Improvement of the bacteriological method for isolation of Listeria monocytogenes
- Authors: Khramov M.V.1, Domotenko L.V.1, Polosenko O.V.1, Mitsevich I.P.1
-
Affiliations:
- State Research Center for Applied Microbiology and Biotechnology
- Issue: Vol 102, No 3 (2025)
- Pages: 362-369
- Section: SCIENCE AND PRACTICE
- URL: https://microbiol.crie.ru/jour/article/view/18795
- DOI: https://doi.org/10.36233/0372-9311-673
- EDN: https://elibrary.ru/QONYBM
- ID: 18795
Cite item
Abstract
Introduction. Currently, listeriosis is regarded as one of the dangerous infections that cannot be prevented by vaccination and is characterized by the severity of the clinical process and high mortality. Improving laboratory diagnostic methods especially in listeriosis meningitis to identify the pathogen in the shortest possible time remains an urgent problem.
The aim of the study was to investigate the behavior of collection and clinical strains of various listeria species on GBM-agar — a nutrient medium for isolating pathogens of purulent bacterial meningitis — in order to improve the bacteriological method for isolating and identifying Listeria monocytogenes.
Materials and methods. In the current study, 1125 samples of clinical material and food produces were used. Of these, 95 were isolated and 5 were reference strains of Listeria spp. The following culture media to isolate listeria were used: Agar Listeria by Ottaviani and Agosti (ALOA); Listeria enrichment broth (LEB), Listeria isolation agar (LIA), GBM-agar.
Results. Of the 1125 samples involved the following strains were isolated using LEB, LIA and ALOA media: L. monocytogenes — 89, L. welshimeri — 2, L. innocua — 3, L. seeligeri — 1. All isolates and reference strains were subcultured by using conventional selective media (LIA, ALOA) and additionally GBM-agar modified by adding a selective additive to isolate L. monocytogenes, and yolk emulsion. Colonies grown on the modified GBM-agar and belonging to the Listeria genus were larger and had distinctive morphological traits making them differ from colonies obtained by means of conventional listeriosis media. This allowed for the primary differentiation of L. monocytogenes from non-pathogenic listeria species and some other pathogens of purulent bacterial meningitis.
Conclusion. It is shown that the algorithm of the culture method can use a new nutrient medium (modified GBM agar) possessing improved growth properties for L. monocytogenes, the introduction of which will serve as an additional effective means for differentiating listeria during research in sanitary and clinical microbiology.
Full Text
Introduction
Listeriosis is not only a medical and social but also an economic problem. Despite the fact that in recent years the incidence of listeriosis has been kept at the level of sporadic cases, listeriosis is considered as one of the dangerous vaccine-uncontrollable infections, characterized by the severity of the clinical process and high mortality (up to 20%) [1, 2].
The incidence of listeriosis is caused by contamination and active multiplication of listeria in foodstuffs, increased susceptibility to listeria in risk groups against the background of cellular immunity disorders [1, 3]. In recent years, data have been obtained on the circulation of Listeria in plant, soil, and water substrates, their high adaptive capabilities in a wide temperature range, humidity, and pH of the environment. There is data on the contamination of water sources with the causative agent of listeriosis near livestock enterprises [4].
In healthy people, infection is usually asymptomatic or in the form of gastroenteritis [1, 2, 5]. In the immunocompromised elderly, pregnant women and newborns, or in patients receiving immunosuppressive therapy, listeriosis may manifest as bacteremia or sepsis, central nervous system involvement, etc., leading to serious or potentially fatal illnesses, including sepsis or meningitis [2, 6, 7–12].
The clinical manifestations of these forms of the disease, including listeriosis meningitis, are nonspecific, mainly fever, headache, vomiting, and disorders of consciousness, which is similar to other types of purulent meningitis [2, 3, 6, 7].
Listeriosis is caused by gram-positive, facultatively anaerobic, enteroinvasive bacteria of the genus Listeria. The main causative agent of the disease in humans is L. monocytogenes, which is capable of causing listeriosis in animals [19]. The main causative agent of listeriosis in animals is L. ivanovii, which in rare cases can lead to the disease in humans [20]. There are isolated cases of listeriosis caused by L. innocua and L. seeligeri [21, 22].
