DNA-protein COVID-19 combination vaccine containing multiepitope T-cell immunogen and receptor binding domain of the SARS-CoV-2 S protein
- Authors: Borgoyakova M.B.1, Rudometov A.P.1, Starostina E.V.1, Yakovlev V.A.1, Tigeeva E.V.1, Zaykovskaya A.V.1, Volosnikova E.A.1, Merkuleva I.A.1, Isaeva A.A.1, Nesmeyanova V.S.1, Shanshin D.V.1, Zaitsev B.N.1, Kisakov D.N.1, Kisakova L.A.1, Shcherbakov D.N.1, Karpenko L.I.1, Ilyichev A.A.1
-
Affiliations:
- State Research Center of Virology and Biotechnology «Vector»
- Issue: Vol 102, No 5 (2025)
- Pages: 571-582
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18834
- DOI: https://doi.org/10.36233/0372-9311-691
- EDN: https://elibrary.ru/WKWCLK
- ID: 18834
Cite item
Abstract
Introduction. During the COVID-19 pandemic, the development of preventive vaccines, including those based on new platforms, became extremely relevant. One such platform is vaccines, which combine, for example, DNA and protein components into a single vaccine.
The aim of this study was to investigate the immunogenicity of a DNA vaccine encoding a polyepitopic T-cell immunogen of the SARS-CoV-2 virus, combined with the recombinant RBD protein (the receptor-binding domain of the SARS-CoV-2 virus S protein, Wuhan-Hu-1 strain) conjugated to a polycationic carrier – polyglucin-spermidine (PGS), and to assess the contribution of individual components to the development of an immune response in BALB/c mice.
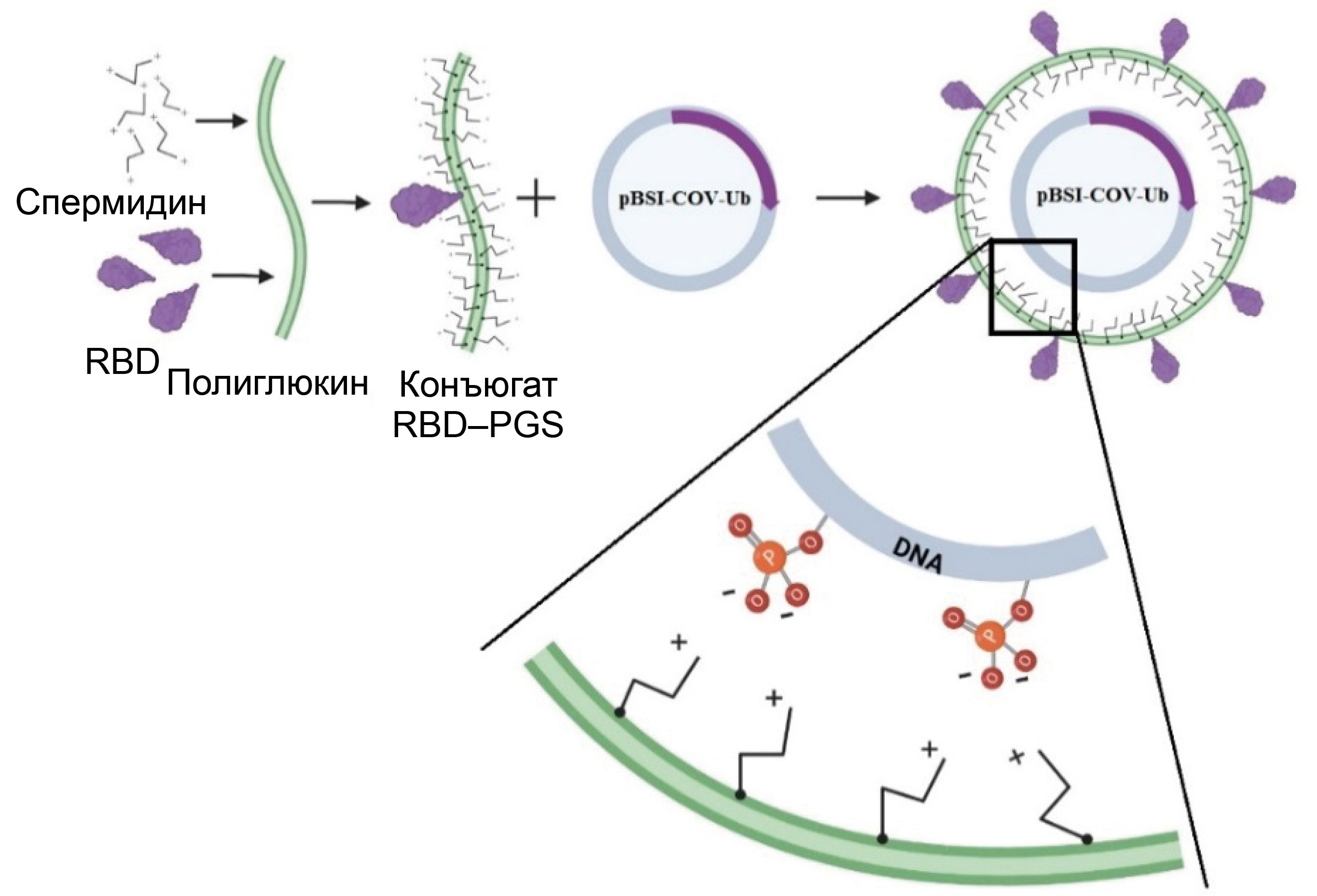
Materials and methods. To create the DNA vaccine (pBSI-COV-Ub), we used a strategy of designing an artificial polyepitope immunogen consisting of conserved immunodominant fragments of various structural proteins of the SARS-CoV-2 virus, containing a large number of T-lymphocyte epitopes: helper and cytotoxic. The recombinant RBD protein was conjugated with the polycation PGS, and upon mixing it with DNA, it formed the vaccine complex CCV–BSI, whose immunogenic properties were investigated in this work.
