation as a promising method for vaccine development
- Authors: Tsyganova M.I.1, Novikov D.V.1, Novikov V.V.1, Karaulov A.V.2
-
Affiliations:
- Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology
- I.M. Sechenov First Moscow State Medical University (Sechenov University)
- Issue: Vol 102, No 4 (2025)
- Pages: 495-506
- Section: REVIEWS
- URL: https://microbiol.crie.ru/jour/article/view/18842
- DOI: https://doi.org/10.36233/0372-9311-696
- EDN: https://elibrary.ru/UYTHOH
- ID: 18842
Cite item
Abstract
Introduction. Bioconjugation, or protein glycan coupling technology, PGCT, is a method for creating carbohydrate-protein composites based on the ability of certain bacteria to perform eukaryotic-type glycosylation. This method allows for the production of glycoproteins directly in the cells of producer bacteria, most often Escherichia coli, bypassing the stage of chemical conjugation. This significantly simplifies the creation and production of conjugated vaccines, consisting of polysaccharide antigens combined with a protein carrier that performs the functions of a T-cell antigen and an adjuvant.
The aim of the review is to analyze and summarize current data on both the bioconjugation method itself and the underlying biochemical processes, as well as on the vaccines being developed using this method.
The preparation of the review involved studies presented in the PubMed, Scopus, Google Scholar, eLIBRARY.RU databases as of February 2025. The following keywords were used for the search: bioconjugation, vaccines, PGCT, conjugated vaccines, bacterial glycosylation.
An analysis of literature sources dedicated to the study of bacterial N-glycosylation, on the basis of which the bioconjugation technology was developed, as well as similar processes occurring in certain bacterial species, was conducted. Reports on the development of new vaccines and the improvement of existing vaccines against the most relevant pathogens have been analyzed. At present, vaccination appears to be the most effective way to combat infectious diseases, including efforts to counter the spread of antibiotic-resistant microorganisms. The diversity of pathogens encountered by the human population compels the search for multiple approaches of creating effective and safe vaccines. Simplifying and reducing the cost of producing new drugs allows for a more confident response to the threat of new epidemics. Bioconjugation helps create new vaccines and improve existing vaccines, although there are certain limitations.
Conclusion. Modern vaccine production is characterized by a variety of approaches united by a single goal — to effectively counter the threats of new epidemics. Bioconjugation is one of the new, yet quite promising methods through which several vaccine candidates are already being developed. The analysis of the current state of these projects may be useful in choosing an approach for developing subsequent preventive immunological drugs.
Full Text
Introduction
In November 2024, the World Health Organization (WHO) updated the list of priority endemic pathogens for which there is the greatest need for vaccines [1], noting that vaccination not only reduces morbidity but also decreases antibiotic use, thereby lowering mortality caused by antibiotic-resistant strains. According to WHO estimates, vaccination against 23 pathogens could reduce the need for antibiotics by 22% [2]. Moreover, the widespread use of existing vaccines against pneumococcus, Haemophilus influenzae type b, and typhoid fever could potentially prevent up to 106,000 deaths annually caused by the spread of antimicrobial resistance. The development and global implementation of new vaccines against Mycobacterium tuberculosis and Klebsiella pneumoniae could potentially prevent more than 500,000 deaths annually caused by antimicrobial resistance. To create the most effective vaccines, a variety of approaches are currently being used. One of them is bioconjugation or protein glycan coupling technology (PGCT).
The aim of this review is to analyze and summarize current data on both the bioconjugation method itself and the biochemical processes underlying it, as well as on the vaccines being developed using this method.
In preparing the review, an analysis was conducted of both English- and Russian-language literature available in the scientific databases PubMed, Scopus, Google Scholar, and eLIBRARY.RU as of February 2025. The following keywords were used for the search: bioconjugation, vaccines, PGCT, bioconjugation, conjugated vaccines, bacterial glycosylation. At the first stage, when searching for "bioconjugation" from 1968 to 2025, more than 6000 sources were found, which were reduced to 250 by combining queries. Initially, studies from 2010 to 2025 were selected, reducing the number to 178, after which a number of relevant articles without time restrictions were added for a more comprehensive coverage of the research issue. Due to the article's length limitation, 59 of the most relevant sources were selected. Also, studies for which it was impossible to obtain the full text of the article, sources not in English, as well as those Russian-language articles in which the issue was mentioned but not discussed in detail, limiting themselves to references to already used foreign sources in the review, were excluded from the selection.
Key features of bioconjugation
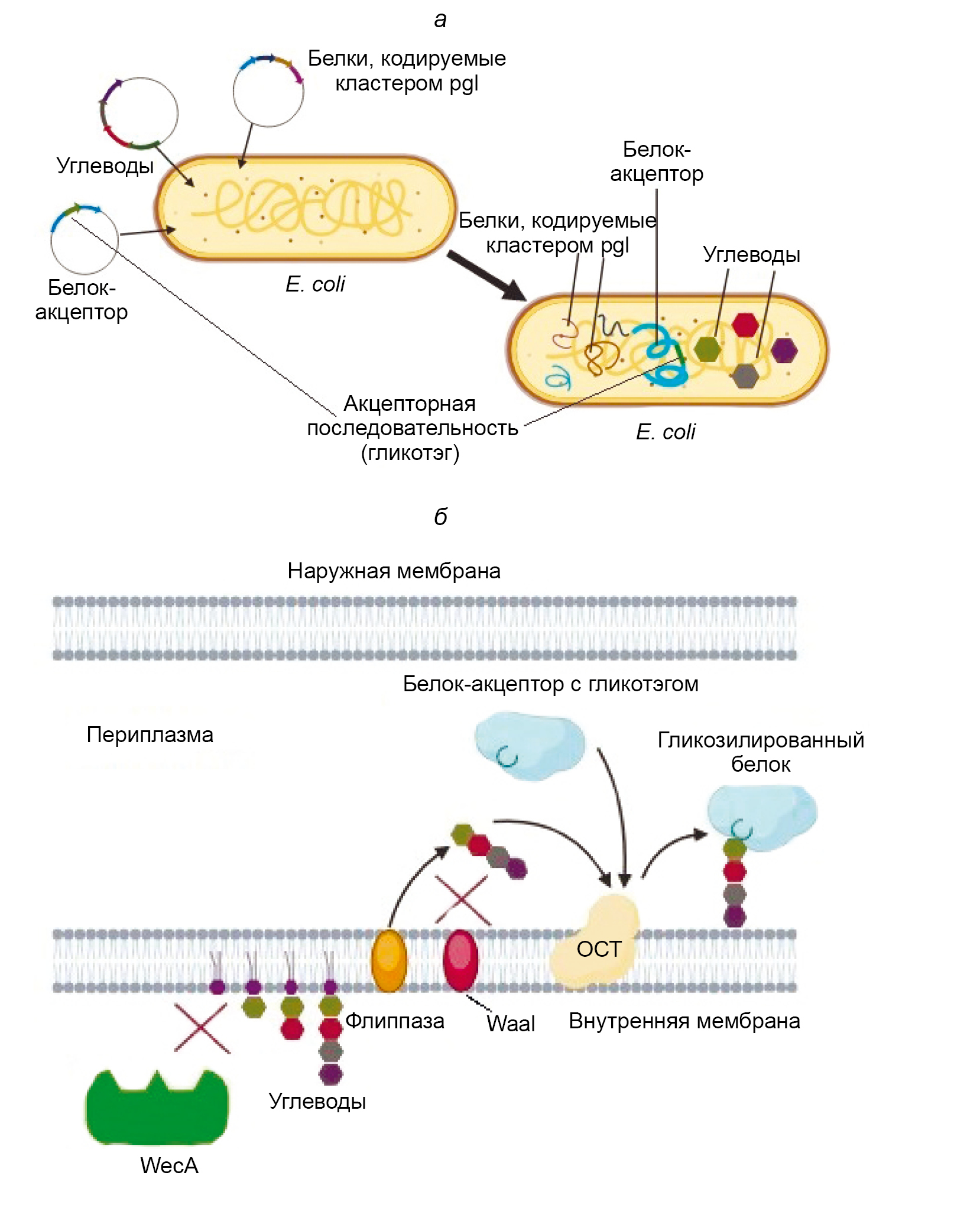
