Surveillance and genotyping of tick-borne pathogens in ixodid ticks in the east of Western Siberia (Russia, 2023)
- Authors: Ilyinskikh E.N.1, Karpova M.R.1, Voronkova O.V.1, Reshetova A.V.1, Filatova E.N.1, Kartashov M.Y.2, Krivosheina E.I.2, Belichenko K.R.2, Ternovoi V.A.2, Loktev V.B.2,3
-
Affiliations:
- Siberian State Medical University, Ministry of Health of Russia
- State Research Center of Virology and Biotechnology "Vector"
- Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences
- Issue: Vol 102, No 3 (2025)
- Pages: 310-324
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18788
- DOI: https://doi.org/10.36233/0372-9311-665
- EDN: https://elibrary.ru/PDFGKO
- ID: 18788
Cite item
Abstract
Introduction. Tomsk region is one of the territories of the Russian Federation with the highest possible incidence of tick-borne infections. However, the spectrum and genetic diversity of tick-borne pathogens remain insufficiently studied.
Materials and methods. The study analyzed 534 ticks: Ixodes persulcatus (n = 107), I. pavlovskyi (n = 234) and Dermacentor reticulatus (n = 193), collected in 13 biotopes of Tomsk and its suburbs during 2023. Detection of genetic material of tick-borne pathogens was carried out by PCR and RT-PCR in individual ticks with subsequent sequencing and phylogenetic analysis of nucleotide sequences.
Results. More than fourfold dominance of I. pavlovskyi and D. reticulatus ticks over the taiga tick was observed. Infection of I. persulcatus ticks with tick-borne encephalitis virus (TBEV) of the Siberian genotype amounted to 1.3%, in ticks of the Ixodes genus, the genetic material of Borrelia burgdorferi s.l. was detected in 8.5%, B. miyamotoi – in 2.1%, Anaplasma phagocytophilum — in 1.5%, and Rickettsia tarasevichiae — in 14.1%. R. raoultii infection of D. reticulatus ticks was identified in 48.7%, and Babesia canis DNA was detected in a single sample. Genotyping and phylogenetic analysis of genomic nucleotide sequences showed the presence of new, unusual for the region genetic variants of B. garinii, B. bavariensis, B. afzelii and the Siberian TBEV genotype (subclade V).
Conclusion. In the territory of Tomsk and its suburbs, genetic material of 9 species of tick-borne pathogens, including their new genetic variants, was detected in ixodes ticks.
Full Text
Introduction
Ixodes ticks are carriers of a number of infectious agents of viral, bacterial and protozoal nature, which play a major role in human infectious pathology. The Tomsk region belongs to the territories of Russia with the highest incidence rates of ixodal tick-borreliosis (ITB) and tick-borne encephalitis [1–4]. In 2020–2023, the incidence rates exceeded the average incidence rates in Russia by 2.0–5.9 times, amounting to 10.3–15.7 per 100,000 population for ITB, and by 4.0 or more times, ranging from 2.9 to 4.5 per 100,000 population, for tick-borne encephalitis. At the same time, the number of applications of the population to medical organizations in the region for medical care for tick bites was more than 1,000 per 100,000 population in 2020–2023.1 During this period, this parameter ranged from 1705.5 to 2390.1 per 100,000 population, exceeding the corresponding average values of the parameter in Russia from 5.0 to 7.4 times.
In general, several tick species are involved in the spread of tick-borne infections in Russia, with Ixodes ricinus and I. persulcatus ticks being the most important [5]. At least 11 species of ixodid ticks have been described in Western Siberia, of which the I. persulcatus tick has the greatest epidemic significance [6–8]. Traditionally, the taiga tick is considered to be the main vector for tick-borne pathogens in the south of Western Siberia, but recently in urban and suburban biotopes of Tomsk unusually widespread ticks I. pavlovskyi (Pomerantzev, 1946) and D. reticulatus (Fabricius, 1794) [8, 9]. It is known that ticks of the Dermacentor genus, prevalent in steppe and forest-steppe zones of Siberia, can be carriers of the Omsk hemorrhagic fever virus, rickettsiae of tick-borne typhus of North Asia, as well as pathogens of Q fever and human granulocytic anaplasmosis [2, 3, 7, 8]. The average number of ixodid ticks on the territory of the suburbs of Tomsk and Tomsk district varied from 26.5 to 57.7 specimens per 1 km of the route [4]. The results of the study of tick viral infection rate by immunoenzyme analysis and polymerase chain reaction methods ranged from 0.6% to 6.1%. Infection with tick-borne encephalitis virus (TBEV) in ticks of the Ixodes genus amounted to 6.5%, and in ticks of the Dermacentor genus — 1.9% [4].
A study of the species composition of ixodid ticks within the city limits of Tomsk, conducted in 2015–2016, showed a significant increase in the number of D. reticulatus on the slopes of the high bank of the Tom River (Camp Garden area), reaching 66 specimens per 1 km of the route. Previously, in 2012–2014, the average seasonal abundance was only 0.17 specimens per 1 km of route [7, 8]. In 2018–2021, the maximum abundance of ticks of the Dermacentor genus reached 20 specimens per 1 survey km. In 2015, the study showed that from the number of ticks of the Ixodes genus captured from vegetation in the suburbs of Tomsk, the percentage of I. pavlovskyi and I. persulcatus amounted to 70.3% and 29.7% respectively. The average seasonal abundance was 3.67 specimens for I. persulcatus and 8.42 specimens for I. pavlovskyi per 1 survey km, respectively [7, 8].
Recently, the significant dominance of I. pavlovskyi and D. reticulatus among the ticks attacking humans was also described in Novosibirsk and its suburbs [6]. At the same time, such a fact has not yet been described in other Siberian regions, where taiga ticks are still associated with the spread of tick-borne infections in the population.
Infection of ixodid ticks in the south of Western Siberia with various pathogens of viral, bacterial and protozoan nature remains insufficiently investigated. At the same time, the number of publications on a wide range of tick-borne pathogens found in various tick species in the territories of Northern Eurasia is increasing [6, 10, 11]. To replenish and update the data on the molecular epidemiology of tick-borne pathogens in the conditions of a large Siberian metropolis, an attempt was made to determine the infection levels of various species of ixodid ticks in urban and suburban biotopes of Tomsk during one summer season. Detection of genetic material of pathogens of various tick-borne infections, including TBEV, orbiviruses (Kemerovo virus), Borrelia spp., Rickettsia spp., Anaplasma spp. and Babesia canis, was performed by PCR and RT-PCR methods for each tick individually with subsequent sequencing of the detected genetic material and genotyping of the identified pathogens.