Results. Immunization of BALB/c mice with the CCV–BSI combined construct resulted in the induction of high antibody titers with neutralizing activity against live SARS-CoV-2 virus, as well as the formation of a virus-specific T-cell response, as demonstrated by ELISA, neutralization assay and ELISpot. It has been shown that the protein component contributes to the humoral immune response, while DNA contributes to the cellular immune response. Administration of the recombinant RBD protein led to the induction of only antibodies, administration of the DNA vaccine led to the induction of only a T-cell response, and administration of the combined preparation led to the induction of both a humoral immune response and specific T cells.
Conclusion. The unique combination of DNA and protein within a single vaccine construct allows for overcoming the limitations of each of these vaccine types and leads to the induction of both arms of immunity. The protein component can be replaced according to the current viral strain, and a universal T-cell immunogen can provide a response to a wide range of circulating variants. This platform can be further used to develop vaccines against various highly variable viruses.
Full Text
Introduction
The COVID-19 pandemic led to the adoption of unprecedented measures in global healthcare. Specifically, for mass vaccination in emergency situations, vaccines based on innovative approaches have been approved: mRNA vaccines [1], DNA vaccines [2] and viral vector vaccines [3, 4]. It has been established that vaccines of these types are capable of inducing T-cell responses that are important for combating viral infection.
For related viruses SARS-CoV (the coronavirus that causes severe acute respiratory syndrome) and MERS-CoV (the coronavirus that causes Middle East respiratory syndrome), it has been shown that the virus-specific T cell response persists for decades, while neutralizing antibody titers decline significantly within six months after the illness [5, 6]. A decrease in the neutralizing activity of sera has also been shown with the emergence of new SARS-CoV-2 virus strains [7]. It should be noted that the long-lasting T cell response is specific not only to the main antigen – the S glycoprotein, the primary target of the humoral immune response – but also to other proteins that are not usually used as immunogens in subunit vaccines [8]. Since proteins N, M, E and others are less prone to accumulating mutations [9], their sequences are more conserved [10], and the T-cell immune response directed against their epitopes can be specific to emerging strains [11]. The promising strategy is creating artificial T-cell polyepitope immunogens containing immunodominant epitopes of viral/bacterial/tumor proteins recognized by CD4+ and CD8+ T cells. The DNA vaccine platform is capable of stimulating cytotoxic and T helper lymphocytes through intracellular synthesis and processing of the immunogen, followed by its presentation by antigen-presenting cells in complex with class I and II major histocompatibility complex (MHC) molecules.
However, in the case of SARS-CoV-2, the importance of the humoral immune response should not be forgotten, since high titers of neutralizing antibodies correlate with vaccine efficacy [12]. The most suitable immunogens for inducing antibody production are recombinant proteins [13]. They are limited in stimulating a specific cytotoxic response, but they activate B cells and T helper cells, especially well in the presence of adjuvants [14]. Such adjuvants can be aluminum salts, various emulsions and polysaccharides. One of the most studied α-glucans used for drug delivery is polyglucin (high molecular weight dextran) [15]. Polyglucin provides prolonged release of the active substance and is also an immunomodulator, which makes it work as an adjuvant. Thanks to the presence of hydroxyl groups, polyglucin is easily modified, particularly by the attachment of substances containing amino groups, such as proteins [16].
Combining different types of immunogens (DNA and protein) in a single construct appears to be a promising approach that can overcome the limitations of each type of vaccine and lead to the induction of both arms of immunity. In most studies investigating the possibility of combining different immunogens, immunization is performed using prime-boost strategies, where the first immunization is most often done with a DNA vaccine, and the booster is with a protein complexed with an adjuvant [17]. Certain studies have shown that co-administration of DNA and protein leads to increased immunogenicity compared to groups receiving the components separately, as well as groups receiving vaccines in a prime-boost system [18–21]. Thus, the administration of a mixture of DNA vaccine encoding the SARS-CoV-2 virus S protein and recombinant S protein, combined with an aluminum hydroxide adjuvant, resulted in the inducing of a strong protective immune response in Syrian hamsters, significantly exceeding the values in groups of animals immunized with individual components [21]. It was also shown that co-administering DNA and protein at the same site is more effective than administering them simultaneously but at different sites (e.g., in different paws) [19].
For other vaccines that induce a T-cell response, such as mRNA or vector vaccines, co-administration with protein is not found in the literature. However, there are studies dedicated to the positive effect of boosting the immune response to these types of vaccines with subunit drugs [22–25].
The aim of this study was to investigate the immunogenicity of a DNA vaccine encoding a polyepitopic T-cell immunogen of the SARS-CoV-2 virus, combined with the recombinant RBD protein (receptor-binding domain of the SARS-CoV-2 virus S protein) conjugated to a polycationic carrier — polyglucin-spermidine (PGS), and to assess the contribution of individual components to the development of an immune response in BALB/c mice.