In the laboratory diagnosis of listeriosis and sanitary-bacteriologic investigations, the leading role is played by the bacteriologic method using nutrient media [13].
The isolation of Listeria from non-sterile clinical material and foodstuffs is only possible using selective nutrient media or enrichment procedures. Listeria enrichment broth (LEB), Fraser broth, UVM broth are used; as selective differential diagnostic media, Listeria isolation agar (LIA), Listeria agar by Ottaviani and Agosti (ALOA), Brilliance Listeria agar, Oxford agar, PALCAM agar, etc. are used as selective enrichment media.
Among the various media, LIA and PALCAM agar media do not provide species differentiation of Listeria. On such media, the isolation of Listeria is based on their ability to hydrolyze esculin to form esculentine, which in the presence of iron ions forms a black complex; as a result, Listeria of all species form grayish colonies with a black zone around them.
Chromogenic media (ALOA and Brilliance Listeria agar) with special selective and chromogenic additives allow to isolate Listeria of different species in the form of blue-green colonies and differentiate L. monocytogenes and certain strains of L. ivanovii from other Listeria species by formation of a characteristic halo around the colonies due to the ability to produce phospholipase C.
The selectivity of the media with respect to associated microflora is ensured by the inclusion of lithium chloride, acriflavine, cycloheximide, nalidixinic acid, polymyxin and other antibiotics [14].
For the isolation of Listeria from normally sterile biological substrates (blood, cerebrospinal fluid, etc.), which is typical in the bacteriological study of meningitis, blood and chocolate agar can be used, as well as GBM-agar, a nutrient medium for the isolation and cultivation of agents of purulent bacterial meningitis without selective additives [15]. Listeria on these media grows as round convex translucent non-pigmented colonies with a smooth surface after 24–48 h of cultivation.
The rich GBM-agar base containing casein hydrolysate, peptone, yeast extract, growth stimulator of hemophilic microorganisms, and glucose is able to support the growth of Listeria of various species.
Since the main purpose of GBM-agar is related to the cultivation and isolation of the three main pathogens of bacterial meningitis: Haemophilus influenzae type B, Streptococcus pneumoniae, Neisseria meningitidis, it contains selective additives only for them. Selective additives for Listeria have a different composition of antibiotics and contain the dye acriflavine, which has antiseptic properties and can give L. monocytogenes colonies a green color.
Preliminary studies of clinical material from meningitis patients have shown that the use of GBM-agar with a selective additive for Listeria allows isolation of the listeriosis pathogen in a shorter time than conventional listeria media.
To introduce the nutrient medium into the scheme of laboratory diagnostics of listeriosis, studies using a wide range of strains belonging to L. monocytogenes and other Listeria species are necessary.
The aim of the study is to investigate the behavior of reference and clinical strains of different Listeria species on GBM agar to improve the bacteriological method for the isolation and identification of L. monocytogenes in clinical and sanitary microbiology.
Materials and methods
The following materials were used: clinical material (CSF, sectional material, blood, cerebrospinal fluid, cervical canal secretion) and food raw materials and food products received by the testing laboratory center of the State Research Center for Applied Microbiology and Biotechnology (SRC AMB) (a total of 1125 samples); reference strains of microorganisms obtained from the State Collection of Pathogenic Microorganisms and Cell Cultures “SCPM-Obolensk”: L. monocytogenes 766, L. monocytogenes NCTC11994, L. ivanovii ATCC19119, L. innocua NCTC 11288, L. seeligeri ATCC 35967, Escherichia coli ATCC 25922, Proteus vulgaris HX 19 222, Staphylococcus aureus Wood-46, S. pneumoniae ATCC 6305, N. meningitidis ATCC 13102, H. influenzae ATCC 49247. Sample preparation and research were carried out using algorithms and methods recommended by SanPiN 3.3686-211, MG 4.2.1122-022, GOST 32031-20223. The study was conducted with the informed consent of the patients or their legal representatives.The research protocol was approved by the Ethics Committee of the SRC AMB (protocol No. ВП-2025/3, May 17, 2025).