Materials and methods
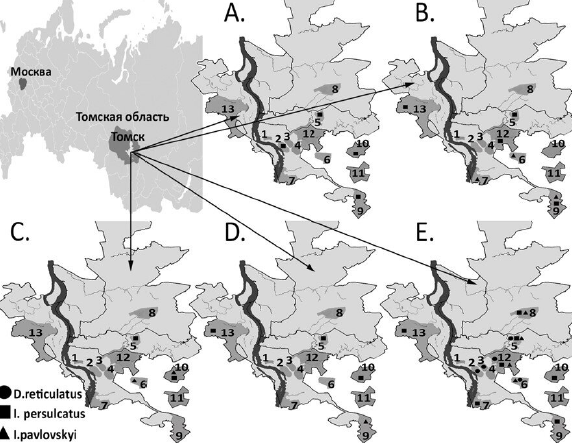
During the study, 534 individual ticks belonging to the following species were collected and analyzed: I. persulcatus (n = 107), I. pavlovskyi (n = 234), and D. reticulatus (n = 193). The ticks were collected using the standard method from vegetation in 13 urban and suburban biotopes of Tomsk (Fig. 1) in the summer of 2023. Species identification of ticks was carried out as described previously [6].
Fig. 1. Map-scheme of Tomsk and suburbs with designation of tick collection areas.
A — TBEV RNA detection sites; B – B. burgdorferi s.l DNA; C — B. miyamotoi DNA; D — A. phagocytophilum DNA; E — Rickettsia spp. DNA.
Designation of collection areas: 1 — Camp Garden; 2 — Burevestnik stadium; 3 — Southern cemetery; 4 — garage cooperative on Continental Street; 5 — Akademgorodok microdistrict; 6 — forest near Zonal Station; 7 — Anikino microdistrict; 8 — forest belt on Irkutsk tract; 9 — Loskutovo village; 10 — Mezheninovka village; 11 — Basandaika settlement; 12 — Stepanovka settlement; 13 — Timiryazevskoye settlement.
To isolate nucleic acids, ticks were treated twice with 70% ethyl alcohol and washed with phosphate-salt buffer to remove external contaminants and external microflora. Homogenization of the obtained samples was performed using a TissueLyserLT laboratory homogenizer (Qiagen) in 300 μL of sterile physiological solution. Total nucleic acids were isolated from 100 μL of homogenate using the AmpliPrime RIBO-prep reagent kit (NextBio) according to the manufacturer's instructions. cDNA was obtained in reverse transcription reaction using a REVERTA-L commercial kit (AmpliSens).
In order to control the stage of nucleic acid isolation and its safety during storage, PCR was performed with all the studied samples to detect the site of cytochrome oxidase subunit I gene localized in the mitochondrial genome of ticks. The primer pairs IpCX-6f/IpCX-9R for ticks of the Ixodes genus and DH_f/DH_r for ticks of the Dermacentor genus were used for this purpose.
Samples were screened for the presence of genetic material of the studied pathogens (TBEV, Kemerovo virus, B.burgdorferi s.l., B. miyamotoi, Rickettsia spp., A.phagocytophilum, Babesia spp.) by real-time PCR or with subsequent electrophoretic detection. PCR was performed in 25 μL of reaction mixture using BioMaster HS-Taq PCR (2×) kit (Biolabmix) and 0.4 pM specific oligonucleotide primers (Table 1). Fusion DNA polymerase Pfu-Sso7d (Biolabmix) was used to perform targeting PCR and to generate amplicons for whole-genome sequencing of TBEV isolates. The 95% confidence interval (CI) of the level of tick infectivity with the pathogens studied was calculated using the online service2.
Table 1. Primers used for isolation of gene fragments of viruses, borrelia, rickettsia and anaplasma from ixodid ticks
Target | Primers | Primer sequence | Size of the fragment | Source |
Ixodes sp. | IpCX-6f | ATTAGGAGCACCTGATATAGCTTTCCC | 660 | |
IpCX-9r | GCTGTAAATAAGCTCGAGTGTCGATA | |||
D. reticulatus | DH_f | TCGAWTAGAAYTAAGACAACCTGG | 610 | [6] |
DH_r | GGTGRCCAAAAAATCAAAATARATG | |||
TBEV | Kgg31 | AAAGGCAGCATTGTGACCTG | 361 | [11] |
Kgg19 | CGTGTCTCCACGGCAGAGCC | |||
ALSV | Miass_gly_3F | TGGATCAGCTCACACCACAC | 333 | |
Miass_gly_3R | TCACCGTCACAGTGGAATGG | |||
YGTV | YGTV_gly_1F | ACTACTGGTTGCCGTCCTCG | ||
YGTV_gly_1R | GTCGCTGCAGTCAAATATCT | |||
Kemerovo | rt_Kem4f | TCCGCCACCCTGGAATGAGAC | 116 | |
rt_Kem4r | TCAGGATCGGTCAAGGCCATTC | |||
Kem_prb4 | FAM–AGCCGTTTCTGTCCACGAGACG–BHQ1 | |||
B. burgdorferi s.l. | F7 | TTCAAAGGGATACTGTTAGAGAG | [13] | |
F10 | AAGAAGGCTTATCTAATGGTGATG | |||
F5 | ACCTGGTGATGTAAGTTCTCC | |||
F12 | CTAACCTCATTGTTGTTAGACTT | |||
B. miyamotoi | Q1 | CACCATTGATCATAGCTCACAG | [13] | |
Q4 | CTGTTGGTGCTTCATTCCAGTC | |||
Q3 | GCTAGTGGGTATCTTCCAGAAC | |||
Q2 | CTTGTTGTTTATGCCAGAAGGGT | |||
Rickettsia spp. | PrF_gltA | GGCTTCGGTCATCGTGT | 120 | [14] |
PrR_gltA | TTGCTATTTGTAAGAGCGGATTG | |||
Z(ROX)_gltA | ROX-CCACGTGCCGCAGTACTTAAAGAAAC-BHQ2 | |||
CS409d | CCTATGGCTATTATGCTTGC | 765 | [15] | |
RP1258n | ATTGCAAAAAGTACAGTGAACA | |||
A. phagocytophilum | MSP2− 3f | CCAGCGTTTAGCAAGATAAGAG | 334 | [13] |
MSP2− 3r | GCCCAGTAACAACATCATAAGC |
Amplification products were analyzed by separation of DNA fragments in a 2% agarose gel in Tris-acetate buffer containing 0.1% ethidium bromide. Purification of amplicons from agarose gel for subsequent sequencing reaction was performed using a microcolumn-based kit (Biosilica) according to the manufacturer's instructions.