PGCT was created based on the ability of the microorganism Campylobacter jejuni to perform N-glycosylation of proteins using the oligosaccharyltransferase (OST) PglB, which is expressed along with other enzymes in a cluster called pgl (protein glycosylation), as described in 1999 [3]. PglB is a membrane enzyme with an active site facing the periplasmic space. It is capable of attaching an oligosaccharide, primarily composed of N-acetylgalactosamine (GalNac) monomers, to the asparagine residue located at the center of the so-called acceptor sequence [4]. The acceptor sequence of PglB is as follows: Asp/Glu — Y — Asn — X — Ser/Thr, where X and Y are any amino acids except proline [5]. The attachment of the oligosaccharide occurs through the amide group of asparagine and is classified as N-glycosylation. The pgl cluster has been successfully cloned into E. coli and has demonstrated the ability to express the entire set of enzymes necessary for glycosylation, as well as perform the glycosylation itself when proteins with the required acceptor sequence are present [6]. Thus, it became possible to obtain glycosylated proteins directly in E. coli. This process was named bioconjugation, or PGCT technology (Figure).
General scheme of PGCT in E. coli cells [18, with added changes].
a — the stage of transforming bacteria with plasmids containing nucleotide sequences that encode, respectively, a carrier protein with an acceptor sequence specific to the used OST, a set of carbohydrates necessary for its glycosylation, and a cluster containing the enzymes required for glycosylation; b — the process of glycosylation itself, occurring on the inner membrane of E. coli cells modified for more efficient bioconjugate synthesis.
WaaL is the O-antigen ligase of E. coli, competing with recombinant OST for oligosaccharides; WecA is the enzyme that catalyzes the biosynthesis of native glycans in the producing bacterium; flippase is the enzyme that transfers the carbohydrate sequence to the periplasm.
Historically, in the production of polysaccharide-based vaccines, carbohydrates are chemically covalently attached to a carrier protein, which serves the functions of a T-cell antigen and partially an adjuvant. The discovery of bacterial N-glycosylation and the possibility of its functional transfer to E. coli allowed for the development of products in which both the production of antigens and their conjugation occur directly in the producing organism, simplifying and reducing the cost of the process.
The first attempts to create a vaccine using PGCT technologies appeared in 2010 [7]. J. Ihssen et al. published a report on the successful production of conjugates in E. coli, consisting of the O-antigen of Shigella dysenteriae serotype 1 and the AcrA proteins of C. jejuni and exotoxin A of Pseudomonas aeruginosa (EPA), respectively. After this report, news about the development of new vaccines against various pathogens based on PGCT began to arrive regularly. However, difficulties immediately arose, primarily due to the fact that PglB is capable of transferring only oligosaccharides with a reducing terminal residue, catalyzing the formation of a bond through the acetamido group at the 2-position, which sharply limits the number of carbohydrate antigens that can be used. To overcome these limitations, researchers began using O-glycosylating bacterial OST, which were found in species such as P. aeruginosa, Neisseria meningitides, Francisella tularensis and Acinetobacter baylyi.
In the case of P. aeruginosa, the function of OST is performed by the PilO enzyme, which transfers glycans to the PilA protein (type IV pilin) [8]. N. meningitides expresses the PglL protein, encoded by the pglL gene, which also glycosylates pili [9]. F. tularensis contains the enzyme PglA, which attaches pentasaccharides to the PilA protein [10]. The PglS enzyme from A. baylyi is an O-glycosyltransferase that transfers carbohydrate residues to the pilin-like protein ComP [11]. All the listed proteins, when cloned in E. coli, demonstrated the ability to glycosylate their native substrates [12]. In this case, PilO transferred only short oligosaccharides, whereas PglL performed glycosylation with long oligosaccharides and, additionally, interacted with glycans that were inaccessible to PglB, such as the O4 O-antigen of Salmonella typhimurium. Regarding PglS, it was found that it is capable of transferring oligosaccharides with glucose as the reducing terminal sugar, which distinguishes it favorably from other bacterial OST [13]. Using these enzymes, researchers were able to develop and obtain bioconjugates capable of eliciting immune responses in both laboratory animals and humans. At present, potential bioconjugate vaccines against Shigella, pathogenic E. coli, Klebsiella, Streptococcus pneumoniae, Brucella, Staphylococcus aureus and other pathogens posing the greatest threat to public health are at various stages of development [2].
Bioconjugate vaccines against Shigella
Shigellosis or bacterial dysentery is caused by microbes of the genus Shigella. These are gram-negative bacteria from the family Enterobacteriaceae that penetrate the gastrointestinal tract, infect the mucous membrane of the large intestine, and cause inflammation. Shigellosis is one of the leading causes of diarrhea-related deaths worldwide. Children under the age of 5 in low- and middle-income countries suffer the most [14]. Currently, four species of Shigella are known: S. flexneri, S. sonnei, S. dysenteriae, and S. boydii. The greatest threat is posed by the species S. flexneri. There are 15 known serotypes of S. flexneri, the most common of which is S. flexneri 2a, followed by S. flexneri 3a and S. flexneri 6. S. sonnei is the prevalent species of Shigella in industrialized countries, and one serotype is known for it. Despite the large number of developments that have reached the clinical trial stage [15] (Table), there is currently no licensed international vaccine against shigellosis.