Nutrient media produced by SRC AMB were used for Listeria accumulation: nutrient broth for cultivation and isolation of Listeria with selective additive (LEB medium) FSR 2010/09161; as differential-diagnostic — nutrient agar for cultivation and isolation of Listeria with selective additive (ALOA medium, SRC AMB, RU No. FSR 2010/09162); Listeria Ottaviani Agosti HiCynth Agar (ALOA, HiMedia, RU No. FSZ 2009/03705) with Enrichment Supplement (FD214), Selective Supplement (FD 212A); nutrient medium for isolation of purulent bacterial meningitis pathogens (GBM-agar, State Research Center AMB, RU No. RZN 2016/4872) with selective additive (polymyxin B sulfate — 0.01 g/L; nalidixic acid — 0.025 g/L; acriflavine — 0.01 g/L) (hereinafter — modified GBM-agar).
To determine lecithinase activity we used nutrient medium for quantitative determination of microbial contamination (medium No. 1 GRM, SRC AMB, RU No. FSR 2011/11415) and modified GBM-agar with ex tempore addition of 5% yolk emulsion, and the same media with the addition of activated carbon at a concentration of 0.5%.
Identification of isolates was performed on a MALDI Biotyper automatic system (Bruker Daltonik).
Results
The studies were conducted in 2 stages. The first stage was devoted to the isolation of listeriosis pathogen from clinical material, food raw materials and food products. The samples prepared for the study were seeded on enrichment medium — LEB. After 24 and 48 h of incubation at 37 ± 1°C from LEB medium, culture fluid was seeded on special nutrient media for the isolation of Listeria (LIA, ALOA). After incubation, characteristic colonies, presumably belonging to Listeria, were selected and subcultured on GRM medium No. 1 for further identification using a MALDI Biotyper automatic system. During the study, 89 isolates of L. monocytogenes, 2 isolates of L. welshimeri, 3 isolates of L. innocua, and 1 isolate of L. seeligeri were obtained.
At the second stage, we studied the behavior of all isolates, including those from meningitis patients, and test strains of Listeria on GBM agar in comparison with their behavior on classical nutrient media (LIA, ALOA) under conditions of equivocation.
On LIA medium, isolated cultures and reference strains of L. monocytogenes formed small, grayish colonies up to 1.0 mm in diameter after 24 h; on the 2nd day their size increased to 1.2–1.4 mm. On ALOA medium, L. monocytogenes grew as blue-green colonies surrounded by an opaque halo 0.6-0.8 mm in diameter on the 1st day of incubation and up to 2.0 mm in diameter on the 2nd day. On modified GBM-agar already after 18 h of L. monocytogenes incubation, the diameter of colonies reached 1.5–2.5 mm (Fig. 1). The colonies acquired a greenish color, in contrast to the colonies obtained on the classical LIA medium.
Fig. 1. Growth of isolated L. monocytogenes cultures on LIA (a), ALOA (b) and modified GBM agar (c).
The growth of other Listeria species on GBM agar differed from that of L. monocytogenes. When studying their growth character, museum test strains: L. ivanovii ATCC19119, L. innocua NCTC 11288, L. seeligeri ATCC 35967 and isolated cultures of Listeria species: L. welshimeri, L. innocua, L. seeligeri were sown on modified GBM-agar and comparison media LIA and ALOA. After 18–24 h of incubation on the modified GBM-agar, the growth of these Listeria species was detected in the form of smooth milk-colored colonies with a diameter of 1.6–2.0 mm, in contrast to LIA and ALOA media, on which the growth of small colonies with a diameter of 0.3–0.8 mm was observed.
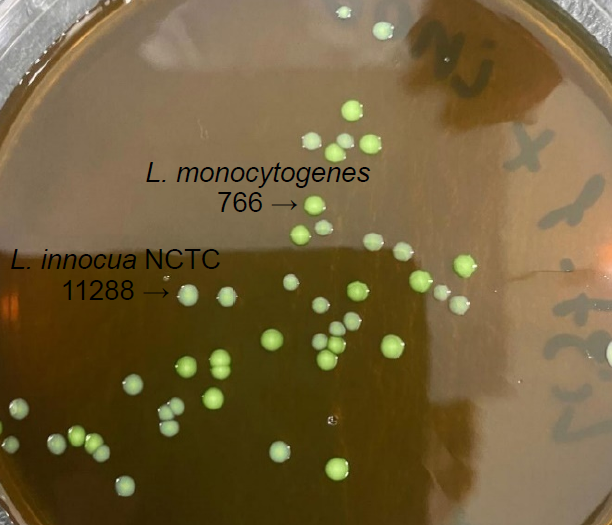
When seeding from a mixture of reference strain L. monocytogenes 766 and L. innocua NCTC 11288 on modified GBM agar and subsequent incubation at 37 ± 1°C for 18 h, the medium was found to have differentiated properties: the growth of reference strain L. monocytogenes 766 was observed as greenish colored colonies with a diameter of 1.8–2.2 mm; L. innocua NCTC 11288 — as milky colored colonies with a diameter of 1.5–1.8 mm (Fig. 2).