Sanger sequencing reaction was performed using the Big Dye Terminator Kit v. 3.1 (Thermo Fisher Scientific). Nucleotide sequences were determined for both strands using a 3130xl Genetic Analyzer automated sequencer (Applied Biosystems), and all nucleotide sequences were determined twice in independent experiments. Whole genome sequencing of identified TBEV isolates was performed using MiSeq technology and appropriate MiSeq reagent kits v2 (Illumina) by analyzing overlapping specific fragments after PCR. Sequence assembly was performed by mapping reads to the reference genome of Zausaev strain (AF527415) with contigs determination using the Geneious Prime program (2024.0.5).
Analysis of the obtained nucleotide sequences, alignments and phylogenetic analysis were performed using the Unipro UGENE v. 1.50 [16] and MEGA X software [17]. Phylogenetic trees were constructed using the maximum likelihood method and the Tamura-Nei evolutionary model (TN93). The statistical significance of phylogenetic tree topology was assessed by Bootstrap analysis; calculations were performed for 500 pseudo-samples.
The nucleotide sequences identified in this study were deposited in the international GenBank database under the following accession numbers: PP942931–PP942934 for whole genome TBEV sequences, PQ126376–PQ126404 for B. burgdorferi s.l. P83/100 gene fragments, PQ126405–PQ126411 for B. miyamotoi glpQ gene fragments, PQ126412–PQ126416 for A. phagocytophilum msp2 gene fragments, PQ123220 for the gene fragment of the detected B. canis isolate.
The study was conducted in compliance with the biosafety rules regulated in SanPiN 3.3686-21 “Sanitary and Epidemiological Requirements for the Prevention of Infectious Diseases” dated 28.01.2021.
Results
Of the 534 studied ixodid ticks, I. persulcatus was represented by 107 (20.0%) specimens (56 females and 51 males), I. pavlovskyi — 234 (43.8%; 133 females and 101 males), D. reticulatus — 193 (36.1%; 120 females and 73 males). All ticks were tested by PCR for the presence of genetic material of 10 species of tick-borne pathogens of viral, bacterial and protozoan etiology (Kemerovo virus, TBEV, B. garinii, B. afzelii, B. bavariensis, B. miyamotoi, R. tarasevichiae, R. raoultii, A. phagocytophilum and B. canis) (Table 2). The Kemerovo virus was not detected during the analysis.
Table 2. Detection of markers of tick-borne infections in D. reticulatus. I. persulcatus and I. pavlovskyi ticks
Маркеры | Number of PCR-positive samples in ixodid ticks, abs./% (95% CI) | |||
Ixodes genus ticks (total) (n = 341) | I. persulcatus (n = 107) | I. pavlovskyi (n = 234) | D. reticulatus (n = 193) | |
TBEV RNA | 4/1.3 (0.3–2.6) | 4/1.3 (0.3–2.6) | 0 | 0 |
DNA R. tarasevichiae/R. raoultii | 48/14.1 (10.8–18.2) | 43/40.2 (31.4–49.7) | 5/2.1 (0.9–4.9) | 94/48.7 (41.8–55.8) |
DNA A. phagocytophilum | 5/1.5 (0.6–3.4) | 4/3.7% (1.5–9.2) | 1/0.4 (0.1–2.4) | 0 |
DNA B. canis | 0 | 0 | 0 | 1/0.5 (0.1–2.9) |
DNA B. miyamotoi | 7/2.1 (1.0–4.2) | 3/2.8 (0.9–7.9) | 4/1.7 (0.6–4.3) | 0 |
DNA B. burgdorferi s.l. | 29/8.5 (5.9–11.9) | 17/15.9 (10.2–23.9) | 12/5.1 (2.9–8.7) | 0 |
Including (n = 29): | ||||
B. garinii | 19/65.5 (47.4–80.1) | 9/8.4 (4.5–15.2) | 10/4.3 (2.3–7.7) | 0 |
B. afzelii | 7/24.1 (12.2–42.1) | 5/4.7 (2.0–10.5) | 2/0.8 (0.2–3.0) | 0 |
B. bavariensis | 3/10.4 (3.6–26.3) | 3/2.8 (0.9–7.9) | 0 | 0 |
TBEV RNA was detected in 4 (1.3%; 95% CI 0.3–2.6) individuals out of 341 ticks of the Ixodes genus. All detected TBEV isolates were attributed to the Siberian genotype when analyzing the full-length nucleotide sequence of the genome. They were characterized by a high level of homology of the nucleotide sequence of the viral genome, which is 94–98% compared to other strains of the Siberian genotype and about 85–86% compared to other TBEV genotypes (Table 3). The levels of homology of the amino acid sequence of the viral polyprotein are about 98–99 and 94–95%, respectively. Tomsk 2-2023 isolate differs by a lower level of homology from the other three sequenced Tomsk isolates.
Table 3. Degree of similarity (%) of nucleotide (nuc.) and amino acid sequences of polyprotein (aa.) of identified TBEV variants compared to reference TBEV strains
Reference strains | Tomsk 1-2023 (PP942931) | Tomsk 2-2023 (PP942932) | Tomsk 3-2023 (PP942933) | Tomsk 4-2023 (PP942934) | ||||
nuc. | aa. | nuc. | aa. | nuc. | aa. | nuc. | aa. | |
Siberian TBEV genotype | ||||||||
Lesopark 11 (KJ701416) | 98.14 | 99.45 | 96.62 | 98.99 | 98.20 | 99.23 | 98.27 | 99.39 |
Zausaev (AF527415) | 98.31 | 99.42 | 96.50 | 99.08 | 98.06 | 99.39 | 98.09 | 99.48 |
Kolarovo-2008 (FJ968751) | 93.98 | 96.47 | 94.06 | 96.47 | 94.02 | 96.47 | 94.22 | 96.57 |
Tomsk-PT122 (KM019545) | 94.16 | 98.31 | 94.47 | 98.28 | 94.24 | 98.28 | 94.42 | 98.41 |
Vasilchenko (AF069066) | 94.23 | 98.31 | 94.55 | 98.25 | 94.38 | 98.28 | 94.58 | 98.44 |
Far Eastern TBEV genotype | ||||||||
Sofjin-HO (AB062064) | 85.66 | 95.34 | 85.52 | 95.37 | 85.64 | 95.40 | 85.51 | 95.43 |
205 (DQ989336) | 85.48 | 95.22 | 85.51 | 95.31 | 85.52 | 95.28 | 85.52 | 95.31 |
Western TBEV genotype | ||||||||
Hypr (U39292) | 85.24 | 94.88 | 85.16 | 94.94 | 85.34 | 94.91 | 85.21 | 94.79 |
Neudoerfl (U27495) | 85.21 | 94.39 | 85.15 | 94.39 | 85.26 | 94.42 | 85.14 | 94.33 |
Baikal TBEV genotype | ||||||||
886-84 (EF469662) | 84.97 | 95.65 | 84.92 | 95.71 | 84.97 | 95.61 | 85.11 | 95.77 |
178-79 (EF469661) | 85.80 | 95.92 | 85.80 | 96.01 | 85.86 | 95.89 | 85.88 | 96.04 |
In phylogenetic analysis, the detected genetic variants are clustered with subclade V of the Siberian TBEV genotype [18]. The detected TBEV isolates have a high level of homology and cluster together with TBEV variants circulating in the southern regions of Siberia, including the regions adjacent to Lake Baikal (Fig. 2). At the same time, isolate Tomsk 2-2023 forms a separate phylogenetic branch, which may be promising for separation into a separate subclade within the Siberian TBEV genotype. Phylogenetic analysis shows that all genomic sequences of the Tomsk 2023 isolates are original and differ from the Kolarovo-2008 and Tomsk-PT122 isolates circulating in Tomsk in 2006–2008, which belong to subclade IV of the Siberian TBEV genotype.
