Results of reconnaissance epizootiological monitoring for West Nile fever in certain regions of European Russia and the Urals in 2024
- Authors: Borodai N.V.1, Nesgovorova A.V.1, Mendygalieva A.K.1, Koloskova A.Y.1, Udovichenko S.K.1, Zarubin N.A.1, Kargashin S.A.1, Gusev Y.A.1, Baturin A.A.1, Khabarova I.A.1, Putintseva E.V.1, Toporkov A.V.1
-
Affiliations:
- Volgograd Plague Control Research Institute
- Issue: Vol 102, No 4 (2025)
- Pages: 413-424
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18767
- DOI: https://doi.org/10.36233/0372-9311-654
- EDN: https://elibrary.ru/VMOBYD
- ID: 18767
Cite item
Abstract
Introduction. Climate warming contributes to the intensification of epizootic and epidemic processes of West Nile fever (WNF). In southern Russia, the activity of the epizootic process is recorded annually, but in the central region of the European part of the country and in the Urals, the enzootic circulation of the West Nile virus (WNV) has not been confirmed in the territory of 20 subjects.
The aim of the study is to investigate zoological and entomological material for WNV infection to confirm the ongoing epizootic process in old WNV foci and in previously non-endemic areas.
Materials and methods. Field samples were collected in 2024 in 19 subjects in accordance with the methods regulated in normative documents. The material was studied using the reverse transcription polymerase chain reaction method.
Results. In total, during the 2024 field season, 5,419 samples of field samples were examined: 684 samples of birds from 74 species, 455 samples of small mammals from 13 species, 45 samples of frogs from 1 species, 3,665 samples of blood-sucking mosquitoes from 33 species (93,438 specimens), and 570 samples of ixodid ticks from 17 species (4,809 specimens). Markers of WNV in field samples were detected in 7 subjects from 3 federal districts. In the Kirov and Chelyabinsk regions and the Republic of Mordovia, evidence of the ongoing epizootic process of WNF has been obtained for the first time. WNV RNA was detected in 6 (0.5%) out of 1184 tested samples of vertebrate animals and in 27 (0.6%) out of 4235 samples of arthropods. The level of individual infection was 0.03% in blood-sucking mosquitoes, 0.06% in ixodid ticks, and 0.9% in birds.
Conclusion. The results of the studies confirm the enzootic circulation of WNV in the territories of the Southern, Volga and Ural Federal Districts.
Full Text
Introduction
West Nile fever (WNF) is an enzootic, natural focal, vector-borne infectious disease caused by the West Nile virus (WNV) from the genus Orthoflavivirus. Certain bird species are the reservoir host of the pathogen, while the vectors are blood-sucking mosquitoes [1].
Climate warming over the past decades has contributed to the transformation of many ecosystems on the planet, leading to changes in the natural habitats of various animal species, including reservoirs and vectors of zoonotic infections. The increase in temperature leads to an increase in the replication rate of the pathogen, an extension of the stay of migratory birds in nesting areas, as well as accelerated development, extended activity periods, and the expansion of the ranges of blood-sucking mosquito vectors [2, 3]. All of the mentioned contributes to the intensification of epizootic and epidemic processes of WNF in natural focal areas and the spread of WNV.
Targeted monitoring for the infection rates in reservoir and vector populations makes it possible to identify signs of the activation of the epizootic process, promptly carry out measures to reduce vector populations, and inform the public about the necessity to use individual and collective protection measures against mosquito bites. Moreover, effective monitoring allows for dynamic observations of the activity of natural foci of zoonotic infections.
At the beginning of the study of WNF, it was commonly accepted that the range of WNV was limited to the territories of the equatorial, subequatorial, tropical, subtropical and southern parts of the temperate climate zones. On the territory of the former USSR, it covered the southern part of the European part of Russia, Belarus, Moldova, Ukraine, Azerbaijan, Georgia, Tajikistan, Kyrgyzstan, Kazakhstan and Turkmenistan. For the first time in the USSR, the virus was isolated in 1963 from Hyalomma plumbeum ixodid ticks (now Hyalomma marginatum) in the Astrakhan region, as well as from a sandpiper and a blackbird from Azerbaijan [4]. In the 1980s, the pathogen was detected in regions located significantly further north: in rooks and nidicolous birds from their nests in the Omsk region, in nidicolous birds from the Novosibirsk region, and in Aedes vexans mosquitoes from the Republic of Tatarstan. During the WNF outbreak in Moscow in 2021, the WNV RNA was detected in 14.2% of samples from the total number of examined blood-sucking mosquitoes, 68.0% of dead birds, and 32.0% of live birds [7]. The obtained data indicated a broader territorial spread of the WNF pathogen than was previously accepted [8]. As of early 2024, markers of WNF have been identified in samples from carriers and vectors in 52 entities across the territory of the Russian Federation. In southern Russia, the activity of the epizootic process is recorded annually. At the same time, the absence of positive findings in certain regions of the central part of European Russia and in the Urals draws attention — endemic circulation of WNV has not been confirmed in 10 entities of the Central Federal District (CFD), 6 in the Volga Federal District (VFD) and 4 in the Ural Federal District (UFD). In certain non-endemic territories, local cases of human WNF have been identified, indicating the presence of foci of this arboviral infection. Therefore, conducting active reconnaissance surveys aimed at clarifying the nosoareal is relevant.