Bioconjugated vaccines against various pathogens currently under development
Pathogen | Drug name/Bioconjugate characteristics | Stage | Reference |
Shigella spp. | GVXN SD133 | Phase I clinical trials | [17] |
Flexyn 2a | Phase 2b clinical trials | [19] | |
S4V | Phase 1/2 clinical trials | NCT04056117 — ClinicalTrials.gov | |
Pathogenic E. coli (E. coli O 157) | Conjugate of O-antigen and E. coli O157 MBP, OST — PglB | Preclinical trials on mice | [23] |
Conjugate of O-antigen and Citrobacter sedlakii CmeA protein NRC6070, OST — PglB | Laboratory experiments | [25] | |
Extraintestinal pathogenic E. coli | ExPEC9V | Phase III clinical trials | NCT04899336 — ClinicalTrials.gov |
ExPEC10V | Phase I and II clinical trials | NCT04306302, NCT03819049 — ClinicalTrials.gov | |
Conjugate of O25B-antigen ExPEC and EPA, ОSТ — PglB | Laboratory experiments | [32] | |
Hypervirulent type K. pneumoniae (hvKp) | Conjugate of capsular polysaccharides of serotypes K1 and K2 with the EPA-ComP protein, OST — PglS | Preclinical trials in mice | [34] |
K. pneumoniae | Conjugate of O-antigens of lipopolysaccharide and protein EPA, OST — PglS | Preclinical trials in mice | [36] |
K. pneumoniaе О1 | KPO1-VLP | Preclinical trials in mice | [37] |
S. pneumonia | Conjugate of serotype 4 capsular polysaccharide and AcrA protein, OST — PglB | Preclinical trials in mice | [39] |
Conjugates of the ST4 capsular polysaccharide and the NanA, Sp-148, PiuA proteins, OST – PglB | Preclinical trials in mice | [43] | |
CPS8-EPAiGTcc | Preclinical trials in mice | [44] | |
S. agalactiae | Conjugate of polysaccharides of serotypes Ia, Ib and III with the EPA-ComP protein, OST — PglS | Preclinical trials in mice | [47] |
B. abortus | Conjugate of Yersinia enterocolitica O-polysaccharides and cholera toxin B, OST — PglL | Preclinical trials in mice | [52] |
Conjugate of Brucella O-polysaccharides and Nano-B5 nanoparticles, OST — PglL | Preclinical trials in mice | [54] | |
S. aureus | CP5-ЕРА, CP8-ЕРА and CP5-Hla | Preclinical trials in mice | [56] |
F. tularensis | Conjugate of the O-antigen of F. tularensis and EPA | Preclinical trials in mice | [58] |
Conjugate of the O-antigen of F. tularensis and the CmeA protein of Citrobacter sedlakii NRC6070, OST — PglB | Laboratory experiments | [25] |
The first bioconjugated vaccine tested in humans was a candidate vaccine against S. dysenteriae [16]. In its development, the O-antigen polysaccharide of S. dysenteriae type O1 was bioconjugated in E. coli with a recombinant version of EPA and the PglB cluster, named GVXN SD133, and underwent Phase I clinical trials [17]. The results showed that regardless of the method of administration, the drug was well tolerated and had an acceptable level of safety. In the blood of the vaccinated, a statistically significant increase in the levels of IgG and IgA class antibodies against the O1 polysaccharide was detected [16].
Then, another monovalent vaccine against Shigella based on the O-antigen of S. flexneri 2a was developed, also based on PglB. The same rEPA was chosen as the protein carrier. The bioconjugate was named Flexyn 2a. The safety and immunogenicity of this vaccine prototype have been confirmed. Similarly, to the results obtained in the study of the vaccine against S. dysenteriae, immunization with the Flexyn 2a drug revealed a significant increase in the titers of IgG and IgA antibodies against LPS of S. flexneri 2a [18]. A randomized double-blind placebo-controlled phase 2b study [19] demonstrated an adequate level of safety and immunogenicity of the vaccine. The efficacy of the Flexyn 2a bioconjugate was further confirmed by other methods, including the assessment of the severity of shigellosis. It was shown that this indicator was lower in the vaccinated individuals than in the patients receiving the placebo [20].
Promising results demonstrated during the development and testing of Flexyn 2a contributed to the development of a polyvalent vaccine. S4V is a quadrivalent bioconjugated vaccine that contains O-antigens of S. flexneri serotypes 2a, 3a, 6, as well as S. sonnei, conjugated to the carrier protein EPA. Currently, a double-blind S4V study is being conducted in Kenya to determine the appropriate dose and age group (adults, children, infants). The data collected during this study will be an important step in the development of a vaccine against Shigella [21].
Bioconjugated vaccines against pathogenic strains of E. coli
Pathogenic strains of E. coli are divided into two groups: extraintestinal pathogenic E. coli (ExPEC) and intestinal pathogenic E. coli (InPEC). ExPEC strains are primarily associated with neonatal meningitis and urinary tract infections in adults (UTIs). InPEC strains cause various diarrheal diseases and are divided into 6 pathotypes, including enter hemorrhagic E. coli strains. One of the most common representatives of the EHEC group is enterohemorrhagic Escherichia coli O157:H7 (E. coli O157), which causes diarrhea, hemorrhagic colitis and hemolytic-uremic syndrome [22]. The need for vaccines to prevent E. coli O157 is very high. Their development has been ongoing for quite some time, and several drugs are undergoing preclinical and clinical trials, including a bioconjugated vaccine [23]. As a carrier protein, the authors chose the maltose-binding protein because recent studies have shown that it is a TLR4 agonist and induces the activation of the NF-κB signaling pathway, as well as the secretion of a number of pro-inflammatory cytokines [24]. Conjugation was carried out using PglB, attaching the O-antigen of E. coli O157 to the protein, with the producer organism being the E. coli W3110 strain. The resulting bioconjugate induced the activation of both the humoral and cellular immune responses [23].