Fig. 2. Phylogenetic tree based on the whole-genome nucleotide sequences of TBEV.
TBEV sequences from this study are shown in bold. Presentation format: GenBank deposit number, isolate name.
B. burgdorferi s.l. DNA was detected in 29 ticks of the Ixodes genus, corresponding to an infection rate of 8.5% (95% CI 5.9–11.9; Table 2). Genotyping by nucleotide sequence of the P83/100 gene fragment showed that the species diversity of Borrelia was represented mainly by B. garinii with 65.5% of cases, B. afzelii was found in 24.1% of cases, and B. bavariensis in 10.4% of cases. The results of phylogenetic analysis of these 3 Borrelia species are presented in Fig. 3. All sequenced variants of B. burgdorferi s.l. on the phylogenetic tree formed compact monophyletic groups within their species, which clustered with previously isolated isolates in northern Eurasia.
Fig. 3. Phylogenetic tree based on the P83/100 gene fragment (325 bp) of the identified B. burgdorferi s.l. complex isolates.
B. miyamotoi DNA was also detected in 7 ticks of the Ixodes genus on the basis of nucleotide sequence analysis of a fragment of the glycerophosphodiester phosphodiesterase (glpQ) gene fragment, which corresponds to an infection rate of 2.1% (95% CI 1.0–4.2; Table 2). No DNA of the B. burgdorferi s.l. and B. miyamotoi complex was detected in ixodid ticks of the Dermacentor genus.
Phylogenetic analysis for the detected B. miyamotoi isolates showed that all of them have a high level of homology with the variants found earlier in Tomsk, Novosibirsk Regions, Khabarovsk and Krasnoyarsk Territories, and together with them are clustered within the Asian subtype of B. miyamotoi (Fig. 4).
Fig. 4. Phylogenetic tree based on the glpQ gene fragment (433 bp) of the identified B. miyamotoi isolates.
A. phagocytophilum DNA was detected in 4 I. persulcatus ticks and 1 I. pavlovskyi tick (Table 2). The nucleotide sequence of the major surface protein 2 (msp2) gene fragment was determined for the detected A. phagocytophilum isolates, and the results of their phylogenetic analysis are presented in Fig. 5. All sequenced A. phagocytophilum isolates form a monophyletic group within their species with isolates previously found in Poland, Kaliningrad and North America.
Fig. 5. Phylogenetic tree based on the msp2 gene fragment (340 bp) of the identified A. phagocytophilum isolates.
The rickettsiae genetic material was most frequently detected by PCR (Table 2). Thus, R. tarasevichiae DNA was detected in 48 (14.1%) out of 341 ticks of the Ixodes genus, R. raoultii DNA —in 94 (48.7%) individuals out of 193 ticks of the Dermacentor genus, in 5 ticks of the Ixodes genus — 1.5%. B. canis DNA was detected in 1 D. reticulatus tick by a fragment of the 18S rRNA gene (0.5% of cases), its phylogenetic tree is presented in Fig. 6. No genetic material of Kemerovo virus was detected in the examined ticks.
Fig. 6. Phylogenetic tree based on the 18S ribosomal RNA gene fragment (394 bp) of the identified B. canis isolate.
In certain cases, several tick-borne pathogens were detected in one tick. Thus, DNA of two tick-borne pathogens was detected in 8 (2.3%) ticks of the Ixodes genus. Genetic material of B. garinii and R. tarasevichiae was detected in 5 ticks, B. garinii and B. miyamotoi — in 2 ticks, B. garinii and A. phagocytophilum — in 1. Moreover, DNA of 3 tick-borne pathogens (B. garinii, R. tarasevichiae and A. phagocytophilum) was detected in 1 tick of the Ixodes genus at once.
Discussion
The I. pavlovskyi and D. reticulatus species were dominant among the studied ixodid ticks (79.9%), which is recently characteristic of Tomsk and Novosibirsk and their suburbs [6–8]. Thus, the increase in the abundance of D. reticulatus more than 200 times in urban biotopes of Tomsk was first recorded in the fall of 2015. When determining the species composition of ticks attacking humans in Novosibirsk and its suburbs, it was also recorded that I. pavlovskyi and D. reticulatus ticks account for 84.8% among ticks removed from patients seeking medical care for tick bites in 2018. The dominance of I. pavlovskyi and D. reticulatus ticks in 2023 in the biotopes of Tomsk and its suburbs indicates the stable nature of this phenomenon and the actual displacement of the taiga tick from the biotopes of these metropolitan areas in the south of Western Siberia. At the same time, an independent study in 2019 of a natural biotope on the Tom River upstream of Tomsk (approximately 125 km) revealed a practically complete dominance of the taiga tick, whose representation amounted to 95.72% in collections from vegetation, while the shares of the I. pavlovskyi tick and its hybrids amounted to only 1.75 and 2.53%, respectively, with a comparable sample size [19]. Such a dramatic difference in the species composition of ticks in urban and natural biotopes in the basin of one river in the south of Western Siberia suggests that anthropogenic impact significantly changes the breeding conditions of ticks and the spectrum of associated tick-borne infections in places of compact human habitation.