The aim of this study is to investigate the zoological-entomological material for the presence of WNF to confirm the ongoing epizootic process in old foci of WNF and in previously non-endemic areas.
Materials and methods
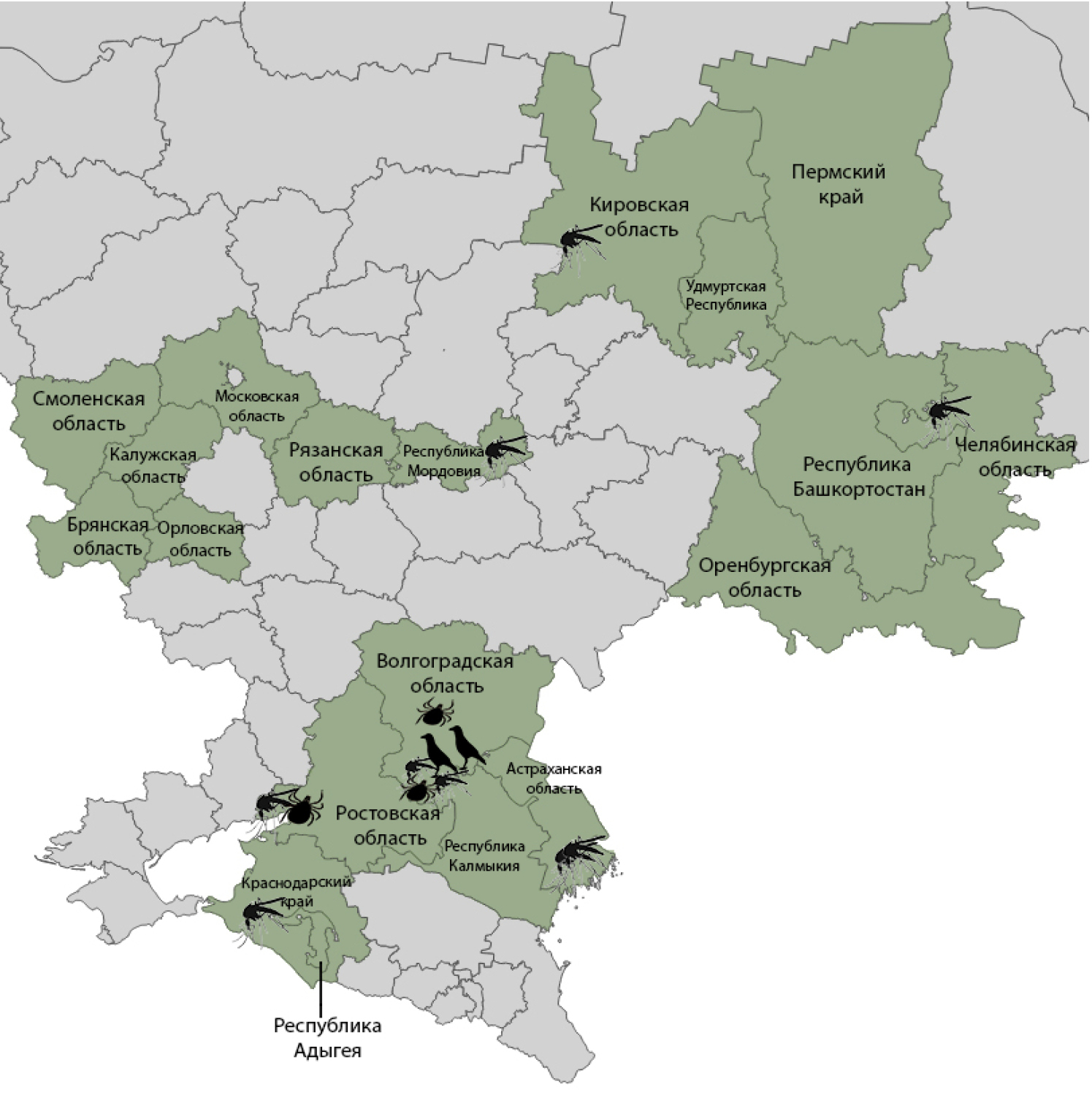
The collection of field samples for research at the Reference Center for monitoring the WNF pathogen in the 2024 season was carried out from April to November in 19 entities of the Russian Federation (Figure) by employees of the Volgograd Research Anti-Plague Institute of Rospotrebnadzor, as well as by anti-plague institutions and the Centers for Hygiene and Epidemiology in the entities of the Russian Federation.