Materials and methods
Construction of the pBSI-COV DNA vaccine
The DNA component was the plasmid pBSI-CoV-Ub, which we obtained earlier [26]. T-cell epitope prediction was performed using the NetMHCpan-4.1 program and the Immune Epitope Database 2.22, IEDB 2.22. The sequences of the S, N, M and E proteins were obtained from genome sequencing the SARS-CoV-2 virus strain Wuhan-Hu-1 (GenBank MN908947). The fragments selected for the study were analyzed for conservation using the GISAID database, then sequentially linked into a single construct, with ubiquitin added to the N-terminus, and the PADRE epitope (Pan DR Epitope, a universal T helper epitope that enhances the induction of B cell-regulating CD4+ T cells and cytotoxic T lymphocytes), and the EPFRDYVDRFYKTLR marker epitope added to the C-terminus. The coding genetic sequence was optimized for efficient translation in mammalian cells using the Jcat program (http://www.jcat.de). The gene synthesis was performed by the DNA Synthesis company. Cloning into the pVAX1 vector was performed at the PspLI and ApaI sites.
Target gene expression was assessed using reverse transcription PCR and Western blotting [26].
Obtaining a combined DNA-protein vaccine
The RBD protein and its conjugate with PGS were obtained as previously described [27]. The RBD sequence was sourced from the genomic data of the Wuhan-Hu-1 isolate (GenBank: MN908947.3). A combined DNA-protein preparation was obtained by mixing 2 parts of plasmid DNA with 1 part of RBD protein conjugated to PGS (by mass ratio of DNA and protein), and then adding an excess of PGS at a mass ratio of 1:10 relative to the DNA. Complex formation was evaluated by the shift in electrophoretic mobility in a 1% agarose gel, as well as by electron microscopy: staining with a 2% aqueous solution of uranyl acetate, JEM-1400 microscope (Jeol).
Animal immunization, sample preparation
Work with laboratory animals was conducted in accordance with the "Guide for the Care and Use of Laboratory Animals" and the principles of humanity outlined in European Community directives (86/609/EEC) and the Helsinki Declaration. The protocols were approved by the Bioethics Committee of the State Research Center of Virology and Biotechnology "Vector" (protocol No. 1 dated March 21, 2023).
To assess the immunogenicity of the constructs, female BALB/c mice weighing 16–18 g, obtained from the nursery of the State Research Center of Virology and Biotechnology "Vector", were used. The animals were divided into groups of 6 mice and immunized intramuscularly twice, 3 weeks apart. Each mouse received 200 μL of the injectable drug into both quadriceps muscles of the hind legs (100 μL into each):
- CCV-BSI group — a combination vaccine containing 100 μg of DNA and 50 μg of protein;
- pBSI-COV-Ub group — 100 μg of plasmid;
- RBD-PGS group — 50 μg of RBD protein conjugated with PGS;
- intact group — non-immunized animals.
Two weeks after the second immunization, blood was taken from the mice to analyze the humoral immune response, and spleens were taken to analyze the cellular response.
Sera were separated from cellular elements by centrifugation (9000g, 15 min), heated for 30 minutes at 56°C, and tested for the presence of antibodies that specifically bind to the RBD protein in an enzyme-linked immunosorbent assay (ELISA), and their virus-neutralizing activity was also analyzed.
Spleens were sequentially minced on 70 and 40 μm pore diameter nylon cell strainers (JET Biofil). Splenocyte preparation was performed as previously described [27].
Enzyme immunoassay
Recombinant RBD protein was adsorbed onto 96-well plates (Nunc) in 2M urea at 4°C overnight (1 μg/mL). The ELISA was performed as previously described [27]. The results were analyzed at a wavelength of 450 nm using a Feyond A-300 spectrophotometer (Allsheng).
Virus neutralization reaction
The neutralizing properties of blood serum antibodies were analyzed in a virus cytopathic effect inhibition assay on cell culture in vitro, as previously described [27]. The study used the SARS-CoV-2 strain nCoV/Victoria/1/2020, obtained from the State Collection of Viral Infection and Rickettsial Pathogens at the State Research Center of Virology and Biotechnology "Vector" of the Federal Service for Surveillance on Consumer Rights Protection and Human Well-being (Rospotrebnadzor). The neutralizing activity of the sera from immunized animals was assessed by serum dilution, at which the protection of cells from the cytopathic effect of the virus was recorded in 50% of the wells.
ELISpot
ELISpot was performed using the ELISpot Plus: Mouse IFN-γ (ALP) kit (Mabtech) according to the manufacturer instructions. Splenocytes were seeded at a density of 2.5 × 105 cells per well and were then treated with RPMI medium containing 10% fetal bovine serum (for the negative control), or a mixture of virus-specific peptides at a concentration of 20 μg/mL for each peptide, or concanavalin A (for the positive control). Spot counting was performed visually using an ELISpot reader (Carl Zeiss). The number of spot-forming units (SFU) per 106 cells was calculated by subtracting the values in the negative control wells.