As a result of the studies, genetic material of 9 species of tick-borne pathogens of viral, bacterial and protozoic nature was detected in ixodid ticks of three species. TBEV was detected in 4 ticks (taiga ticks), and all detected TBEV isolates were attributed to the Siberian genotype. It is known that in northern Eurasia TBEV is mainly represented by three main genotypes — Far Eastern, Siberian and European, the first of which most often causes severe clinical forms of TBEV, and the second one is more often found in Western Siberia [5]. The isolates of the Siberian TBEV genotype that we have detected can be attributed to subclade V, for which the Zausaev strain is considered to be the prototype virus. Initially, the Zausaev strain was isolated in Moscow from the brain of a deceased patient with a chronic form of tick-borne encephalitis (within 2 years), who was presumably infected in the Tomsk region and became ill 10 years after a tick bite in 1973. [20].
However, in Tomsk and its suburbs, circulation of TBEV variants of the Siberian subtype in 2006–2008, belonging to subclade IV, strains Kolarovo-2008 (FJ968751) and Tomsk-PT122 (KM019545), as well as variants of the Far Eastern genotype, was previously detected [7, 9, 21]. Phylogenetic analysis of full-genome sequences showed that isolates of TBEV 2023 are new to Tomsk, with the unusual isolate Tomsk 2023-2 forming a separate phylogenetic branch that may be promising for isolation into a separate subclade within the Siberian TBEV genotype. We failed to detect the genetic material of the Kemerovo virus in the studied ticks, although there are reports of its detection in Western Siberia and Kazakhstan [12].
Infection of ticks of the Ixodes genus with B. burgdorferi s.l. amounted to 8.5%, which is below the average values of recent years [1, 2, 6, 22, 23]. B. garinii was detected most frequently (65.5% of cases), B. afzelii (24.1%) and B. bavariensis (10.4%) were detected less frequently. The population of borreliae pathogens of ITB is heterogeneous, counting more than 20 species of borreliae, and the species diversity of borreliae is significantly influenced by the diversity of reservoir hosts, which ensure circulation and persistence of ITB pathogens. B. afzelii and B. bavariensis are commonly associated with small rodents and B. garinii with birds [23]. It is likely that the dominance of B. garinii can be attributed to the fact that birds may be an important feeder of ixodid ticks in urban areas [1, 8, 13, 24]. B. burgdorferi s.l. DNA in the ixodid ticks of the Dermacentor genus was unable to be detected, but the possibility of the presence of borrelia in D. reticulatus ticks is confirmed by a number of publications [23, 24]. Phylogenetic analysis of sequenced borrelia DNA fragments showed that borrelia isolates formed compact monophyletic groups within their species. Moreover, B. garinii isolates were grouped into 3 different phylogenetic branches. At the same time, one of these groups, including 9 isolates, was not associated with isolates previously found in Tomsk. Probably, we can speak about the appearance of new genetic variants of B. garinii in urban biotopes of Tomsk. This assumption applies equally to B. afzelii and B. bavariensis isolates, which also form new phylogenetic groups.
B. miyamotoi DNA was detected in 2.1% of the examined ticks of the Ixodes genus. This species of Borrelia belongs to the causative agents of tick-borne relapsing fevers, which are widespread in various regions of the world, including Russia [25–28]. This species of borrelia is associated with erythematous forms of ITB, while the pathogen is capable of causing severe forms of disease, including meningoencephalitis, in immunocompromised people, and mixt-infections with other tick-borne pathogens. Infection of ixodid ticks with B. miyamotoi is usually much lower than with Borrelia of other species and ranges from 0.3–16% [27, 28]. In 2023, we were unable to detect B. miyamotoi DNA in ixodid ticks of the Dermacentor genus, although in 2021 in the Tomsk region it was detected in 2% of D. reticulatus ticks [3]. Phylogenetic analysis of 7 B. miyamotoi isolates based on the glpQ gene fragment showed that all of them form a rather compact genetic group within the Asian subtype and cluster together with the previously detected variants in the Tomsk, Novosibirsk Regions, Khabarovsk and Krasnoyarsk Krai.
The highest infection rate of the studied ticks was found for rickettsiae (48.7% for R. raoultii and 14.1% for R. tarasevichiae), with R. tarasevichiae occurring only in ticks of the Ixodes genus and R. raoultii in ticks of the Dermacentor genus. These rickettsiae are capable of causing tick-borne rickettsioses in humans, and their circulation has been established in different regions of the Russian Federation, mainly in Siberia and Kazakhstan [29, 30]. In the Tomsk region, only single cases of rickettsiosis are registered annually [2, 4]. R. raoultii is known to occur in many European countries, in different regions of Russia, such as Novosibirsk, Omsk, Irkutsk, in the Republics of Altai and Buryatia, in Primorsky and Khabarovsk Krai and is usually associated with ticks of the Dermacentor genus [6, 7, 10, 30]. It is currently accepted that R. raoultii is capable of causing the development of TIBOLA syndrome (tick-borne lymphadenopathy), which is characterized by a primary affect in the form of erythema developing at the site of tick sucking and painful regional lymph nodes [31, 32]. Detection of genetic material of R. raoultii in meadow ticks, for which an explosive increase in numbers (more than 200 times) in urban biotopes has been registered, requires special attention to the diagnosis of TIBOLA syndrome in patients in the Tomsk region. R. tarasevichiae is characterized by infection of ticks of the Ixodes genus (more often I. persulcatus, less often I. pavlovskyi). This species of rickettsiae is widespread in the Asian part of Russia, and cases of human infection with R. tarasevichiae were recorded in the Novosibirsk region [6].
Genetic markers of A. phagocytophilum were detected in 1.5% of ticks of the Ixodes genus and were not found in ticks of the Dermacentor genus, although earlier the genetic material of A. phagocytophilum was detected in ticks of D. reticulatus in the territory of Tomsk [7]. All sequenced isolates of A. phagocytophilum form a monophyletic group within their species with isolates previously detected in Poland, Kaliningrad, and North America, which, in all probability, demonstrates the conservatism of the msp2 gene used for genotyping. Babesia infections of ixodid ticks were also previously registered in the Tomsk region [2]. As a result of this study, we managed to detect Babesia canis in only 1 tick of the Dermacentor genus.
Mixed infections associated with various tick-borne infections are quite common and can affect the course and clinical manifestations of diseases [33]. We detected different combinations of tick-borne pathogens in ixodid ticks, with B. garinii occurring in all cases of mixed infection.
Comparing the infection rates of different tick species, a significantly higher infection rate of Borrelia was found (OR = 3.1; 95% CI 1.43–6.72; F = 0.004; χ2 = 8.9), rickettsiae (OR = 18.81; 95% CI 7.25–48.82; F = 0.000; χ2 = 60.17) and anaplasmas (OR = 8.75; 95% CI 0.97–79.2; F = 0.038; χ2 = 5.35) of I. persulcatus ticks compared to I. pavlovskyi. A wider range of pathogens (TBEV, B. burgdorferi s.l., B. miyamotoi, R. tarasevichiae and A. phagocytophilum) was recorded in I. pavlovskyi ticks which were dominant in urban biotopes. Ticks of the Dermacentor genus were predominantly infected with R. raoultii, and the other tick-borne pathogens were found in them much less frequently.