Entities where zoological and entomological material sampling was conducted in 2024, and points of positive WNV RNA findings from birds and arthropods.
The capture of small mammals was carried out with the help of snap traps. Birds were hunted by employees of hunting farms through shooting, and the collection of fallen individuals was carried out by staff of zoological groups and researchers. Sampling of blood-sucking mosquitoes in open biotopes in household plots, along water bodies, in cemeteries, and in forests was carried out using automatic traps such as BG-sentinel-2 (Biogents AG), LovKom (ProTechnoSystems), Mosquito Magnet Executive (Woodstream), Black Kill M3000 (Black Kill), and an entomological net. Mosquitoes were captured in enclosed biotopes (chicken coops, pigsties, basements of multi-story buildings) with the help of battery-operated vacuum cleaners (BLV 18-200, Karcher and Tefal X-PERT 3.60 Versatile Handstick TY6975WO, Tefal) and exhausters. The collection of ixodid ticks was carried out using classical methods: in nature — with an entomological flag on vegetation, in populated areas — from animals (small and large livestock, dogs, cats).
The collected arthropods were delivered to the laboratory in thermal containers with cold packs, identified using the Stemi 2000C (Karl Zeiss) and MSP-1 (LOMO) stereomicroscopes on a cooled surface to the species level according to standard keys [9–12]. They were placed in 2 mL cryotubes.
Field samples were transported on dry ice or in car refrigerators at –20℃. For the analysis of climatic factors, data from the Federal Service for Hydrometeorology and Environmental Monitoring of the Russian Federation were used1.
Field samples were examined using the reverse transcription polymerase chain reaction method at the stationary laboratory of the Volgograd Research Anti-Plague Institute of Rospotrebnadzor. For the extraction of WNV RNA, suspensions of blood-sucking arthropods, as well as organs from birds, small mammals, and frogs (in a pooled sample from each individual – brain, kidneys, spleen) were prepared. The detection of WNV RNA was carried out using the AmpliSens WNV-FL reagent kit (Central Research Institute of Epidemiology of Rospotrebnadzor) according to the manufacturer's instructions. The determination of the WNV lineage in positive samples was carried out using the Ampligen-WNV-genotype-1/2/4 reagent kit (Volgograd Research Anti-Plague Institute of Rospotrebnadzor).
The infection rate of vertebrates was determined by calculating the proportion of positive samples from the total number of samples examined (%), and the individual infection rate of arthropods was calculated using the formula by V.N. Beklemishev [13]. Statistical processing of the materials and calculations were carried out using the Microsoft Excel program.
Results
A total of 5,419 field samples were examined during the 2024 field season: 684 bird samples from 74 species, 455 small mammal samples from 13 species, 45 frog samples from 1 species, 3,665 blood-sucking mosquito samples from 33 species (93,438 specimens), and 570 ixodid tick samples from 17 species (4,809 specimens). Table 1 presents the amount of birds, small mammals, mosquitoes and ixodid ticks in each entity. In the Volgograd region, 45 frogs were also collected for the study.