Fig. 2. Growth of a mixture of reference strains of L. monocytogenes 766 and L. innocua NCTC 11288 on modified GBM agar.
Furthermore, the addition of yolk emulsion to the modified GBM-agar medium provided a clear differentiation of the reference strain L. ivanovii ATCC19119, which has lecithinase activity, from the reference strain L. innocua NCTC 11288, which does not have lecithinase (Fig. 3).
Fig. 3. Growth of a mixture of reference strains of L. ivanovii ATCC19119 and L. innocua NCTC 11288 on modified GBM agar with yolk emulsion addition.
Studies of clinical material, food raw materials and food products for the presence of Listeria include a stage to determine the lecithinase activity, the presence of which is an indicator of pathogenicity, to confirm the affiliation of isolated bacteria to the species L. monocytogenes. The originality of the bacteriological method is based on the comparison of lecithinase activity of the culture in the presence or absence of activated carbon. In this study, an attempt was made to determine the lecithinase activity of Listeria in accordance with the method regulated by the regulatory documents MG 4.2.1122-02 and GOST 32031-2022 using modified GBM-agar with the addition of yolk emulsion with and without activated carbon. The isolated cultures of L. monocytogenes and reference strains L. monocytogenes 766 and L. ivanovii ATCC19119 were seeded on medium from dilutions 10–6. Seeds were incubated at 37 ± 1°C for 24 h and viewed under transmitted light. The traditionally used for this purpose medium No. 1 GRM medium was used as a control medium of comparison.
On medium No. 1 GRM with the addition of yolk emulsion in the presence of charcoal after 24 h, a dense zone of turbidity around the colonies of isolated strains of L. monocytogenes, reference strain L. monocytogenes 766 and L. ivanovii ATCC19119 with a width of more than 2.0 mm, characteristic of the lecithinase activity of cultures, was observed. On the same medium without activated charcoal, no zones of opacity were observed around L. monocytogenes colonies, in contrast to L. ivanovii ATCC19119 colonies.
On modified GBM-agar with yolk emulsion without charcoal, a clearly distinguishable lecithinase activity was also observed only around L. ivanovii ATCC19119 colonies (Fig. 4).
Fig. 4. Growth of reference strain L. ivanovii ATCC19119 on modified GBM-agar medium with yolk emulsion addition.
Addition of activated carbon to the modified GBM-agar in the same concentrations resulted in more intense blackening of the medium, which did not allow us to consider and correctly interpret the results of lecithinase activity detection.
When determining the selective properties of modified GBM-agar, growth suppression of reference strains of E. coli ATCC 25922, P. vulgaris HX 19 222, S. aureus Wood-46, S. pneumoniae ATCC 6305, N. meningitidis ATCC 13102, H. influenzae ATCC 49247 from dilutions 10–4 was detected.
Discussion
Meningitis caused by L. monocytogenes is a serious and life-threatening disease. In recent years, L. monocytogenes is the third most common cause of bacterial meningitis in the elderly, after S. pneumoniae and N. meningitidis, because of a decrease in the incidence of meningitis caused by H. influenzae type B due to vaccination [18]. The disease is more common in elderly people, clinical manifestations in this type of meningitis are not specific, so for the correct diagnosis it is important to take into account the results of bacteriological examination aimed at isolation and identification of the pathogen.