The obtained data confirm the necessity of monitoring the circulation in natural and anthropourgical foci of tick-borne infections in Tomsk and Tomsk region along with TBEV and pathogens of other tick-borne infections: B. miyamotoi, Rickettsia spp., A. phagocytophilum, Babesia spp. It is necessary to further improve methods of diagnostics and prevention of these infections, including identification of possible human cases and mixtinfection. It is important to emphasize that currently 3 species of ticks (I. persulcatus, I. pavlovskyi, D. reticulatus), infected with at least 9 species of tick-borne pathogens, dominate in urban biotopes and take part in the formation of urban foci of tick-borne infections in the park zone of Tomsk.
Conclusion
In the territory of Tomsk and its suburbs, I. pavlovskyi and D. reticulatus predominate among ixodid ticks collected from vegetation. As a result of PCR analysis, 9 species of tick-borne pathogens of viral, bacterial and protozoan nature were detected in the ixodid ticks of 3 species, which apparently take part in the formation of urban foci of tick-borne infections. Higher levels of infection with borrelia, rickettsiae and anaplasma were found in I. persulcatus ticks compared to I. pavlovskyi. A wider range of pathogens (TBEV, B. burgdorferi s.l., B. miyamotoi, R. tarasevichiae and A. phagocytophilum) was recorded in I. pavlovskyi and I. persulcatus ticks than in D. reticulatus ticks (TBEV, R. raoultii and Babesia canis).
Infection of taiga ticks with TBEV amounted to 1.3%. Infection of ticks of the Ixodes genus amounted to the following: B. burgdorferi s.l. — 8.5%, B. miyamotoi — 2.1%, A. phagocytophilum — 1.5%, R. tarasevichiae — 14.1%. Furthermore, the rate of occurrence of R. raoultii in D. reticulatus ticks amounted to 48.7%, and Babesia canis DNA was detected in a single sample. Genotyping of tick-borne pathogens was carried out on the basis of sequencing of isolated gene fragments of TBEV, B. burgdorferi s.l., B. miyamotoi, A. phagocytophilum and Babesia canis. All detected TBEV isolates were assigned to the Siberian genotype, subclade V, by analyzing the full-length nucleotide sequence of the genome. The sequences were deposited in GenBank.
1 On the state of sanitary and epidemiological welfare of the population in the Russian Federation in 2022: State Report. Moscow; 2023. 368 p. (In Russ.)
2 URL: https://www.pedro.org.au/english/downloads/confidence-interval-calculator
About the authors
Ekaterina N. Ilyinskikh
Siberian State Medical University, Ministry of Health of Russia
Email: infconf2009@mail.ru
ORCID iD: 0000-0001-7646-6905
Dr. Sci. (Med.), Associate Professor, Professor, Department of infectious diseases and epidemiology
Russian Federation, TomskMaria R. Karpova
Siberian State Medical University, Ministry of Health of Russia
Author for correspondence.
Email: karpova.mr@ssmu.ru
ORCID iD: 0000-0002-7109-9955
Dr. Sci. (Med.), Professor, Head, Department of microbiology and virology
Russian Federation, TomskOlga V. Voronkova
Siberian State Medical University, Ministry of Health of Russia
Email: voronkova-ov@yandex.ru
ORCID iD: 0000-0001-9478-3429
Dr. Sci. (Med.), Associate Professor, Head, Department of biology and genetics
Russian Federation, TomskAlina V. Reshetova
Siberian State Medical University, Ministry of Health of Russia
Email: wind_of_change95@mail.ru
ORCID iD: 0000-0001-5195-3897
postgraduate student, Department of infectious diseases and epidemiology
Russian Federation, TomskEvgenia N. Filatova
Siberian State Medical University, Ministry of Health of Russia
Email: synamber@mail.ru
ORCID iD: 0000-0001-9951-8632
postgraduate student, Department of infectious diseases and epidemiology
Russian Federation, TomskMikhail Yu. Kartashov
State Research Center of Virology and Biotechnology "Vector"
Email: kartashov_myu@vector.nsc.ru
ORCID iD: 0000-0002-7857-6822
Cand. Sci. (Biol.), senior researcher, Department of molecular virology for flaviviruses and viral hepatitis
Russian Federation, KoltsovoEkaterina I. Krivosheina
State Research Center of Virology and Biotechnology "Vector"
Email: krivosheina_ei@vector.nsc.ru
ORCID iD: 0000-0001-5181-0415
researcher, Department of molecular virology for flaviviruses and viral hepatitis
Russian Federation, KoltsovoKsenia R. Belichenko
State Research Center of Virology and Biotechnology "Vector"
Email: belichenko_kr@vector.nsc.ru
ORCID iD: 0009-0003-2330-025X
research assistant, Department of development and production of diagnostic tools for viral diseases
Russian Federation, KoltsovoVladimir A. Ternovoi
State Research Center of Virology and Biotechnology "Vector"
Email: tern@vector.nsc.ru
ORCID iD: 0000-0003-1275-171X
Cand. Sci. (Biol.), leading researcher, Head, Department of molecular virology for flaviviruses and viral hepatitis
Russian Federation, KoltsovoValery B. Loktev
State Research Center of Virology and Biotechnology "Vector"; Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences
Email: valeryloktev@gmail.com
ORCID iD: 0000-0002-0229-321X
Dr. Sci (Biol.), Professor, chief researcher, Head, Department of molecular virology for flaviviruses and viral hepatitis, State Research Center of Virology and Biotechnology "Vector"
Russian Federation, Koltsovo; NovosibirskReferences
- Полторацкая Н.В., Полторацкая Т.Н., Панкина Т.М., и др. Анализ эпидемиологической ситуации по клещевому энцефалиту и иксодовому клещевому боррелиозу в Томской области. Медицинская паразитология и паразитарные болезни. 2021;(1):9‒15. Poltoratskaya N.V., Poltoratskaya T.N, Pankina T.M., et al. Analysis of the epidemiological situation on tick-borne encephalitis and ixodid tick-borne borreliosis in Tomsk region. Medical Parasitology and Parasitic Diseases 2021;(1):9‒15. DOI: https://doi.org/10.33092/0025-8326mp2021.1.9-15 EDN: https://elibrary.ru/mfwclv