Table 1. Amount of field samples collected in 2024
Entity | Birds (specimens) | Small mammals (specimens) | Mosquitos | Ixodid ticks | ||
specimens | samples | specimens | samples | |||
Astrakhan region | – | – | 7.231 | 254 | 559 | 81 |
Volgograd region | 332 | – | 11.355 | 423 | 350 | 75 |
Rostov region | – | – | 3.189 | 124 | 5 | 2 |
Republic of Kalmykia | – | – | 598 | 25 | – | |
Republic of Adygea | 7 | 9 | 2.926 | 112 | 370 | 67 |
Krasnodar Krai | 1 | – | 9.736 | 341 | – | – |
Chelyabinsk region | – | – | 2.430 | 97 | – | – |
Orenburg region | 20 | 35 | 8.855 | 312 | 230 | 23 |
Republic of Bashkortostan | 5 | 30 | 4.102 | 158 | 400 | 40 |
Perm Krai | 24 | 54 | 3.034 | 142 | 779 | 70 |
Kirov region | – | 75 | 7.067 | 276 | 200 | 21 |
Republic of Mordovia | 44 | 60 | 5.252 | 254 | 109 | 24 |
Republic of Udmurt | 23 | 73 | 2.995 | 120 | 222 | 20 |
Bryansk region | 166 | – | 2.493 | 97 | – | – |
Smolensk region | 4 | 35 | 5.650 | 214 | 400 | 20 |
Orlov region | 20 | 15 | 2.736 | 117 | 310 | 33 |
Moscow region | 17 | 18 | 4.054 | 168 | 57 | 8 |
Kaluga region | 21 | 16 | 3.941 | 180 | 403 | 43 |
Ryazan region | 0 | 35 | 5.794 | 251 | 415 | 43 |
Total | 684 | 455 | 93.438 | 3665 | 4809 | 570 |
The species composition, amount of samples and results of the studies are presented in Table 2 and Table 3.
Table 2. Results of WNV RNA testing in vertebrate species in 2024
Species | Amount of studied specimens | Amount of positive specimens |
Birds | ||
White stork — Ciconia ciconia Linnaeus, 1758 | 1 | 0 |
Gray partridge — Perdix perdix Linnaeus, 1758 | 4 | 0 |
Common pheasant — Phasianus colchicus Linnaeus, 1758 | 5 | 0 |
Black grouse — Lyrurus tetrix Linnaeus, 1758 | 1 | 0 |
Rock dove — Columba livia Gmelin, 1789 | 84 | 0 |
Wood pigeon — Columba palumbus Linnaeus, 1758 | 2 | 0 |
Mottled duck — Anas fulvigula Ridgway, 1874 | 2 | 0 |
Gadwall — Mareca strepera Linnaeus, 1758 | 4 | 0 |
Tufted duck — Aythya fuligula Linnaeus, 1758 | 1 | 0 |
Greater white-fronted goose — Anser albifrons Scopoli, 1769 | 12 | 0 |
Greylag goose — Anser anser Linnaeus, 1758 | 6 | 0 |
Garganey — Spatula querquedula Linnaeus, 1758 | 7 | 0 |
Eurasian teal — Anas crecca Linnaeus, 1758 | 12 | 0 |
Mallard — Anas platyrhynchos Linnaeus, 1758 | 157 | 0 |
Common pochard — Aythya ferina Linnaeus, 1758 | 3 | 0 |
Northern Shoveler — Spatula clypeata Linnaeus, 1758 | 2 | 0 |
Eurasian Wigeon — Mareca penelope Linnaeus, 1758 | 2 | 0 |
Common merganser — Mergus merganser Linnaeus, 1758 | 2 | 0 |
Ruddy shelduck — Tadorna ferruginea Pallas, 1764 | 1 | 0 |
Common goldeneye — Bucephala clangula Linnaeus, 1758 | 3 | 0 |
Eurasian woodcock — Scolopax rusticola Linnaeus, 1758 | 54 | 0 |
Common snipe — Gallinago gallinago Linnaeus, 1758 | 1 | 0 |
Black-headed gull — Chroicocephalus ridibundus Linnaeus, 1766 | 9 | 0 |
Common tern — Sterna hirundo Linnaeus, 1758 | 1 | 0 |
Little tern — Sternula albifrons Pallas, 1764 | 2 | 0 |
Caspian gull — Larus cachinnans Pallas, 1811 | 1 | 0 |
Northern Lapwing — Vanellus vanellus Linnaeus, 1758 | 1 | 0 |
Eurasian dotterel — Eudromias morinellus Linnaeus, 1758 | 1 | 0 |
Common sandpiper — Actitis hypoleucos Linnaeus, 1758 | 2 | 0 |
Corncrake — Crex crex Linnaeus, 1758 | 1 | 0 |
Eurasian coot — Fulica atra Linnaeus, 1758 | 17 | 0 |