Stimulating peptides were identified using the IEDB Analysis Resource tools and synthesized by AtaGenix Laboratories. The purity of the peptides was over 80%: VYAWNRKRI, FERDISTEI, CGPKKSTNL, RFASVYAWNRKRISN, VGGNYNYLYRLFRKS, GGNYNYLYRLFRKSN, YNYKLPDDFTGCVIA, KNKCVNFNFNGLTGT, QPTESIVRF, VSPTKLNDL, LLHAPATVCGPKKST, ASVYAWNRKRISN, YNYLYRLFRKSNL, AYSNNSIAI, QYIKWPWYI, LPPLLTDEM, SAPHGVVFL, WPWYIWLGF, YYRRATRRIRGGDGK, GTWLTYTGAIKLDDK, DDQIGYYRRATRRIR, VKPSFYVYSRVKNLN, CFVLAAVYRINWITG, YYRRATRRI, TPSGTWLTY, KHIDAYKTF, SPDDQIGYY.
Statistical processing
The data were analyzed using GraphPad Prism 6.0 software. Differences between groups were determined using the non-parametric Mann–Whitney method; differences were considered significant at p < 0.05.
Results
Design of an artificial polyepitopic T-cell immunogen
The T-cell immunogen was designed using an approach that allows for the selection of immunodominant epitopes and then combines them into a single sequence. Based on the analysis, 5 fragments from the S protein, 2 fragments each from the M and N proteins, and 1 fragment from the E protein were selected, which contain the highest number of CD4+ and CD8+ epitopes restricted by human and mouse MHC class I and II molecules. Homology analysis of these fragments for different virus strains, including variants relevant for 2024, showed a high degree of conservation (85.4–100%). A sequence encoding ubiquitin was added to the construct to increase the efficiency of intracellular processing of the synthesized protein. The amino acid sequences of the selected fragments and the overall structure of the immunogen are presented in Fig. 1. The designed immunogen was named BSI-COV-Ub.
Fig. 1. Schematic representation of the artificial polyepitopic T-cell immunogen BSI-COV-Ub, composed of fragments of SARS-CoV-2 virus proteins
The pBSI-COV-Ub plasmid (Fig. 2, a) was produced in preparative quantities and characterized previously [26]. The expression of the target gene encoding the artificial BSI-COV-Ub immunogen was determined at the RNA and protein levels using reverse transcription polymerase chain reaction (RT-PCR) and Western blotting. According to RT-PCR data, the size of the amplified fragment was approximately 1470 bp, which corresponds to the theoretically calculated fragment when using specific primers (Fig. 2, b). Immunoblotting revealed discrete proteins, the largest of which corresponded to the theoretically calculated product of the BSI-COV-Ub gene (54.5 kDa; Fig. 2, c). The presence of a ladder of discrete proteins indicates effective processing of the T-cell immunogen within the cell.
Fig. 2. Structure of plasmid pBSI-CoV-Ub (a) and target gene expression analysis after transfection of HEK293T cells.
b — RT-PCR products using total RNA from HEK293T cells transfected with plasmid pBSI-CoV-Ub (lane 1) (electrophoresis in a 1% agarose gel). Lane 2: PCR products obtained from total RNA preparations without reverse transcription.
c — protein products identified in HEK293T cells transfected with pBSI-CoV-Ub plasmid by Western blotting using monoclonal antibody 29F2 against the marker epitope.
The recombinant RBD protein and its conjugate with PGS were produced, purified and characterized previously [27].
Obtaining a DNA–protein complex
The combined complex of DNA and the RBD–PGS conjugate was obtained by mixing the components, and their interaction scheme in the mixture is presented in Fig. 3, a. Complex formation was determined by the change in the electrophoretic mobility of plasmid DNA in an agarose gel: the encapsulated plasmid showed significantly less mobility in an electric field compared to the naked plasmid (Fig. 3, b).
Fig. 3. DNA-protein complex.
a — schematic representation of the complex: the negative phosphate groups in the plasmid DNA electrostatically bind to the positive amino groups in spermidine conjugated with polyglucin.
b — confirmation of DNA encapsulation within the RBD–PGS shell by electrophoresis in a 1% agarose gel: 1 — CCV–BSI; 2 — pBSI-COV-Ub.
c — electron micrographs of CCV-BSI particles.
Electron microscopy (Fig. 3, c) showed that the particle sizes range is from 50 to 200 nm. A number of researchers suggest that nanoparticles of this size are optimal for vaccine development because they accumulate in B-cell follicles and elicit a strong immune response [28].
Analysis of the humoral immune response
To assess the immunogenicity of the created vaccine constructs, BALB/c mice were immunized twice on days 0 and 21, and were sacrificed at the endpoint (day 35). The sera were tested for the presence of RBD-specific antibodies using ELISA, as well as for their ability to neutralize live virus. Sera from the mice immunized with pBSI-COV-Ub plasmid DNA and RBD protein conjugated to PGS, as well as sera from intact mice, were used as controls (Fig. 4, a).
Fig. 4. Humoral immune response in BALB/c mice immunized with CCV–BSI, pBSI-COV-Ub, and RBD–PGS constructs. Intact, unvaccinated mice are the negative control.
a — schematic representation of the immunogenicity study experiment: mice were immunized twice with a 3-week interval, and samples were taken from the mice for analysis 2 weeks after the second immunization.
b — titers of SARS-CoV-2 RBD-specific IgG, determined by ELISA.
c — titers of neutralizing antibodies, determined using the SARS-CoV-2 strain nCoV/Victoria/1/2020.
The data for fragments b and c are presented as geometric means of the inverse titers with a geometric standard deviation, and individual values are marked with dots. The significance of the differences between the groups was calculated using the non-parametric Mann–Whitney method (*p < 0.01, **p < 0.05, n.s. — statistically non-significant).