- Воронкова О.В., Романенко В.Н., Симакова А.В. и др. Анализ множественной инфицированности иксодовых клещей Dermacentor reticulatus в сочетанном природном очаге трансмиссивных инфекций в Томской области. Проблемы особо опасных инфекций. 2023;(2):106–11. Voronkova O.V., Romanenko V.N., Simakova A.V., et al. Analysis of multiple infection in ixodic ticks Dermacentor reticulatus in a combined natural focus of vector-borne infections in the Tomsk region. Problems of Particularly Dangerous Infections. 2023;(2):106-11. DOI: https://doi.org/10.21055/0370-1069-2023-2-106-111 EDN: https://elibrary.ru/xluwjc
- Воронкова О.В., Лукашова Л.В., Карпова М.Р. и др. Циркуляция возбудителя возвратной клещевой лихорадки Borrelia miyamotoi в природном очаге Томской области. Эпидемиология и инфекционные болезни. 2022;27(1):15–22. Voronkova O.V., Lukashova L.V., Karpova M.R., et al. Distribution of the causative agent of relapsing tick-borne fever Borrelia miyamotoi in natural focus in the Tomsk region. Epidemiology and Infectious Diseases. 2022;27(1):15–22. DOI: https://doi.org/10.17816/EID109070 EDN: https://elibrary.ru/hxuspn
- Полторацкая Н.В., Полторацкая Т.Н., Панкина Т.М. и др. Характеристика природных очагов клещевого энцефалита на территории Томской области. Медицинская паразитология и паразитарные болезни. 2021;(3):3–11. Poltoratskaya N.V., Poltoratskaya T.N, Pankina T.M., et al. Characteristics of natural tick-borne encephalitis foci in the Tomsk region. Medical Parasitology and Parasitic Diseases 2021;(3):3–11. DOI: https://doi.org/10.33092/0025-8326mp2021.3.3-11 EDN: https://elibrary.ru/jcdonv
- Gritsun T.S., Lashkevich V.A., Gould E.A. Tick-born encephalitis. Antiviral Res. 2003;57(1-2):129–46. DOI: https://doi.org/10.1016/S0166-3542(02)00206-1
- Карташов М.Ю., Кривошеина Е.И., Свирин К.А. и др. Генотипирование возбудителей клещевых инфекций и определение видового состава клещей, нападающих на людей в Новосибирске и его пригородах. Инфекция и иммунитет. 2022;12(6):1103–12. Kartashov M.Yu., Krivosheina E.I., Svirin K.A., et al. Genotyping of tick-borne pathogens and determination of human attacking tick species in Novosibirsk and its suburbs. Russian Journal of Infection and Immunity. 2022;12(6):1103–12. DOI: https://doi.org/10.15789/2220-7619-GOT-1979 EDN: https://elibrary.ru/zwdbcf
- Карташов М.Ю., Микрюкова Т.П., Кривошеина Е.И. и др. Генотипирование возбудителей клещевых инфекций в клещах Dermacentor reticulatus, собранных в городских биотопах г. Томска. Паразитология. 2019;53(5):355–69. Kartashov M.Yu., Mikryukova T.P., Krivosheina E.I., et al. Genotyping of tick-borne pathogens in Dermacentor reticulatus ticks collected in urban biotopes of Tomsk. Parazitologiya. 2019;53(5):355–69. DOI: https://doi.org/10.1134/S0031184719050016 EDN: https://elibrary.ru/xodhop
- Романенко В.Н., Соколенко В.В., Максимова Ю.В. Локальное формирование высокой численности клещей Dermacentor reticulatus (Parasitiformes, Ixodidae) в Томске. Паразитология. 2017;51(4):345–53. Romanenko V.N., Sokolenko V.V., Maksimova Yu.V. Local formation of high abundance of Dermacentor reticulatus (Parasitiformes, Ixodidae) ticks in Tomsk. Parazitologiya. 2017;51(4):345–53. EDN: https://elibrary.ru/zgoydh
- Mikryukova T.P., Moskvitina N.S., Kononova Y.V., et al. Surveillance of tick-borne encephalitis virus in wild birds and ticks in Tomsk city and its suburbs (Western Siberia). Ticks Tick Borne Dis. 2014;5(2):145–51. DOI: https://doi.org/10.1016/j.ttbdis.2013.10.004
- Карташов М.Ю., Микрюкова Т.П., Кривошеина Е.И. и др. Генотипирование возбудителей клещевого энцефалита и лихорадки Кемерово в таежных клещах, собранных в Республике Коми. Инфекция и иммунитет. 2020;10(1):159–66. Kartashov M.Yu., Mikryukova T.P., Krivosheina E.I., et al., Genotyping of tick-borne encephalitis and Kemerovo viruses in taiga ticks collected in the Komi Republic. Russian Journal of Infection and Immunity. 2020;10(1):159–66. DOI: https://doi.org/10.15789/2220-7619-GOT-1147 EDN: https://elibrary.ru/ycyhgg
- Del Cerro A., Oleaga A., Somoano A., et al. Molecular identification of tick-borne pathogens (Rickettsia spp., Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Coxiella burnetii and piroplasms) in questing and feeding hard ticks from North-Western Spain. Ticks Tick Borne Dis. 2022;13(4):101961. DOI: https://doi.org/10.1016/j.ttbdis.2022.101961
- Kholodilov I.S., Belova O.A., Ivannikova A.Y., et al. Distribution and characterisation of tick-borne Flavi-, Flavi-like, and Phenuiviruses in the Chelyabinsk region of Russia. Viruses. 2022;14(12):2699. DOI: https://doi.org/10.3390/v14122699
- Tkachev S.E., Tikunov A.Y., Babkin I.V., et al. Occurrence and genetic variability of Kemerovo virus in Ixodes ticks from different regions of Western Siberia, Russia and Kazakhstan. Infect. Genet. Evol. 2017;47:56–63. DOI: https://doi.org/10.1016/j.meegid.2016.11.007
- Rar V., Livanova N., Tkachev S., et al. Detection and genetic characterization of a wide range of infectious agents in Ixodes pavlovskyi ticks in Western Siberia, Russia. Parasit. Vectors. 2017; 10(1):258. DOI: https://doi.org/10.1186/s13071-017-2186-5