Gray heron — Ardea cinerea Linnaeus, 1758 | 12 | 2 |
Black-crowned Night heron — Nycticorax nycticorax Linnaeus, 1758 | 2 | 0 |
Purple heron — Ardea purpurea Linnaeus, 1766 | 4 | 0 |
Glossy ibis — Plegadis falcinellus Linnaeus, 1766 | 1 | 0 |
Great cormorant — Phalacrocorax carbo Linnaeus, 1758 | 43 | 2 |
Hooded crow — Corvus cornix Linnaeus, 1758 | 50 | 1 |
Common raven — Corvus corax Linnaeus, 1758 | 1 | 0 |
Rook — Corvus frugilegus Linnaeus, 1758 | 79 | 0 |
Eurasian magpie — Pica pica Linnaeus, 1758 | 22 | 0 |
Western jackdaw — Coloeus monedula Linnaeus, 1758 | 2 | 0 |
Eurasian jay — Garrulus glandarius Linnaeus, 1758 | 6 | 0 |
Eurasian tree sparrow — Passer montanus Linnaeus, 1758 | 3 | 0 |
House sparrow — Passer domesticus Linnaeus, 1758 | 3 | 0 |
Great tit — Parus major Linnaeus, 1758 | 7 | 0 |
Red-backed shrike — Lanius collurio Linnaeus, 1758 | 1 | 0 |
Song thrush — Turdus philomelos Brehm, 1831 | 2 | 0 |
American robin — Turdus migratorius Linnaeus, 1766 | 1 | 0 |
Common blackbird — Turdus merula Linnaeus, 1758 | 2 | 0 |
Fieldfare — Turdus pilaris Linnaeus, 1758 | 7 | 0 |
Garden warbler — Sylvia borin Boddaert, 1783 | 1 | 0 |
Common redstart — Phoenicurus phoenicurus Linnaeus, 1758 | 1 | 0 |
European greenfinch — Chloris chloris Linnaeus, 1758 | 1 | 0 |
Bohemian waxwing — Bombycilla garrulus Linnaeus, 1758 | 1 | 0 |
Barn swallow — Hirundo rustica Linnaeus, 1758 | 1 | 0 |
Yellowhammer — Emberiza citrinella Linnaeus, 1758 | 1 | 0 |
European robin — Erithacus rubecula Linnaeus, 1758 | 5 | 0 |
Eurasian chaffinch — Fringilla coelebs Linnaeus, 1758 | 1 | 0 |
Blyth’s reed warbler — Acrocephalus dumetorum Blyth, 1849 | 2 | 0 |
Arctic warbler — Phylloscopus borealis Blasius, 1858 | 2 | 0 |
Eurasian wren — Troglodytes troglodytes Linnaeus, 1758 | 1 | 0 |
Common grasshopper warbler — Locustella naevia Boddaert, 1783 | 1 | 1 |
Spotted flycatcher — Muscicapa striata Pallas, 1764 | 1 | 0 |
Barred warbler — Curruca nisoria Bechstein, 1795 | 1 | 0 |
Common swift — Apus apus Linnaeus, 1758 | 2 | 0 |
Black woodpecker — Dryocopus martius Linnaeus, 1758 | 1 | 0 |
Great spotted woodpecker — Dendrocopos major Linnaeus, 1758 | 2 | 0 |
European Nightjar — Caprimulgus europaeus Linnaeus, 1758 | 1 | 0 |
Ural owl — Strix uralensis Pallas, 1771 | 1 | 0 |
Tawny owl — Strix aluco Linnaeus, 1758 | 4 | 0 |
Red-footed falcon — Falco vespertinus Linnaeus, 1766 | 1 | 0 |
Western marsh harrier — Circus aeruginosus Linnaeus, 1758 | 1 | 0 |
Black kite — Milvus migrans Boddaert, 1783 | 1 | 0 |
Common buzzard — Buteo buteo Linnaeus, 1758 | 1 | 0 |
Total | 684 | 6 |
Small mammals |
|
|
Bank vole — Myodes glareolusи Schreber, 1780 | 186 | 0 |
East European vole — Microtus majori Satunin, 1907 | 1 | 0 |
Common vole — Microtus arvalis Pallas, 1779 | 40 | 0 |
Feldmäuse – Microtus Schrank, 1798, sp. | 7 | 0 |
European water vole — Arvicola amphibius Linnaeus, 1758 | 1 | 0 |
House mouse — Mus musculus Linnaeus, 1758 | 8 | 0 |
Yellow-necked mouse — Apodemus flavicollis Melchior, 1834 | 9 | 0 |
Ural field mouse — Sylvaemus uralensis Pallas, 1811 | 134 | 0 |
Striped field mouse — Apodemus agrarius Pallas, 1771 | 35 | 0 |
Short-tailed field vole — Microtus agrestis Linnaeus, 1761 | 1 | 0 |
Brown rat — Rattus norvegicus Berkenhout, 1769 | 5 | 0 |
European mole — Talpa europaea Linnaeus, 1758 | 1 | 0 |