According to the results of the RBD-specific ELISA two weeks after the second immunization, the average titers of specific antibodies in animals immunized with the CCV–BSI vaccine, which combines protein and DNA, were 1 : 1,557,215. In the group immunized with the RBD protein conjugated to PGS, the average titer was 1 : 391,951, which was not significantly different from the first group (p = 0.2739). In the sera of mice that received only the pBSI-COV-Ub plasmid and in the sera of intact animals, antibodies specifically binding to the RBD protein were detected at background levels (Fig. 4, b).
The results of the serum neutralization analysis using the nCoV/Victoria/1/2020 strain of the SARS-CoV-2 virus on cell culture in vitro also showed no significant differences between the groups immunized with the CCV–BSI combined construct and the RBD protein conjugated to PGS: the mean neutralizing titers were 1:238 and 1 : 263, respectively (p = 0.7780). Animal sera from those who received only the pBSI-COV-Ub plasmid and sera from intact animals did not show virus-neutralizing activity (Fig. 4, c).
Analysis of the cellular immune response
To assess the ability of the created constructs to induce cellular immunity in mice, spleens were taken at the end of the experiment, homogenized, and examined using the ELISpot method. Splenocytes from mice immunized with plasmid DNA pBSI-COV-Ub and RBD protein conjugated to PGS, as well as splenocytes from intact mice, were used as controls. The response was evaluated based on the ability of splenocytes to respond with interferon-γ secretion to specific stimulation, which was carried out using a pool of peptides from the RBD protein and the BSI-COV-Ub immunogen (Fig. 5, a).
Fig. 5. Cellular immune response in BALB/c mice.
a — images of representative wells in ELISpot.
b — number of splenocytes releasing interferon-γ in response to specific stimulation with peptides from the RBD protein and the artificial BSI-COV-Ub immunogen, counted using ELISpot. Data are presented as geometric means with geometric standard deviation, individual values are marked with dots. The significance of the differences between the groups was calculated using the non-parametric Mann–Whitney method (*p < 0.01, **p < 0.05, n.s. — statistically non-significant).
ELISpot showed that the highest cellular immunity responses were achieved in the groups immunized with the DNA vaccine or the combined complex (Fig. 5, b). The average number of splenocytes responding to stimulation in both groups was 46 and 54 SFU per 106 cells, respectively (p > 0.9999 between groups and p < 0.01 compared to the other two groups). In the group receiving the RBD–PGS conjugate, a low response was recorded at the negative control level (2 SFU).
Discussion
The global spread of SARS-CoV-2 necessitated the rapid development of a vaccine. The global population vaccination campaign provided a unique opportunity to compare different strategies and platforms of the developed vaccines. Almost all existing approaches used by researchers were employed in the development, and as a result, not only classic vaccine types (inactivated, subunit) but also new-generation vaccines (mRNA, vector, DNA vaccines) have entered the world market.
It is not only the induction of a humoral immune response and neutralizing antibodies in particular that is critically important, but also the activation of a virus-specific T-cell response [29]. In this work, to create a vaccine construct, we combined two platforms: a DNA vaccine and a recombinant protein. The resulting mixture is DNA surrounded by positively charged PGS molecules conjugated to the RBD protein. The RBD was chosen as the antigen because this region of the S protein is the dominant target for the neutralizing response during COVID-19 infection.
Previously, we developed a polyepitope artificial immunogen containing CTL and Th epitopes from the SARS-CoV-2 virus proteins S, N, M and E (Fig. 1) and obtained the pBSI-COV-Ub DNA vaccine encoding this immunogen (Fig. 2) [26]. The fragments selected for inclusion in the final construct contain epitopes restricted by a wide range of human and mouse MHC class I and II molecules, which have been confirmed to be immunodominant in studies of SARS-CoV-2-specific cellular immunity and are conserved across different virus variants [30–32]. After the fragments were joined into a single sequence, ubiquitin was added to its N-terminus. The attachment of ubiquitin to a protein facilitates its targeting to the proteasome, leading to efficient processing and the release of peptide epitopes that are presented by MHC class I on the surface of an antigen-presenting cell and contribute to the activation of cytotoxic T lymphocytes [33], and through cross-presentation, they also activate T-helper cells.
The PGS conjugate used in this study was previously used to deliver candidate DNA vaccines against HIV-1, Ebola and COVID-19 [27, 34, 35]. In studies of these constructs, their safety and effective enhancement of the immunogenicity of DNA vaccines through the use of PGS have been proven. PGS protects DNA from the action of nucleases, promotes prolonged release of the immunogen, and has an immunomodulatory effect [15, 16]. PGS components are biodegradable and allow for the lyophilization of the vaccine preparation. The RBD–PGS conjugate is positively charged and, when DNA is added, begins to interact with its negatively charged phosphate groups, forming a complex (Fig. 3, a). Complex formation was confirmed by a decrease in DNA mobility in an agarose gel (Fig. 3, b).
The study of the humoral immune response in BALB/c mice after two intramuscular injections of the CCV–BSI complex showed that the combined construct, along with the RBD protein conjugated to PGS, induces the production of high titers of specific antibodies with neutralizing activity against the nCoV/Victoria/1/2020 strain of the SARS-CoV-2 virus in cell culture in vitro (Fig. 4, a, b). Recombinant RBD protein without an adjuvant has less pronounced immunogenicity compared to the conjugate [36]. The higher RBD-specific response in the group immunized with CCV–BSI compared to the group immunized with RBD–PGS (mean titer 1 : 1.5 million versus 1 : 0.3 million) may indicate the contribution of antigen multimerization resulting from DNA interaction with multiple molecules of the RBD–PGS conjugate, as well as the stimulation of a specific T-helper response by the DNA vaccine. A similar synergistic effect on the induction of a humoral immune response was noted by most researchers who studied the co-administration of DNA and subunit vaccines [18–20, 34].