- Карташов М.Ю., Микрюкова Т.П., Терновой В.А. и др. Высокоэффективная детекция ДНК риккетсий методом ПЦР в реальном времени. Клиническая лабораторная диагностика. 2015;60(12):39‒43. Kartashov M.Yu., Mikryukova T.P., Ternovoi V.A., et al. The highly effective detection of DNA ricketsia using technique of polymerase chain reaction in real-time. Clinical Laboratory Diagnostics. 2015;60(12):39‒43. EDN: https://elibrary.ru/vhthvt
- Roux V., Rydkina E., Eremeeva M., Raoult D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int. J. Syst. Bacteriol. 1997;47(2): 252–61. DOI: https://doi.org/10.1099/00207713-47-2-252
- Okonechnikov K., Golosova O., Fursov M.; UGENE team. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics. 2012;28(8):1166–7. DOI: https://doi.org/10.1093/bioinformatics/bts091
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018;35(6):1547–9. DOI: https://doi.org/10.1093/molbev/msy096
- Boldbaatar B., Cleary N.G., Paoli J.E., et al. Characterization of tick-borne encephalitis virus isolates from Ixodes persulcatus ticks collected during 2020 in Selenge, Mongolia. Pathogens. 2024;13(12):1086. DOI: https://doi.org/10.3390/pathogens13121086
- Kovalev S., Okulovskaya V. The first record of Omsk hemorrhagic fever virus and tick-borne encephalitis virus of Baltic lineage from the Kemerovo region of Russia. Vector Borne Zoonotic Dis. 2024;24(7):443–50. DOI: https://doi.org/10.1089/vbz.2023.0156
- Gritsun T.S., Frolova T.V., Zhankov A.I., et al. Characterization of a siberian virus isolated from a patient with progressive chronic tick-borne encephalitis. J. Virol. 2003;77(1):25–36. DOI: https://doi.org/10.1128/jvi.77.1.25-36.2003
- Чаусов Е.В., Терновой В.А., Протопопова Е.В. и др. Генетическое разнообразие инфекционных агентов, переносимых иксодовыми клещами в г. Томске и его пригородах. Паразитология. 2009;43(5):374–88. Chausov E.V., Ternovoi V.A., Protopopova E.V., et al. Genetic diversity of ixodid tick-borne pathogens in Tomsk city and suburbs. Parazitologiya. 2009;43(5):374–88. EDN: https://elibrary.ru/oihzdh
- Воронкова О.В., Ильинских Е.Н., Рудиков А.А. и др. Клинико-эпидемиологические проявления очагов иксодового клещевого боррелиоза в Томской области. Эпидемиология и вакцинопрофилактика. 2022;21(4):70–9. Voronkova O.V., Ilyinskikh E.N., Rudikov A.A., et al. Clinical and epidemiological manifestations of Ixodic tick-borne borreliosis foci in the Tomsk region. Epidemiology and Vaccinal Prevention. 2022;21(4):70–9. DOI: https://doi.org/10.31631/2073-3046-2022-21-4-70-79 EDN: https://elibrary.ru/jbkbzw
- Рудакова С.А., Теслова О.Е., Муталинова Н.Е. и др. Молекулярно генетический надзор на основе индикации и идентификации боррелий в иксодовых клещах. Фундаментальная и клиническая медицина. 2023;8(1):63–70. Rudakova S.A., Teslova O.T., Mutalinova N.E., et al. Molecular genetic surveillance based on the identification of Borrelia in ixodid ticks. Fundamental and Clinical Medicine. 2023;8(1):63–70. DOI: https://doi.org/10.23946/2500-0764-2023-8-1-63-70 EDN: https://elibrary.ru/nzddjy
- Zajac V., Wojcik-Fatla A., Sawczyn A., et al. Prevalence of infections and co-infections with 6 pathogens in Dermacentor reticulatus ticks collected in eastern Poland. Ann. Agric. Environ. Med. 2017;24(1):26–32. DOI: https://doi.org/10.5604/12321966.1233893
- Kim C.M., Seo J.W., Kim D.M., et al. Detection of Borrelia miyamotoi in Ixodes nipponensis in Korea. PLoS One. 2019;14(7):e0220465. DOI: https://doi.org/10.1371/journal.pone.0220465
- Cleveland D.W., Anderson C.C., Brissette C.A. Borrelia miyamotoi: a comprehensive review. Pathogens 2023;12(2):267. DOI: https://doi.org/10.3390/pathogens12020267
- Cutler S., Vayssier-Taussat M., Estrada-Peña A., et al. A new Borrelia on the block: Borrelia miyamotoi – a human health risk? Euro Surveill. 2019;24(18):1800170. DOI: https://doi.org/10.2807/1560-7917.es.2019.24.18.1800170
- Тупота Н.Л., Терновой В.А., Карташов М.Ю. и др. Детекция Borrelia miyamotoi в иксодовых клещах, собранных на юге Западной Сибири. Проблемы особо опасных инфекций. 2021;(3):129–33. Tupota N.L., Ternovoy V.A., Kartashov M.Yu., et al. Detection of Borrelia miyamotoi in Ixodidae ticks collected in the South of Western Siberia. Problems of Particularly Dangerous Infections. 2021;(3):129–133. DOI: https://doi.org/10.21055/0370-1069-2021-3-129-133 EDN: https://elibrary.ru/jkhmuj
- Яковчиц Н.В., Бондаренко Е.И., Адельшин Р.В. и др. Выявление ДНК возбудителей клещевого риккетсиоза в клещах на территории Иркутской области. Эпидемиология и вакцинопрофилактика. 2015;14(6):43–6. Yakovchitc N., Bondarenko E., Adelshin R., et al. Detection of rickettsial DNA in ticks in Irkutsk region. Epidemiology and Vaccinal Prevention. 2015;14(6):43–6. DOI: https://doi.org/10.31631/2073-3046-2015-14-6-43-46 EDN: https://elibrary.ru/vbiajp
- Igolkina Y., Krasnova E., Rar V., et al. Detection of causative agents of tick-borne rickettsioses in Western Siberia, Russia: identification of Rickettsia raoultii and Rickettsia Sibirica DNA in clinical samples. Clin. Microbiol. Infect. 2018;24(2):199.e9–12. DOI: https://doi.org/10.1016/j.cmi.2017.06.003
- Świtaj K., Chmielewski T., Borkowski P., et al. Spotted fever rickettsiosis caused by Rickettsia raoultii — case report. Przegl. Epidemiol. 2012;66(2):347–50.
- Silva-Pinto A., Santos M. de L., Sarmento A. Tick-borne lymphadenopathy, an emerging disease. Ticks Tick Borne Dis. 2014;5(6):656–9. DOI: https://doi.org/10.1016/j.ttbdis.2014.04.016
- Pukhovskaya N.M., Morozova O.V., Vysochina N.P., et al., Prevalence of Borrelia burgdorferi sensu lato and Borrelia miyamotoi in ixodid ticks in the Far East of Russia. Int. J. Parasitol. Parasites Wildl. 2019;8:192–202. DOI: https://doi.org/10.1016/j.ijppaw.2019.01.005
Supplementary files