Another prototype of a bioconjugated vaccine against E. coli O157 was created using the experimental MAGIC technology (Mobile-element Assisted Glycoconjugation by Insertion on Chromosome) [25]. The essence of the method lies in the use of mobile genetic elements, specifically the tn5 transposon, to integrate constructed genetic sequences into the E. coli chromosome. The developers of MAGIC claim that such a construct significantly alleviates the metabolic burden and directly promotes the increase in producer biomass and the yield of the bioconjugate. To achieve this result, they used the tn5 transposon, into which segments encoding PglB, the C. jejuni AcrA carrier protein, and enzymes involved in polysaccharide biosynthesis [26] were integrated into the nucleotide sequence. The bioconjugated prototype of the MAGIC vaccine was obtained in the non-pathogenic bacterium Citrobacter sedlakii NRC6070. The carrier protein used to create the preparation was CmeA 6xHis, the polysaccharide component was the O-antigen of C. sedlakii, which is similar to that of E. coli O157, and PglB was used as the OST. In terms of its biochemical parameters, the bioconjugate met the stated requirements. Unfortunately, there is currently no data on any clinical trials of the obtained drug.
Extraintestinal strains of pathogenic E. coli (ExPEC) are also quite dangerous, as they are capable of causing various types of diseases. ExPEC strains are classified into three main pathotypes: uropathogenic E. coli (UPEC), sepsis-causing E. coli (SEPEC), and E. coli associated with neonatal meningitis [27]. Unfortunately, UTIs caused by ExPEC are extremely difficult to treat. The creation of effective vaccines that prevent such developments is an extremely important task. To address this, along with other approaches, PGCT methods were also applied. The study began with the creation of a quadrivalent prototype, which included 4 EPA-conjugated variants of the O-antigen. High tolerability of the prototype and a significant increase in IgG antibody levels against all antigens, as well as a reduction in the number of reported UTI cases among trial participants, have been demonstrated [28, 29]. Based on the 4-valent prototype, a 9-valent vaccine ExPEC9V has been developed, containing a conjugated polysaccharide and currently undergoing phase III clinical trials (NCT04899336).
The safety and immunogenicity of the 10-valent ExPEC10V vaccine among elderly individuals aged 60–85 years are still being studied (Phases I and II, NCT04306302, NCT03819049). Like the quadrivalent prototype, this drug is well tolerated and induces the production of antigen-specific antibodies in the majority of participants, despite their advanced age [30].
A similar approach was used in the development of the O25B antigen ExPEC vaccine prototype. Although the O-antigen of E. coli has more than 180 serotypes, a significant number of isolates obtained from UTI carriers belong to the O25B serotype [31]. Therefore, a group of researchers attempted to create a vaccine based specifically on this antigen. The O-antigen cluster was integrated into the E. coli W3110 genome, after which the expressed polysaccharide was enzymatically conjugated with the EPA enzyme PglB. Detailed characterization of the O25B-EPA conjugate using physicochemical methods, including nuclear magnetic resonance and gas chromatography-mass spectrometry, confirmed its correspondence to the O25B structure, thereby opening up the possibility for the development of a polyvalent conjugate vaccine against ExPEC [32].
Bioconjugated vaccines against Klebsiella pneumoniae
The Gram-negative bacterium Klebsiella pneumoniae is the second most common opportunistic pathogen after E. coli. It causes neonatal sepsis, UTI and nosocomial pneumonias that are poorly treatable due to antimicrobial resistance — the bacteria acquire resistance factors such as extended-spectrum β-lactamases and K. pneumoniae carbapenemases. The WHO has assigned the highest danger levels to isolates containing these factors [33], confirming the urgent need for the development of an effective and safe vaccine.
Using PGCT technology, several vaccine formulations are being developed. M.F. Feldman and co-authors focused on developing a vaccine against hypervirulent K. pneumoniae (hvKp) because this particular strain of the pathogen is the most dangerous. If other serotypes generally cause diseases in hospitalized patients, the elderly, infants, or people with various types of immunodeficiencies, hvKp pose a threat even to healthy individuals [34]. The mechanisms of hypervirulence are not fully understood, but it is suggested that the main reason is the excess of capsular polysaccharide, which hinders the elimination of the pathogen from the body. As the main O-antigen synthase, the researchers chose PglS from A. baylyi, and as the carrier protein, they used EPA fused with the ComP protein. As the carbohydrate component, the capsular polysaccharides of the most common serotypes K1 and K2 were used, the synthesis clusters of which were cloned into E. coli producer cells with partially blocked natural glycosyltransferases. The obtained glycoproteins, when administered to mice, demonstrated the ability to induce the synthesis of protective IgG1 antibodies, significantly increasing the survival rate of mice upon subsequent infection.
Another vaccine is being developed against the classical serotypes of K. pneumonia. As a carbohydrate component, O-antigens of bacterial lipopolysaccharides are used. Unlike capsular polysaccharides, only 11 serotypes of O-antigens expressed by K. pneumoniae are currently known [35]. Based on the 7 most common O-antigen serotypes, a heptavalent bioconjugate vaccine was constructed. The PglS enzyme was chosen as the OST, and the carrier protein was recombinant EPA with an inserted acceptor sequence for PglS. The E. coli CLM24 strain was used as the producer. After isolation and purification, glycoproteins of all 7 types were used for mouse immunization, which was accompanied by the production of a high level of IgG antibodies to all glycoproteins. However, the bactericidal activity of the antibodies against various strains of K. pneumoniae was low, indicating the need for further development of the vaccine. In this regard, the authors suggest incorporating capsular antigens into the vaccine composition [36].
Another vaccine against K. pneumoniae using PGCT is being developed based on PglL. As an acceptor protein, a universal recombinant protein SpyCatcher4573 and a specially modified strain of E. coli, in which both key components SC4573 and PglL are integrated into the genome, were used. Glycoproteins obtained in this way can spontaneously bind to protein nanocarriers in vitro using the SpyTag system, forming conjugated nanovaccines. To enhance the efficiency of glycoprotein expression, the yfdGHI gene cluster was removed. The obtained conjugated nanovaccine against K. pneumoniae O1 (KPO1-VLP) demonstrated its effectiveness in experiments, where high antibody titers and 100% protection against infection with a virulent strain were observed after three immunizations [37].
Bioconjugated vaccines against Streptococcus pneumoniaе
S. pneumoniae, pneumococcus, is one of the most common and harmful causative agents of bacterial pneumonia, meningitis, and sepsis. Despite the availability of vaccines, S. pneumoniae still causes over 1 million deaths annually, primarily among children under 5 years old in low- and middle-income countries [38]. Since most of the capsular polysaccharides of S. pneumoniae contain a terminal sugar that is not transferred by PglB, the initial attempts to create a bioconjugated vaccine focused on serotype 4, where the terminal residue was the recognizable GalNac. The acceptor chosen was the native C. jejuni AcrA protein, and PglB, cloned into the chromosome of E. coli W3110, was used as the OST. The obtained preparation protected mice upon subsequent infection with S. pneumoniae serotype 4 [39].