When assessing the cellular response by determining the number of splenocytes producing IFN-γ in response to stimulation with viral peptides using the ELISpot method, the highest level of specific cellular immunity was found in the groups immunized with preparations containing the pBSI-COV-Ub DNA construct. This indicates that the DNA vaccine, both in its free state and when encapsulated, is capable of inducing a specific T-cell immune response against a wide range of viral strains, whereas immunization with the RBD protein conjugated to PGS does not induce a T-cell response (Fig. 5). In many studies, the use of DNA vaccines in conjunction with subunit vaccines leads to the induction of a T-cell response. Moreover, its development is only slightly dependent on the administration regimen, meaning that a DNA vaccine, whether used as a prime immunization or co-administered with protein, stimulates T-cells to a level comparable to that achieved by administering the DNA vaccine alone [20, 21, 34].
Conclusion
The unique combination of DNA and protein within a single vaccine construct allows for overcoming the limitations of each of these vaccine types and leads to the induction of both arms of immunity. The protein component can be replaced according to the current viral strain, and a universal T-cell immunogen can provide a response to a wide range of circulating variants. This platform can be further used to develop vaccines against various highly variable viruses.
About the authors
Mariya B. Borgoyakova
State Research Center of Virology and Biotechnology «Vector»
Author for correspondence.
Email: borgoyakova_mb@vector.nsc.ru
ORCID iD: 0000-0002-0768-1561
Cand. Sci. (Biol.), senior researcher, Department of bioengineering
Russian Federation, Koltsovo, Novosibirsk regionAndrey P. Rudometov
State Research Center of Virology and Biotechnology «Vector»
Email: rudometov_ap@vector.nsc.ru
ORCID iD: 0000-0003-2808-4309
Cand. Sci. (Biol.), leading researcher, Acting Head, Department of bioengineering
Russian Federation, Koltsovo, Novosibirsk regionEkaterina V. Starostina
State Research Center of Virology and Biotechnology «Vector»
Email: starostina_ev@vector.nsc.ru
ORCID iD: 0000-0002-1733-9524
Cand. Sci. (Biol.), senior researcher, Department of bioengineering
Russian Federation, Koltsovo, Novosibirsk regionVladimir A. Yakovlev
State Research Center of Virology and Biotechnology «Vector»
Email: yakovlev_va@vector.nsc.ru
ORCID iD: 0009-0009-4563-2099
junior researcher, Department of bioengineering
Russian Federation, Koltsovo, Novosibirsk regionElena V. Tigeeva
State Research Center of Virology and Biotechnology «Vector»
Email: tigeeva_ev@vector.nsc.ru
ORCID iD: 0009-0001-5203-2130
junior researcher, Department of bioengineering
Russian Federation, Koltsovo, Novosibirsk regionAnna V. Zaykovskaya
State Research Center of Virology and Biotechnology «Vector»
Email: zaykovskaya_av@vector.nsc.ru
ORCID iD: 0000-0002-0450-5212
Cand. Sci. (Biol.), senior researcher, Department “Collection of microorganisms”
Russian Federation, Koltsovo, Novosibirsk regionEkaterina A. Volosnikova
State Research Center of Virology and Biotechnology «Vector»
Email: volosnikova_av@vector.nsc.ru
ORCID iD: 0000-0001-5028-5647
Cand. Sci. (Biol.), leading researcher, Head, Laboratory of the production and analysis of biosubstances
Russian Federation, Koltsovo, Novosibirsk regionIuliia A. Merkuleva
State Research Center of Virology and Biotechnology «Vector»
Email: merkuleva_iua@vector.nsc.ru
ORCID iD: 0000-0002-6974-0686
Cand. Sci. (Biol.), senior researcher, Department of biophysics and environmental research
Russian Federation, Koltsovo, Novosibirsk regionAnastasya A. Isaeva
State Research Center of Virology and Biotechnology «Vector»
Email: isaeva_aa@vector.nsc.ru
ORCID iD: 0000-0002-4085-2887
Cand. Sci. (Chem.), researcher, Department of biophysics and environmental research
Russian Federation, Koltsovo, Novosibirsk regionValentina S. Nesmeyanova
State Research Center of Virology and Biotechnology «Vector»
Email: nesmeyanova_vs@vector.nsc.ru
ORCID iD: 0000-0003-1091-3586
junior researcher, Department of biophysics and environmental research
Russian Federation, Koltsovo, Novosibirsk regionDaniil V. Shanshin
State Research Center of Virology and Biotechnology «Vector»
Email: shanshin_dv@vector.nsc.ru
ORCID iD: 0000-0001-9985-1582
Cand. Sci. (Biol.), junior researcher, Department of biophysics and environmental research
Russian Federation, Koltsovo, Novosibirsk regionBoris N. Zaitsev
State Research Center of Virology and Biotechnology «Vector»