Common shrew — Sorex araneus Linnaeus, 1758 | 27 | 0 |
Total | 455 | 0 |
Amphibians |
|
|
Marsh frog — Pelophylax ridibundus Pallas, 1771 | 45 | 0 |
Table 3. Results WNV RNA testing in arthropod species in 2024
Species | Amount of specimens | Amount of studied samples | Amount of positive samples |
Blood-sucking mosquitoes | |||
Anopheles algeriensis Theobald, 1903 | 172 | 7 | 0 |
Anopheles claviger Meigen, 1804 | 695 | 36 | 0 |
Anopheles hyrcanus Pallas, 1771 | 4096 | 146 | 0 |
к. Anopheles maculipennis Meigen, 1818 | 38 292 | 1577 | 2 |
Anopheles plumbeus Stephens, 1828 | 8 | 2 | 0 |
Aedes albopictus Skuse, 1895 | 30 | 1 | 0 |
Aedes annulipes Meigen, 1830 | 111 | 6 | 0 |
Aedes behningi Martini, 1926 | 201 | 11 | 0 |
Aedes cantans Meigen, 1818 | 4687 | 164 | 0 |
Aedes caspius Pallas, 1771 | 2865 | 109 | 0 |
Aedes cataphylla Dyar, 1916 | 204 | 8 | 0 |
Aedes cinereus Meigen, 1818 | 2066 | 80 | 1 |
Aedes communis De Geer, 1776 | 964 | 36 | 0 |
Aedes cyprius Ludlow, 1920 | 5 | 1 | 0 |
Aedes dorsalis Meigen, 1830 | 878 | 34 | 0 |
Aedes excrucians Walker, 1856 | 709 | 25 | 0 |
Aedes flavescens Muller, 1764 | 1014 | 52 | 0 |
Aedes geniculatus Olivier, 1791 | 214 | 11 | 0 |
Aedes intrudens Dyar, 1919 | 380 | 14 | 0 |
Aedes nigrinus Eckstein, 1918 | 15 | 1 | 0 |
Aedes pulcritarsis Rondani, 1872 | 83 | 4 | 0 |
Aedes punctor Kirby, 1837 | 132 | 5 | 0 |
Aedes riparius Dyar et Knab, 1907 | 63 | 3 | 0 |
Aedes sticticus Meigen, 1838 | 3974 | 143 | 0 |
Aedes subdiversus Martini, 1926 | 1 | 1 | 0 |
Aedes vexans Meigen, 1830 | 8344 | 293 | 0 |
Culex modestus Ficalbi, 1890 | 5197 | 188 | 2 |
Culex pipiens Linnaeus, 1758 | 13 428 | 477 | 11 |
Culiseta alaskaensis Ludlow, 1906 | 322 | 24 | 0 |
Culiseta annulata Schrank, 1776 | 480 | 46 | 0 |
Culiseta longiareolata Macquart, 1838 | 59 | 6 | 0 |
Coquillettidia richiardii Ficalbi, 1889 | 3573 | 146 | 2 |
Uranotaenia unguiculata Edwards, 1913 | 176 | 8 | 6 |
Total | 93 438 | 3665 | 24 |
Ixodid ticks | |||
Dermacentor marginatus Sulzer, 1776 | 96 | 13 | 0 |
Dermacentor niveus Neumann, 1897 | 33 | 3 | 0 |
Dermacentor reticulatus Fabricius, 1794 | 1688 | 193 | 0 |
Haemaphysalis punctata Canestrini and Fanzago, 1878 | 9 | 1 | 0 |
Hyalomma detritum Schulze, 1919 | 20 | 1 | 0 |
Hyalomma marginatum Koch, 1844 | 197 | 50 | 2 |
Hyalomma scupense Schulze, 1919 | 181 | 39 | 0 |
Ixodes persulcatus Schulze, 1930 | 960 | 86 | 0 |
Ixodes ricinus Linnaeus, 1758 | 895 | 77 | 0 |
Rhipicephalus annulatus Say, 1821 | 342 | 64 | 0 |
Rhipicephalus niveus Yamazaki (1919) | 15 | 2 | 0 |
Rhipicephalus rossicus Yakimov et Kohl-Yakimova, 1911 | 169 | 18 | 1 |
Rhipicephalus sanguineus Latreille, 1806 | 66 | 9 | 0 |
Rhipicephalus turanicus Pomerantsev 1936 | 20 | 2 | 0 |
Rhipicephalus pumilio Schulze, 1935 | 118 | 12 | 0 |
Total | 4809 | 570 | 3 |
Markers of the WNF pathogen were found in field samples in 7 entities from 3 federal districts (figure), including in 3 entities (Kirov, Chelyabinsk regions and the Republic of Mordovia), where evidence of the epizootic process of WNF was obtained for the first time. In the specified territories, local cases of WNF were registered in the Chelyabinsk region in 2010 and 2011 [14], however, from 2010 to 2023, WNV markers were not detected in field samples.