The next variant of the bioconjugated vaccine was developed based on native S. pneumoniae proteins. It was assumed that this would create heterologous protection against vaccine-uncovered serotypes and enhance mucosal immune protection by stimulating Th17 activation. The authors tested the efficacy of a trivalent bioconjugate in mouse models, which included the ST4 capsular polysaccharide and three protein antigens of S. pneumoniae: the N-terminal fragment NanA, a virulence factor that promotes growth and survival in the nasopharyngeal tract, invasion of brain endothelial cells [40], the Th17-stimulating antigen Sp0148 [41], and the ABC transporter lipoprotein PiuA [42]. The bioconjugates obtained using PglB in E. coli induced the synthesis of anti-capsular antibodies in mice at a level comparable to existing vaccines, and also elicited strong responses to protein antigens, which extended to other, heterologous serotypes. The authors also noted that the expression of several serotypes of capsular polysaccharides in E. coli opens up new possibilities for designing vaccines against S. pneumoniae. For example, glycosylated outer membrane vesicles (glyOMV) can be used as a platform [43].
Another prototype, this time polyvalent, was created using the PglS enzyme from A. baylyi as the main OST, capable of transferring the glucose terminal residue. As a protein acceptor, the natural substrate of PglS, the pilin-like protein ComP, was used, which in E. coli was glycosylated with the capsule polysaccharides S. pneumoniae CPS8, CPS9V and CPS14. The obtained vaccine showed immunogenicity in preliminary tests comparable to the immunogenicity of the Prevnar13 vaccine. Moreover, the sera of mice immunized with the obtained vaccine exhibited bactericidal activity against S. pneumoniae serotypes 14 and 8. Developing the idea further, the authors constructed a bioconjugate based on the protein carrier EPA, modifying its C-terminus by attaching to it an acceptor sequence of 23 amino acids from the ComP protein, and the pneumococcal polysaccharide CPS8. The obtained bioconjugate induced the active formation of IgG antibodies in mice and exhibited protective effects [13]. In 2022, it was shown that the bioconjugate obtained by the authors, named CPS8-EPAiGTcc, possesses high immunogenicity, induces the formation of IgM and IgG antibodies specific to serotype CPS8 in mice, and provides protection against infection with S. pneumoniae serotype 8 [44].
Bioconjugated vaccines against other types of streptococci
Bioconjugated vaccines against pathogenic streptococci are also being developed. Group B Streptococcus (GBS, Streptococcus agalactiae, β-hemolytic streptococcus B) is a gram-positive opportunistic bacterium that most often colonizes the lower parts of the gastrointestinal tract and the urogenital system. Approximately 10–35% of women are infected with GBS, which can lead to various acute diseases in pregnant and postpartum women, as well as to stillbirth [45]. GBS can also be transmitted to the newborn. It usually manifests as Group B Streptococcal disease and can cause meningitis, sepsis and pneumonia. Moreover, recent studies have shown that GBS is also responsible for a significant number of illnesses in adults, especially those over 65 years old [46]. All of this makes the development of a vaccine against GBS extremely necessary. In this regard, a vaccine based on PGCT was developed. The characterization of the trivalent bioconjugate vaccine targeting the three most clinically prevalent serotypes of GBS: Ia, Ib, III, is included in a study by J.A. Duke et al. [47]. The authors introduced loci necessary for the expression of the PglS protein from A. baylyi into E. coli, which allowed for the glycosylation of the constructed EPA-based carrier protein and the ComP protein with sialic acid residues according to the GBS serotypes Ia, Ib, and III. Further immunization of mice with the obtained vaccine showed that the trivalent bioconjugated vaccine against S. pneumoniae induces the production of IgG antibodies specific to the involved serotypes, which possess neutralizing ability. However, the effectiveness of antibodies against different serotypes used in the creation of the vaccine varied, and the authors suggested that this effect could be eliminated by altering the degree of glycosylation of the carrier protein.
PGCT technologies can also be applied to create a vaccine against group A streptococcus (Streptococcus pyogenes or group A strep). S. pyogenes is an extremely common pathogen, causing a wide range of diseases from acute pharyngitis and impetigo to scarlet fever and invasive diseases such as toxic shock syndrome or necrotizing fasciitis. They lead to the development of secondary autoimmune diseases, such as rheumatic heart disease [48]. Moreover, humans are the only natural hosts of S. pyogenes, therefore, blocking the transmission of this pathogen could lead to its complete elimination. Streptococcus A, like S. pneumoniae, has high antigenic heterogeneity. Serotypes are determined by differences in the main virulence factor, protein M. Due to such heterogeneity in the surface proteins of S. pyogenes, researchers have focused on developing conjugated vaccines based on the external polysaccharides of the pathogen, particularly group A polysaccharide. However, R. Di Benedetto et al. showed that for greater effectiveness of the future vaccine, it is necessary to preserve the protein epitopes of the carriers, as random conjugation did not affect the synthesis of IgG to the group A polysaccharide component but significantly reduced the response to the protein component [49]. As a result of the random conjugation of group A polysaccharide with three S. pyogenes proteins (SLO, SpyAD and SpyCEP), conjugates were obtained, and immunization with them led to the production of antibodies that did not block the activity of one of the proteins used for conjugation — SpyCEP. It retained the ability to cleave interleukin-8. Apparently, to create an effective vaccine based on the native proteins of S. pyogenes and its own group A polysaccharide, it will be necessary to ensure an extremely high precision in attaching the polysaccharide to specific sites on the proteins, which can be quite effectively achieved using PGCT [50].
Other bioconjugate vaccines
PGCT technologies are used in the development of a vaccine against B. abortus [51]. This pathogen, although primarily a cause of diseases in livestock, nevertheless poses a threat to humans as well. There is currently no licensed vaccine that protects against B. abortus infection. There are attenuated vaccines used to protect large (S19 and RB51) and small (Rev1) cattle [52]. However, these vaccines are pathogenic to humans, have residual toxicity for animals, and do not protect against all known species of the pathogen. Moreover, working with Brucella cultures requires high-level biosafety equipment due to the risk of aerosol transmission. To avoid these difficulties, Y. enterocolitica, a less dangerous opportunistic pathogen, is often used for the synthesis of glycosylated glycoproteins similar to B. abortus, as the O-polysaccharides of Brucella and Y. enterocolitica are similar [53]. At present, prototypes of vaccines based on cholera toxin B as a protein carrier and O-polysaccharides of Y. enterocolitica synthesized in genetically modified E. coli [52], as well as those based on Nano-B5 nanoparticles as a platform and O-polysaccharides of Brucella [54], are at a high level of readiness. The producer in the latter case is Y. enterocolitica. Both vaccines used an O-glycosylation system with the central OST PglL from N. meningitides. In both cases, the researchers report successful application of the obtained prototypes in preclinical studies on mice. When administered to animals, both increased antibody production and activation of cellular immunity were observed. Moreover, both prototypes demonstrated a strong protective effect of immunization followed by infection in mice, and in the case of the nanovaccine, even against several species of Brucella. Further clinical studies will obviously demonstrate the applicability of the obtained drugs for human immunization.