Email: zaitsev@vector.nsc.ru
ORCID iD: 0000-0001-6359-465X
Cand. Sci. (Phys.-Math.), senior researcher, Department of microscopic research
Russian Federation, Koltsovo, Novosibirsk regionDenis N. Kisakov
State Research Center of Virology and Biotechnology «Vector»
Email: kisakov_dn@vector.nsc.ru
ORCID iD: 0000-0003-4889-6865
junior researcher, Department of bioengineering
Russian Federation, Koltsovo, Novosibirsk regionLyubov A. Kisakova
State Research Center of Virology and Biotechnology «Vector»
Email: orlova_la@vector.nsc.ru
ORCID iD: 0000-0001-7214-1855
junior researcher, Department of bioengineering
Russian Federation, Koltsovo, Novosibirsk regionDmitry N. Shcherbakov
State Research Center of Virology and Biotechnology «Vector»
Email: scherbakov_dn@vector.nsc.ru
ORCID iD: 0000-0001-8023-4453
Cand. Sci. (Biol.), leading researcher, Department of biophysics and environmental research
Russian Federation, Koltsovo, Novosibirsk regionLarisa I. Karpenko
State Research Center of Virology and Biotechnology «Vector»
Email: lkarpenko1@ya.ru
ORCID iD: 0000-0003-4365-8809
Dr. Sci. (Biol.), leading researcher, Head, Laboratory of recombinant vaccines, Department of bioengineering
Russian Federation, Koltsovo, Novosibirsk regionAlexander A. Ilyichev
State Research Center of Virology and Biotechnology «Vector»
Email: ilyichev@vector.nsc.ru
ORCID iD: 0000-0001-5356-0843
Dr. Sci. (Biol.), chief researcher, Department of bioengineering
Russian Federation, Koltsovo, Novosibirsk regionReferences
- Pawlowski C., Lenehan P., Puranik A., et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med. 2021;2(8):979–92.e8. DOI: https://doi.org/10.1016/j.medj.2021.06.007
- Khobragade A., Bhate S., Ramaiah V., et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet. 2022;399(10332):1313–21. DOI: https://doi.org/10.1016/S0140-6736(22)00151-9
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–81. DOI: https://doi.org/10.1016/s0140-6736(21)00234-8
- Ramasamy M.N., Minassian A.M., Ewer K.J., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–93. DOI: https://doi.org/10.1016/S0140-6736(20)32466-1
- Ng O.W., Chia A., Tan A.T., et al. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine. 2016;34(17):2008–14. DOI: https://doi.org/10.1016/j.vaccine.2016.02.063
- Alhabbab R.Y., Algaissi A., Mahmoud A.B., et al. Middle East respiratory syndrome coronavirus infection elicits long-lasting specific antibody, T and B cell immune responses in recovered individuals. Clin. Infect. Dis. 2023;76(3):e308–18. DOI: https://doi.org/10.1093/cid/ciac456
- Zhang Y., Ndzouboukou J.B., Lin X., et al. SARS-CoV-2 evolves to reduce but not abolish neutralizing action. J. Med. Virol. 2023;95(1):e28207. DOI: https://doi.org/10.1002/jmv.28207
- Le Bert N., Tan A.T., Kunasegaran K., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–62. DOI: https://doi.org/10.1038/s41586-020-2550-z
- Arya R., Tripathi P., Nayak K., et al. Insights into the evolution of mutations in SARS-CoV-2 non-spike proteins. Microb. Pathog. 2023;185:106460. DOI: https://doi.org/10.1016/j.micpath.2023.106460
- Srinivasan S., Cui H., Gao Z., et al. Structural genomics of SARS-CoV-2 indicates evolutionary conserved functional regions of viral proteins. Viruses. 2020;12(4):360. DOI: https://doi.org/10.3390/v12040360
- Choi S.J., Kim D.U., Noh J.Y., et al. T cell epitopes in SARS-CoV-2 proteins are substantially conserved in the Omicron variant. Cell. Mol. Immunol. 2022;19(3):447–8. DOI: https://doi.org/10.1038/s41423-022-00838-5
- Cromer D., Steain M., Reynaldi A., et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3(1):e52–61. DOI: https://doi.org/10.1016/S2666-5247(21)00267-6
- Chavda V.P., Ghali E.N.H.K., Balar P.C., et al. Protein subunit vaccines: Promising frontiers against COVID-19. J. Control. Release. 2024;366:761–82. DOI: https://doi.org/10.1016/j.jconrel.2024.01.017
- Mekonnen D., Mengist H.M., Jin T. SARS-CoV-2 subunit vaccine adjuvants and their signaling pathways. Expert. Rev. Vaccines. 2022;21(1):69-81. DOI: https://doi.org/10.1080/14760584.2021.1991794
- Lin H., Han R., Wu W. Glucans and applications in drug delivery. Carbohydr. Polym. 2024;332:121904. DOI: https://doi.org/10.1016/j.carbpol.2024.121904
- Huang S., Huang G. The dextrans as vehicles for gene and drug delivery. Future Med. Chem. 2019;11(13):1659–67. DOI: https://doi.org/10.4155/fmc-2018-0586
- An Y., Zhao G., Duan H., et al. Robust and protective immune responses induced by heterologous prime-boost vaccination with DNA-protein dimeric RBD vaccines for COVID-19. J. Med. Virol. 2023;95(7):e28948. DOI: https://doi.org/10.1002/jmv.28948