WNV RNA was detected in 6 (0.5%) out of 1184 tested samples of vertebrate animals and in 27 (0.6%) out of 4235 samples of arthropods. In 1 sample, the RNA was typed as lineage 1 (Rostov region), in 25 samples as lineage 2 (Rostov, Volgograd, Kirov, Chelyabinsk, Astrakhan regions, Republic of Mordovia, Krasnodar Krai), and in 6 samples as lineage 4 (Volgograd region).
The overall level of individual infection rate (infection of each individual) among blood-sucking mosquitoes was 0.03%, among ixodid ticks it was 0.06%, and among birds it was 0.9%. When examining samples from small mammals and frogs, no markers of WNV were found.
From the CFD with negative results, 228 birds, 119 small mammals, 1,027 mosquito samples and 147 ixodid tick samples were examined.
The largest number of samples from the total number examined came from the Southern Federal District (SFD) and the VFD. From the SFD, 340 birds, 9 small mammals, 45 frogs, 1279 mosquito samples, and 225 ixodid tick samples were tested. WNV RNA was detected in 20 samples of mosquitoes (individual infection rate was 0.06%), 3 samples of ixodid ticks (0.24%), and 6 samples of birds (1.76%). Vertebrates from the VFD in the studies were represented by 116 birds and 327 small mammals. No positive findings from vertebrates were detected. Out of 1,262 mosquito samples collected in this district, WNV RNA was detected in 3 (individual infection rate — 0.01%). The results of the studies on 198 samples of ixodid ticks are negative.
97 samples of mosquitoes were delivered from the UFD for research. RNA markers of WNV were detected in 1 sample. The level of individual mosquito infection rate was 0.04%.
Discussion
One of the leading factors contributing to the activation of the epizootic process of WNF is the high air temperature readings. In 2024, averaged anomalies were significant across most of Russia in April, throughout the summer months, and in September, which contributed to an increase in the replication rate of the pathogen, accelerated the development stages of vectors, and prolonged the stay of migratory birds in their breeding areas.
When examining the material from birds, all positive samples were found only in the Volgograd region. Among them, there are sedentary birds (gray crow) and migratory birds (common cricket, great cormorants, gray herons). A fallen common cricket was discovered by us in the area of high-rise buildings in the center of Volgograd in mid-August during the period of maximum activity of the pathogen and Culex mosquitoes. At the same time, over the many years of studying WNF in southern Russia, official epizootics among birds with fatal outcomes (in Volgograd and Astrakhan regions) have not been recorded. This fact was explained by the possible adaptation of local bird populations as a result of long-term interaction with the pathogen population [15]. Confirmation of the etiological role of the WNF pathogen in the occurrence of fatal disease in birds in the territory of the old disease focus in the 2024 season suggests that active monitoring for the morbidity of wild and synanthropic birds, as well as targeted examination for the presence of WNV markers in deceased individuals in accordance with regulatory documents, is not being carried out2.
The remaining birds with detected WNV RNA were captured in fish farming ponds and lakes of the Volga-Akhtuba floodplain in the Volgograd region from September 28 to November 3. Great cormorants and gray herons use these biotopes as stops for rest and feeding during the autumn migration to wintering grounds, while resident gray crows are attracted to these water bodies due to the constant presence of food remnants from other birds on their shores.
Markers of WNV in Russia have been found in mosquitoes of 19 species over the entire observation period [16–18]. According to the results of our research in 2024, mosquitoes of 6 species tested positive for the presence of WNV RNA. Among the positive findings, C. pipiens accounted for 45.8%, U. unguiculata for 25.0%, C. richiardii, Cx. modestus and A. maculipennis mosquitoes for 8.3% each, and Ae. cinereus for 4.2%.