The GBM-agar nutrient medium designed for isolation of the main pathogens of purulent bacterial meningitis has proven itself in the study of clinical material for the presence of S. pneumoniae, N. meningitidis, and H. influenzae type B [15]. Therefore, it was of interest to study the possibility of using this nutrient medium for the isolation of Listeria, but using a special selective additive for Listeria. The antibiotics polymyxin B, nalidixic acid, ceftazidime, and acriflavine included in the medium inhibit the growth of the main pathogens of GBM, as well as a number of other microorganisms (e.g., E. coli, P. vulgaris, S. aureus). The medium with the selective additive is called modified GBM agar.
In our study, we examined the behavior of different Listeria species on modified GBM agar: 89 isolates of L. monocytogenes, 2 isolates of L. welshimeri, 3 isolates of L. innocua, 1 isolate of L. seeligeri, and 5 reference strains of Listeria spp. On modified GBM-agar, L. monocytogenes colonies acquired a greenish color and after 18 h of incubation significantly exceeded the colonies on the classical LIA medium and even ALOA. At the same time, non-pathogenic Listeria formed small colonies of milky/cream color. This ability of modified GBM-agar will accelerate the isolation and differentiation of the main listeriosis pathogen — L. monocytogenes — from other Listeria species by morphological features of colonies (color and size).
Addition of yolk emulsion to modified GBM-agar provided clear differentiation of the test strain L. ivanovii ATCC19119, possessing lecithinase activity, from the reference strain L. innocua NCTC 11288, lacking lecithinase. However, the addition of activated charcoal, which activates the manifestation of lecithinase activity in L. monocytogenes on GRM medium No. 1, no zones of turbidity around L. monocytogenes colonies were observed. Perhaps, this situation is explained by more intensive blackening of GBM-agar, which is already colored dark brown due to the growth stimulant of hemophilic microorganisms included in its composition. And on such a dark nutrient medium it is difficult to consider and correctly interpret the results of lecithinase activity detection.
I.S. Tartakovsky and coauthors noted that identification of L. monocytogenes by lecithinase activity detection is often difficult due to morphological and biochemical features of the listeriosis pathogen [13]. In the studies of domestic and foreign authors it is mentioned that when cultured on nutrient media containing yolk emulsion, the lecithinase activity of Listeria is detected extremely weakly or is not observed at all. The addition of activated carbon can result in the sorption of the product secreted by Listeria that inhibits the production of lecithinase enzyme [13, 16, 17].
Conclusion
The possibility of using a new nutrient medium (modified GBM agar) with improved growth properties against L. monocytogenes in the algorithm of the culture method is shown. Its implementation will serve as an additional effective means for differentiating Listeria during research in sanitary and clinical microbiology.
1 SanPiN 3.3686-21 Sanitary and epidemiologic requirements for the prevention of infectious diseases” (enacted on 01.09.2021).
2 MG 4.2.1122-02 Organization of control and methods of detection of Listeria monocytogenes bacteria in food products: methodological guidelines. Moscow; 2002.
3 GOST 32031-2022 Food products. Methods of detection of bacteria Listeria monocytogenes and other Listeria species (Listeria spp.).
About the authors
Mikhail V. Khramov
State Research Center for Applied Microbiology and Biotechnology
Email: khramov@obolensk.org
ORCID iD: 0000-0002-4553-3826
Cand. Sci. (Med.), Deputy director for quality and development
Russian Federation, ObolenskLyubov V. Domotenko
State Research Center for Applied Microbiology and Biotechnology
Email: domotenko@obolensk.org
ORCID iD: 0000-0002-4785-6418
Cand. Sci. (Chem.), leading researcher, Nutrient medium development laboratory
Russian Federation, ObolenskOlga V. Polosenko
State Research Center for Applied Microbiology and Biotechnology
Author for correspondence.