- Jalah R., Kulkarni V., Patel V., et al. DNA and protein co-immunization improves the magnitude and longevity of humoral immune responses in macaques. PLoS One. 2014;9(3):e91550. DOI: https://doi.org/10.1371/journal.pone.0091550
- Felber B.K., Lu Z., Hu X., et al. Co-immunization of DNA and protein in the same anatomical sites induces superior protective immune responses against SHIV challenge. Cell Rep. 2020;31(6):107624. DOI: https://doi.org/10.1016/j.celrep.2020.107624
- Li Y., Bi Y., Xiao H., et al. A novel DNA and protein combination COVID-19 vaccine formulation provides full protection against SARS-CoV-2 in rhesus macaques. Emerg. Microbes Infect. 2021;10(1):342–55. DOI: https://doi.org/10.1080/22221751.2021.1887767
- Liao H.C., Huang M.S., Chiu F.F., et al. Co-delivery of a trimeric spike DNA and protein vaccine with aluminum hydroxide enhanced Th1-dominant humoral and cellular immunity against SARS-CoV-2. J. Med. Virol. 2023;95(8):e29040. DOI: https://doi.org/10.1002/jmv.29040
- He Q., Mao Q., An C., et al. Heterologous prime-boost: breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg. Microbes Infect. 2021;10(1):629–37. DOI: https://doi.org/10.1080/22221751.2021.1902245
- Peng D., Zhao T., Hong W., et al. Heterologous vaccination with subunit protein vaccine induces a superior neutralizing capacity against BA.4/5-included SARS-CoV-2 variants than homologous vaccination of mRNA vaccine. MedComm (2020). 2023;4(2):e238. DOI: https://doi.org/10.1002/mco2.238
- Kim E., Khan M.S., Ferrari A., et al. SARS-CoV-2 S1 subunit booster vaccination elicits robust humoral immune responses in aged mice. Microbiol. Spectr. 2023;11(3):e0436322. DOI: https://doi.org/10.1128/spectrum.04363-22
- Park H.J., Bang Y.J., Kwon S.P., et al. Analyzing immune responses to varied mRNA and protein vaccine sequences. NPJ Vaccines. 2023;8(1):84. DOI: https://doi.org/10.1038/s41541-023-00684-0
- Borgoyakova M.B., Karpenko L.I., Rudometov A.P., et al. Artificial COVID-19 T-cell immunogen. Bull. Exp. Biol. Med. 2023;175(6):804–9. DOI: https://doi.org/10.1007/s10517-023-05951-7
- Borgoyakova M.B., Karpenko L.I., Rudometov A.P., et al. Self-assembled particles combining SARS-CoV-2 RBD protein and RBD DNA vaccine induce synergistic enhancement of the humoral response in mice. Int. J. Mol. Sci. 2022;23(4):2188. DOI: https://doi.org/10.3390/ijms23042188
- Singh A. Eliciting B cell immunity against infectious diseases using nanovaccines. Nat. Nanotechnol. 2021;16(1):16–24. DOI: https://doi.org/10.1038/s41565-020-00790-3
- Toor S.M., Saleh R., Sasidharan Nair V., et al. T-cell responses and therapies against SARS-CoV-2 infection. Immunology. 2021;162(1):30–43. DOI: https://doi.org/10.1111/imm.13262
- Safavi A., Kefayat A., Mahdevar E., et al. Exploring the out of sight antigens of SARS-CoV-2 to design a candidate multi-epitope vaccine by utilizing immunoinformatics approaches. Vaccine. 2020;38(48):7612–28. DOI: https://doi.org/10.1016/j.vaccine.2020.10.016
- Mahajan S., Kode V., Bhojak K., et al. Immunodominant T-cell epitopes from the SARS-CoV-2 spike antigen reveal robust pre-existing T-cell immunity in unexposed individuals. Sci. Rep. 2021;11(1):13164. DOI: https://doi.org/10.1038/s41598-021-92521-4
- Bhattacharya M., Sharma A.R., Ghosh P., et al. A next-generation vaccine candidate using alternative epitopes to protect against wuhan and all significant mutant variants of SARS-CoV-2: An immunoinformatics approach. Aging Dis. 2021;12(8):2173–95. DOI: https://doi.org/10.14336/AD.2021.0518
- de Oliveira L.M., Morale M.G., Chaves A.A., et al. Expression, polyubiquitination, and therapeutic potential of recombinant E6E7 from HPV16 antigens fused to ubiquitin. Mol. Biotechnol. 2017;59(1):46–56. DOI: https://doi.org/10.1007/s12033-016-9990-6
- Bazhan S.I., Karpenko L.I., Lebedev L.R., et al. A synergistic effect of a combined bivalent DNA-protein anti-HIV-1 vaccine containing multiple T- and B-cell epitopes of HIV-1 proteins. Mol. Immunol. 2008;45(3):661–9. DOI: https://doi.org/10.1016/j.molimm.2007.07.016
- Karpenko L.I., Apartsin E.K., Dudko S.G., et al. Cationic polymers for the delivery of the Ebola DNA vaccine encoding artificial T-cell immunogen. Vaccines (Basel). 2020;8(4):718. DOI: https://doi.org/10.3390/vaccines8040718
- Volosnikova E.A., Merkuleva I.A., Esina T.I., et al. SARS-CoV-2 RBD conjugated to polyglucin, spermidine, and dsRNA elicits a strong immune response in mice. Vaccines (Basel). 2023;11(4):808. DOI: https://doi.org/10.3390/vaccines11040808
Supplementary files