Staphylococcus aureus is responsible for numerous human diseases, including endocarditis, pneumonia and wound infections. The methicillin-resistant S. aureus (so-called MRSA strains) [55] poses a particular danger. In this regard, there is an urgent need for effective vaccination against staphylococcal infection. In a study by M. Wacker et al., the results of testing 3 conjugates obtained using PGCT technologies on mice were presented. By the names of the components they contain, they are called CP5-ETA, CP8-ETA and CP5-Hla, where CP5 and CP8 are capsular polysaccharides of S. aureus serotypes 5 and 8, respectively, ETA is exotoxin A of P. aeruginosa, and Hla is α-toxin of S. aureus. In the study, PglB from C. jejuni was used. Bioconjugates were synthesized in E. coli and then administered to mice. All three prototypes induced a high level of antibody production. When evaluating the protective efficacy of the drugs, the best results were shown by the conjugate CP5-Hla; the administration of CP5-Epa and CP8-Epa significantly reduced bacteremia; the bioconjugated vaccine CP5-Hla protected against both bacteria and fatal pneumonia.
The PGCT technology was applied to create vaccine prototypes against Francisella tularensis, an intracellular pathogen causing tularemia — a potentially fatal disease. For humans, the two most dangerous subspecies are F. tularensis tularensis (type A) and F. tularensis holarctica (type B) [57]. The authors used PglB to obtain a bioconjugate in E. coli consisting of the O-antigen of F. tularensis and EPA, and tested it on a mouse model. The obtained recombinant bioconjugate exhibited high yield, stimulated the production of specific antibodies, and provided protection against subsequent infection with the virulent wild-type strain F. tularensis subsp. holarctica [58]. The authors subsequently modified the EPA carrier by adding 8 additional acceptor sequences to increase its glycosylation degree [59]. The new bioconjugate actually stimulated the formation of specific antibodies more effectively, protecting rats from developing the disease when infected with F. tularensis. The researchers plan to continue working on the presented vaccine prototype, intending to replace the carrier protein EPA with native protein antigens of F. tularensis.
Another attempt to create a new vaccine against F. tularensis was associated with the use of the already mentioned experimental technology MAGIC [25]. PglB was used as the OST, the carrier protein was periplasmic Cmea from C. jejuni, equipped with a 6His tag for ease of purification, and the polysaccharide was the O-antigen of F. tularensis. The producing organism was E. coli, and all components were integrated into the bacterial chromosome. The study demonstrated the effectiveness of the MAGIC technology in obtaining highly immunogenic bioconjugates.
Conclusion
Thus, bioconjugation, like other modern technologies, is actively used in the development of new vaccines and the improvement of existing vaccines. Despite the current limitations, this method can be used to create drugs that prevent infectious diseases, thereby reducing the spread of antibiotic-resistant microorganisms.
About the authors
Maria I. Tsyganova
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology
Author for correspondence.
Email: maria_che@mail.ru
ORCID iD: 0000-0002-2811-6844
Cand. Sci. (Biol.), leading researcher, Laboratory of immunochemistry
Russian Federation, Nizhny NovgorodDmitry V. Novikov
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology
Email: novikov.dv75@mail.ru
ORCID iD: 0000-0001-7049-6935
Cand. Sci. (Biol.), leading researcher, Laboratory of immunochemistry
Russian Federation, Nizhny NovgorodViktor V. Novikov
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology
Email: mbre@mail.ru
ORCID iD: 0000-0002-2449-7213
Dr. Sci. (Biol.), Professor, Head, Laboratory of immunochemistry
Russian Federation, Nizhny NovgorodAlexander V. Karaulov
I.M. Sechenov First Moscow State Medical University (Sechenov University)
Email: drkaraulov@mail.ru
ORCID iD: 0000-0002-1930-5424
Dr. Sci. (Med.), Professor, RAS Full Member, Head, Laboratory of immunopathology, Institute of Molecular Medicine, Head, Department of clinical immunology and allergy, N.V. Sklifosovsky Institute of Clinical Medicine
Russian Federation, MoscowReferences
- Hasso-Agopsowicz M., Hwang A., Hollm-Delgado M.G., et al. Identifying WHO global priority endemic pathogens for vaccine research and development (R&D) using multi-criteria decision analysis (MCDA): an objective of the Immunization Agenda 2030. EBioMedicine. 2024;110:105424. DOI: https://doi.org/10.1016/j.ebiom.2024.105424
- WHO. Estimating the impact of vaccines in reducing antimicrobial resistance and antibiotic use;2024.