Email: polosenko.olga@yandex.ru
ORCID iD: 0000-0001-5961-9041
Cand. Sci. (Biol.), leading researcher, Microbiology research sector
Russian Federation, ObolenskIrina P. Mitsevich
State Research Center for Applied Microbiology and Biotechnology
Email: mitsevichev@gmail.com
ORCID iD: 0000-0003-2324-502X
senior researcher, Аntimicrobial drugs laboratory
Russian Federation, ObolenskReferences
- Тюкавкина С.Ю., Котиева И.М., Додохова М.А. и др. Патогенез и клинические формы листериоза человека. Южно-Российский журнал терапевтической практики. 2024;5(1):99–111. Tyukavkina S.Yu., Kotieva I.M., Dodokhova M.A., et al. Pathogenesis and clinical forms of human listeriosis. South-Russian Journal of Therapeutic Practice. 2024;5(1):99–111. DOI: https://doi.org/10.21886/2712-8156-2024-5-1-99-111 EDN: https://elibrary.ru/kztkts
- Тагирова З.Г., Понежева Ж.Б., Макашова В.В. и др. Менингоэнцефалит листериозной этиологии. Случай из практики. Лечащий врач. 2023;11(26):21–5. Tagirova Z.G., Ponezheva Zh.B., Makashova V.V., et al. Meningoencephalitis of listeriosis etiology. A case from practice. Lechaschi Vrach Journal. 2023;11(26):21–5. DOI: https://doi.org/10.51793/OS.2023.26.11.003 EDN: https://elibrary.ru/jfslpg
- Нагибина М.В., Бессараб Т.П., Венгеров Ю.Я. и др. Листериозный менингоэнцефалит как оппортунистическое заболевание при ВИЧ-инфекции. Журнал инфектологии. 2023; 15(1):68–77. Nagibina M.V., Bessarab T.P., Vengerov Yu.Ya., et al. Listeriosis meningoencephalitis as an opportunistic disease in HIV infection. Journal Infectology. 2023;15(1):68–77. DOI: https://doi.org/10.22625/2072-6732-2023-15-1-68-77 EDN: https://elibrary.ru/ifevbe
- Алексеева E.A., Полосенко О.В., Фурсова Н.К. и др. Первый случай выявления Listeria monocytogenes сиквенс-типов ST7, ST20, ST425 в сточных водах при обследовании водных объектов Вологодской области. Журнал микробиологии, эпидемиологии и иммунобиологии. 2022;99(4):453–64. Alekseeva E.A., Polosenko O.V., Fursova N.K. et al. The first case of detection of Listeria monocytogenes sequence types ST7, ST20, ST425 in wastewater during an investigation of water bodies in the Vologda region. Journal of Microbiology, Epidemiology and Immunobiology 2022; 99(4): 453–464. DOI: https://doi.org/10.36233/0372-9311-266 EDN: https://elibrary.ru/gashhr
- Hobbs J.L., Lee C., Thompson B., et al. Two Listeria monocytogenes outbreaks in a cancer centre: onsite food premises and their potential health risk to patients. BMC Public Health. 2023;23(1):1443. DOI: https://doi.org/10.1186/s12889-023-16371-7
- Сорокина М.Н., Иванова В.В., Скрипченко Н.В. Бактериальные менингиты у детей. М.;2003. Sorokina M.N., Ivanova V.V., Skripchenko N.V. Bacterial Meningitis in Children. Moscow;2003.
- Тагирова З.Г., Нагибина М.В., Макашова В.В. и др. Листериозный менингоэнцефалит: особенности течения и диагностики (клиническое наблюдение). РМЖ. Медицинское обозрение. 2023;7(11):766–70. Tagirova Z.G., Nagibina M.V., Makashova V.V., et al. Listeria meningoencephalitis: specifics of its course and diagnosis (case report). Russian Medical Inquiry. 2023;7(11):766–770. DOI: https://doi.org/10.32364/2587-6821-2023-7-11-9 EDN: https://elibrary.ru/xfibcs
- Воронина О.Л., Рыжова Н.Н., Кунда М.С., и др. Динамика спектра генотипов Listeria monocytogenes, вызвавшей инвазивный листериоз в период циркуляции вариантов SARS-CoV-2 Omicron. Молекулярная генетика, микробиология и вирусология. 2024;42(3):29–36. Voronina O.L., Ryzhova N.N., Kunda M.S., et al. Dynamics of the spectrum of genotypes of Listeria monocytogenes, which caused invasive listeriosis during the period of circulation of SARS-CoV-2 Omicron variants. Molecular Genetics, Microbiology and Virology. 2024;42(3):29–36. DOI: https://doi.org/10.17116/molgen20244203129 EDN: https://elibrary.ru/mjgjvp
- Алексеева E.A., Шепелин А.П., Полосенко О.В. Опыт выделения Listeria monocytogenes на территории Вологодской области. Бактериология. 2019;4(2):31–6. Alekseeva E.A., Shepelin А.Р., Polosenko O.V. Experience a selection of Listeria monocytogenes in the territory of the Vologda region. Bacteriology. 2019;4(2):31–6. DOI: https://doi.org/10.20953/2500-1027-2019-2-31-36 EDN: https://elibrary.ru/humtek
- Меньшиков В.В., ред. Клиническая лабораторная аналитика. Частные аналитические технологии в клинической лаборатории. Том 6. М.;2003:578–82. Men'shikov V.V., ed. Clinical Laboratory Analysis. Private Analytical Technologies in the Clinical Laboratory. Volume 6. Moscow;2003:578–82.