- Szymanski C.M., Yao R., Ewing C.P., et al. Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol. Microbiol. 1999;32(5):1022–30. DOI: https://doi.org/10.1046/j.1365-2958.1999.01415.x
- Kowarik M., Numao S., Feldman M.F., et al. N-linked glycosylation of folded proteins by the bacterial oligosaccharyltransferase. Science. 2006;314(5802):1148–50. DOI: https://doi.org/10.1126/science.1134351
- Bacon D.J., Szymanski C.M., Burr D.H., et al. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol. Microbiol. 2001;40(3):769–77. DOI: https://doi.org/10.1046/j.1365-2958.2001.02431.x
- Wacker M., Linton D., Hitchen P.G., et al. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science. 2002;298(5599):1790–3. DOI: https://doi.org/10.1126/science.298.5599.1790
- Ihssen J., Kowarik M., Dilettoso S., et al. Production of glycoprotein vaccines in Escherichia coli. Microb. Cell Fact. 2010;9:61. DOI: https://doi.org/10.1186/1475-2859-9-61
- Castric P. pilO, a gene required for glycosylation of Pseudomonas aeruginosa 1244 pilin. Microbiology (Reading). 1995;141(Pt. 5):1247–54. DOI: https://doi.org/10.1099/13500872-141-5-1247
- Power P.M., Seib K.L., Jennings M.P. Pilin glycosylation in Neisseria meningitidis occurs by a similar pathway to wzy-dependent O-antigen biosynthesis in Escherichia coli. Biochem. Biophys. Res. Commun. 2006;347(4):904–8. DOI: https://doi.org/10.1016/j.bbrc.2006.06.182
- Egge-Jacobsen W., Salomonsson E.N., Aas F.E., et al. O-linked glycosylation of the PilA pilin protein of Francisella tularensis: identification of the endogenous protein-targeting oligosaccharyltransferase and characterization of the native oligosaccharide. J. Bacteriol. 2011;193(19):5487–97. DOI: https://doi.org/10.1128/JB.00383-11
- Harding C.M., Nasr M.A., Kinsella R.L., et al. Acinetobacter strains carry two functional oligosaccharyltransferases, one devoted exclusively to type IV pilin, and the other one dedicated to O-glycosylation of multiple proteins. Mol. Microbiol. 2015;96(5):1023–41. DOI: https://doi.org/10.1111/mmi.12986
- Faridmoayer A., Fentabil M.A., Haurat M.F., et al. Extreme substrate promiscuity of the Neisseria oligosaccharyl transferase involved in protein O-glycosylation. J. Biol. Chem. 2008;283(50):34596–604. DOI: https://doi.org/10.1074/jbc.M807113200
- Harding C.M., Nasr M.A., Scott N.E., et al. A platform for glycoengineering a polyvalent pneumococcal bioconjugate vaccine using E. coli as a host. Nat. Commun. 2019;10(1):891. DOI: https://doi.org/10.1038/s41467-019-08869-9
- Platts-Mills J.A., Liu J., Rogawski E.T., et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob. Health. 2018;6(12):e1309–18. DOI: https://doi.org/10.1016/S2214-109X(18)30349-8
- Lόpez-Vélez R., Lebens M., Bundy L., et al. Bacterial travellers' diarrhoea: a narrative review of literature published over the past 10 years. Travel Med. Infect. Dis. 2022;47:102293. DOI: https://doi.org/10.1016/j.tmaid.2022.102293
- Ravenscroft N., Haeuptle M.A., Kowarik M., et al. Purification and characterization of a Shigella conjugate vaccine, produced by glycoengineering Escherichia coli. Glycobiology. 2016;26(1): 51–62. DOI: https://doi.org/10.1093/glycob/cwv077
- Hatz C.F., Bally B., Rohrer S., et al. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella dysenteriae type 1 administered to healthy adults: a single blind, partially randomized Phase I study. Vaccine. 2015;33(36):4594–601. DOI: https://doi.org/10.1016/j.vaccine.2015.06.102
- Martin P., Alaimo C. The ongoing journey of a Shigella bioconjugate vaccine. Vaccines (Basel). 2022;10(2):212. DOI: https://doi.org/10.3390/vaccines10020212
- Talaat K.R., Alaimo C., Martin P., et al. Human challenge study with a Shigella bioconjugate vaccine: analyses of clinical efficacy and correlate of protection. EBioMedicine. 2021;66:103310. DOI: https://doi.org/10.1016/j.ebiom.2021.103310
- Clarkson K.A., Porter C.K., Talaat K.R., et al. Shigella-specific immune profiles induced after parenteral immunization or oral challenge with either Shigella flexneri 2a or Shigella sonnei. mSphere. 2021;6(4):e0012221. DOI: https://doi.org/10.1128/mSphere.00122-21
- Lu T., Das S., Howlader D.R., et al. Shigella vaccines: the continuing unmet challenge. Int. J. Mol. Sci. 2024;25(8):4329. DOI: https://doi.org/10.3390/ijms25084329
- Rojas-Lopez M., Monterio R., Pizza M., et al. Intestinal pathogenic Escherichia coli: insights for vaccine development. Front. Microbiol. 2018;9:440. DOI: https://doi.org/10.3389/fmicb.2018.00440
- Ma Z., Zhang H., Shang W., et al. Glycoconjugate vaccine containing Escherichia coli O157:H7 O-antigen linked with maltose-binding protein elicits humoral and cellular responses. PLoS One. 2014;9(8):e105215. DOI: https://doi.org/10.1371/journal.pone.0105215
- Fernandez S., Palmer D.R., Simmons M., et al. Potential role for Toll-like receptor 4 in mediating Escherichia coli maltose-binding protein activation of dendritic cells. Infect. Immun. 2007; 75(3):1359–63. DOI: https://doi.org/10.1128/IAI.00486-06
- Abouelhadid S., Atkins E.R., Kay E.J., et al. Development of a novel glycoengineering platform for the rapid production of conjugate vaccines. Microb. Cell Fact. 2023;22(1):159. DOI: https://doi.org/10.1186/s12934-023-02125-y
- Wren B., Cuccui J., Abouelhadid S. Glycosylation method. Patent № US 2015/0344928 A1. London;2015.
- Dale A.P., Woodford N. Extra-intestinal pathogenic Escherichia coli (ExPEC): Disease, carriage and clones. J. Infect. 2015;71(6):615–26. DOI: https://doi.org/10.1016/j.jinf.2015.09.009
- Inoue M., Ogawa T., Tamura H., et al. Safety, tolerability and immunogenicity of the ExPEC4V (JNJ-63871860) vaccine for prevention of invasive extraintestinal pathogenic Escherichia coli disease: A phase 1, randomized, double-blind, placebo-controlled study in healthy Japanese participants. Hum. Vaccin. Immunother. 2018;14(9):2150–7. DOI: https://doi.org/10.1080/21645515.2018.1474316
- Frenck R.W.Jr., Ervin J., Chu L., et al. Safety and immunogenicity of a vaccine for extra-intestinal pathogenic Escherichia coli (ESTELLA): a phase 2 randomised controlled trial. Lancet Infect. Dis. 2019;19(6):631–40. DOI: https://doi.org/10.1016/S1473-3099(18)30803-X
- Fierro C.A., Sarnecki M., Doua J., et al. Safety, reactogenicity, immunogenicity, and dose selection of 10-valent extraintestinal pathogenic Escherichia coli bioconjugate vaccine (VAC52416) in adults aged 60–85 years in a randomized, multicenter, interventional, first-in-human, phase 1/2a study. Open Forum Infect. Dis. 2023;10(8):ofad417. DOI: https://doi.org/10.1093/ofid/ofad417
- Phan M.D., Peters K.M., Sarkar S., et al. The serum resistome of a globally disseminated multidrug resistant uropathogenic Escherichia coli clone. PLoS Genet. 2013;9(10):e1003834. DOI: https://doi.org/10.1371/journal.pgen.1003834
- Kowarik M., Wetter M., Haeuptle M.A., et al. The development and characterization of an E. coli O25B bioconjugate vaccine. Glycoconj. J. 2021;38(4):421–35. DOI: https://doi.org/10.1007/s10719-021-09985-9
- CDC. Antibiotic resistance threats in the United States;2019.