- Xu X., Shan Y., Cen Y., et al. Clinical characteristics and treatment of Listeria monocytogenes infections in the central nervous system. Infect. Drug Resist. 2023;16:5899–909. DOI: https://doi.org/10.2147/idr.s424012
- Paranjape N. Rhombencephalitis due to Listeria monocytogenes. IDCases. 2021;24:e01081. DOI: https://doi.org/10.1016/j.idcr.2021.e01081
- Тартаковский И.С., Малеев В.В., Ермолаева С.А. Листерии: роль в инфекционной патологии человека и лабораторная диагностика. М.;2002. Tartakovsky I.S., Maleev V.V., Ermolaeva S.A. Listeria: its role in human infectious pathology and laboratory diagnostics. Moscow;2002. EDN: https://elibrary.ru/pbdion
- Алексеева Е.А., Миронов А.Ю., Полосенко О.В. и др. Выделение и идентификация листерий из клинического материала. Клиническая лабораторная диагностика. 2022;67(6):362–8. Alekseeva E.A., Mironov A.Yu., Polosenko O.V., et al. Isolation and identification of Listeria in clinical material. Clinical Laboratory Diagnostics. 2022;67(6):362–8. DOI: https://doi.org/10.51620/0869-2084-2022-67-6-362-368 EDN: https://elibrary.ru/hiaphn
- Подкопаев Я.В., Домотенко Л.В., Круглов А.Н. и др. Сравнительная оценка питательных сред для выделения возбудителей гнойных бактериальных менингитов. Инфекция и иммунитет. 2016;6(4):389–94. Podkopaev Ya.V., Domotenko L.V., Kruglov A.N., et al. Comparative evaluation of culture media for pathogen isolation of purulent bacterial meningitis. Russian Journal of Infection and Immunity. 2016;6(4):389–94. EDN: https://elibrary.ru/xetljd
- Омарова С.М., Исаева Р.И., Багандова Д.Ш. и др. Разработка и изучение питательных сред для определения биологических свойств листерий. Клиническая лабораторная диагностика. 2022;67(2):110–4. Omarova S.M., Isaeva R.I., Bagandova D.Sh.. et al. Development and study of nutrient media for determining the biological properties of Listeria. Clinical Laboratory Diagnostics. 2022;67(2):110–4. DOI: https://doi.org/10.51620/0869-2084-2022-67-2-110-114 EDN: https://elibrary.ru/vuylev
- Vazquez-Boland J.A., Kocks C., Dramsi S., et al. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect. Immun. 1992;60(1):219–30. DOI: https://doi.org/10.1128/iai.60.1.219-230.1992
- Amaya-Villar R., García-Cabrera E., Sulleiro-Igual E., et al. Three-year multicenter surveillance of community-acquired Listeria monocytogenes meningitis in adults. BMC Infect. Dis. 2010;10:324. DOI: https://doi.org/10.1186/1471-2334-10-324
- Guillet C., Join-Lambert O., Le Monnier A., et al. Human listeriosis caused by Listeria ivanovii. Emerg. Infect. Dis. 2010;16(1):136–8. DOI: https://doi.org/10.3201/eid1601.091155
- Perrin M., Bemer M., Delamare C. Fatal case of Listeria innocua bacteremia. J. Clin. Microbiol. 2003;41(11):5308–9. DOI: https://doi.org/10.1128/jcm.41.11.5308-5309.2003
- Rocourt J., Hof H., Schrettenbrunner A., et al. Acute purulent Listeria seelingeri meningitis in an immunocompetent adult. Schweiz. Med. Wochenschr. 1986;116(8):248–51. (in French)
Supplementary files