- Feldman M.F., Mayer Bridwell A.E., Scott N.E., et al. A promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae. Proc. Natl. Acad. Sci. USA. 2019;116(37):18655–63. DOI: https://doi.org/10.1073/pnas.1907833116
- Follador R., Heinz E., Wyres K.L., et al. The diversity of Klebsiella pneumoniae surface polysaccharides. Microb. Genom. 2016;2(8):e000073. DOI: https://doi.org/10.1099/mgen.0.000073
- Wantuch P.L., Knoot C.J., Robinson L.S., et al. Capsular polysaccharide inhibits vaccine-induced O-antigen antibody binding and function across both classical and hypervirulent K2:O1 strains of Klebsiella pneumoniae. PLoS Pathog. 2023;19(5):e1011367. DOI: https://doi.org/10.1371/journal.ppat.1011367
- Liu Y., Pan C., Wang K., et al. Preparation of a Klebsiella pneumoniae conjugate nanovaccine using glycol-engineered Escherichia coli. Microb. Cell Fact. 2023;22(1):95. DOI: https://doi.org/10.1186/s12934-023-02099-x
- Sari R.F., Fadilah F., Maladan Y., et al. A narrative review of genomic characteristics, serotype, immunogenicity, and vaccine development of Streptococcus pneumoniae capsular polysaccharide. Clin. Exp. Vaccine Res. 2024;13(2):91–104. DOI: https://doi.org/10.7774/cevr.2024.13.2.91
- Herbert J.A., Kay E.J., Faustini S.E., et al. Production and efficacy of a low-cost recombinant pneumococcal protein polysaccharide conjugate vaccine. Vaccine. 2018;36(26):3809–19. DOI: https://doi.org/10.1016/j.vaccine.2018.05.036
- Wren J.T., Blevins L.K., Pang B., et al. Pneumococcal neuraminidase A (NanA) promotes biofilm formation and synergizes with influenza A virus in nasal colonization and middle ear infection. Infect. Immun. 2017;85(4):e01044–16. DOI: https://doi.org/10.1128/IAI.01044-16
- Moffitt K.L., Gierahn T.M., Lu Y.J., et al. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe. 2011;9(2):158–65. DOI: https://doi.org/10.1016/j.chom.2011.01.007
- Ogunniyi A.D., Mahdi L.K., Trappetti C., et al. Identification of genes that contribute to the pathogenesis of invasive pneumococcal disease by in vivo transcriptomic analysis. Infect. Immun. 2012; 80(9):3268–78. DOI: https://doi.org/10.1128/IAI.00295-12
- Reglinski M., Ercoli G., Plumptre C., et al. A recombinant conjugated pneumococcal vaccine that protects against murine infections with a similar efficacy to Prevnar-13. NPJ Vaccines. 2018;3:53. DOI: https://doi.org/10.1038/s41541-018-0090-4
- Aceil J., Paschall A.V., Knoot C.J., et al. Immunogenicity and protective efficacy of a prototype pneumococcal bioconjugate vaccine. Vaccine. 2022;40(42):6107–13. DOI: https://doi.org/10.1016/j.vaccine.2022.09.018
- Russell N.J., Seale A.C., O'Driscoll M., et al. Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin. Infect. Dis. 2017;65(Suppl. 2):S100–S11. DOI: https://doi.org/10.1093/cid/cix658
- McLaughlin J.M., Peyrani P., Furmanek S., et al. Burden of adults hospitalized with group B Streptococcal infection. J. Infect. Dis. 2021;224(7):1170–8. DOI: https://doi.org/10.1093/infdis/jiaa110
- Duke J.A., Paschall A.V., Robinson L.S., et al. Development and immunogenicity of a prototype multivalent group B Streptococcus bioconjugate vaccine. ACS Infect. Dis. 2021;7(11):3111–23. DOI: https://doi.org/10.1021/acsinfecdis.1c00415
- Watkins D.A., Johnson C.O., Colquhoun S.M., et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N. Engl. J. Med. 2017;377(8):713–22. DOI: https://doi.org/10.1056/NEJMoa1603693
- Di Benedetto R., Mancini F., Carducci M., et al. Rational design of a glycoconjugate vaccine against group A Streptococcus. Int. J. Mol. Sci. 2020;21(22):8558. DOI: https://doi.org/10.3390/ijms21228558
- Burns K., Dorfmueller H.C., Wren B.W., et al. Progress towards a glycoconjugate vaccine against group A Streptococcus. NPJ Vaccines. 2023;8(1):48. DOI: https://doi.org/10.1038/s41541-023-00639-5
- Li S., Huang J., Wang K., et al. A bioconjugate vaccine against Brucella abortus produced by engineered Escherichia coli. Front. Bioeng. Biotechnol. 2023;11:1121074. DOI: https://doi.org/10.3389/fbioe.2023.1121074
- Oliveira S.C., Giambartolomei G.H., Cassataro J. Confronting the barriers to develop novel vaccines against brucellosis. Expert. Rev. Vaccines. 2011;10(9):1291–305. DOI: https://doi.org/10.1586/erv.11.110
- Skurnik M., Biedzka-Sarek M., Lübeck P.S., et al. Characterization and biological role of the O-polysaccharide gene cluster of Yersinia enterocolitica serotype O:9. J. Bacteriol. 2007;189(20):7244–53. DOI: https://doi.org/10.1128/JB.00605-07
- Huang J., Guo Y., Yu S., et al. One-step preparation of a self-assembled bioconjugate nanovaccine against Brucella. Virulence. 2023;14(1):2280377. DOI: https://doi.org/10.1080/21505594.2023.2280377
- Bassetti M., Nicco E., Mikulska M. Why is community-associated MRSA spreading across the world and how will it change clinical practice? Int. J. Antimicrob. Agents. 2009;34(Suppl. 1): S15–9. DOI: https://doi.org/10.1016/S0924-8579(09)70544-8
- Wacker M., Wang L., Kowarik M., et al. Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J. Infect. Dis. 2014;209(10):1551–61. DOI: https://doi.org/10.1093/infdis/jit800
- McLendon M.K., Apicella M.A., Allen L.A. Francisella tularensis: taxonomy, genetics, and immunopathogenesis of a potential agent of biowarfare. Annu. Rev. Microbiol. 2006;60:167–85. DOI: https://doi.org/10.1146/annurev.micro.60.080805.142126
- Cuccui J., Thomas R.M., Moule M.G., et al. Exploitation of bacterial N-linked glycosylation to develop a novel recombinant glycoconjugate vaccine against Francisella tularensis. Open Biol. 2013;3(5):130002. DOI: https://doi.org/10.1098/rsob.130002
- Prior J.L., Prior R.G., Hitchen P.G., et al. Characterization of the O antigen gene cluster and structural analysis of the O antigen of Francisella tularensis subsp. tularensis. J. Med. Microbiol. 2003;52(Pt. 10):845–51. DOI: https://doi.org/10.1099/jmm.0.05184-0
Supplementary files