The level of individual infection rate in mosquitoes feeding on birds and mammals, including humans, was 0.08% for C. pipiens, 0.06% for C. richiardii, 0.04% each for Cx. modestus and Ae. cinereus, and 0.006% for An. maculipennis. This indicator for Uranotaenia unguiculata mosquitoes, whose main hosts are frogs — carriers of the lineage 4 WNV — reached 6.3%. The pathogenicity of lineage 4 WNV for humans remains unproven.
All C. pipiens, An. maculipennis and C. richiardii mosquitoes in which WNV markers were detected were collected in populated areas. Ae. cinereus and Cx. modestus mosquitoes were caught on the shores of water bodies in areas where waterfowl concentrate. This indicates a high risk of WNV infection for the population in both urbanized and natural habitats.
Territorially, as expected, the maximum number (20) of positive findings from mosquitoes was identified in the SFD: 8 in Astrakhan region, 10 in Volgograd region, and 1 each in Rostov region and Krasnodar Krai. In the VFD, WNV markers were found in mosquitoes: in 2 samples from the Republic of Mordovia and 1 from the Kirov region. Out of 97 samples collected in the Southern UFD in the Chelyabinsk region, the pathogen was found in 1 sample from C. modestus thermophilic mosquitoes, which reach high numbers only in the Southern UFD. In central Russia, however, they are usually found in small numbers and not in all regions. And although the average summer temperatures in the Southern Urals are 2.0–3.5°C lower than in the CFD as a whole, the presence of one of the main carriers of WNV in Europe in this area and the detection of the pathogen RNA from it indicate a sufficiently high risk of infection for the population in the Chelyabinsk region.
In the other surveyed regions, infected carriers of WNV were not found, which does not rule out the presence of WNV foci and requires conducting repeated studies with the selection of other biotopes for field sample collection.
Positive samples for the presence of WNV RNA from ixodid ticks were found only in the SFD: 2 from H. marginatum in the Volgograd region and 1 from Rhipicephalus rossicus in the Rostov region. Their individual infection rates were 1.0% and 1.8%, respectively.
The detection of WNV RNA in mid-April from ticks and in June from mosquitoes and ticks indicates an early activation of the epizootic process in 2024. Moreover, the April findings may also indicate the preservation of WNV in ticks during the winter period.
The established combined presence of lineages 1 and 2 WNV in the Rostov region is of scientific interest.
Conclusion
In southern Russia, the enzootic circulation of WNV in 2024 has been confirmed in the territories of Volgograd, Astrakhan, Rostov regions and Krasnodar Krai. The beginning of the epizootic process of WNF was registered in these territories (with the exception of Astrakhan region) during the spring–early summer period, which was a precursor to possible epidemiological instability. The presence of positive findings in the Republic of Mordovia, Kirov and Chelyabinsk regions confirms the circulation of the pathogen in the territories of the VFD and the UFD. Information on the spread of WNV in Russia has been supplemented with data from three new regions, and the Kirov region was the northernmost point where the pathogen RNA was detected in field samples in our study.
1 Newsletter of the Federal Service for Hydrometeorology and Environmental Monitoring: overview of the state and trends of climate change in Russia 2024 (December 2023 — November 2024). URL: http://downloads.igce.ru/climate_change_2/monitoring-klimata/Russia/2024/2024.pdf (In Russ.)
2 Paragraphs 5.18, 5.5, 8.5.1 Epidemiological surveillance, laboratory diagnostics and prevention of West Nile fever. MU 3.1/4.2.4063-24. Moscow; 2024. 46 p. (In Russ.)
About the authors
Natalia V. Borodai
Volgograd Plague Control Research Institute
Author for correspondence.
Email: borodai.nat@yandex.ru
ORCID iD: 0000-0002-2076-5276
Researcher, Laboratory of epidemiological analysis and epizootological monitoring
Russian Federation, VolgogradAnna V. Nesgovorova
Volgograd Plague Control Research Institute
Email: info@vnipchi.rospotrebnadzor.ru
ORCID iD: 0000-0001-5810-8864
Researcher, Laboratory of epidemiological analysis and epizootological monitoring
Russian Federation, VolgogradAina K. Mendygalieva
Volgograd Plague Control Research Institute
Email: info@vnipchi.rospotrebnadzor.ru
ORCID iD: 0000-0002-3862-9133
Researcher, Laboratory of epidemiological analysis and epizootological monitoring
Russian Federation, VolgogradAnna Yu. Koloskova
Volgograd Plague Control Research Institute
Email: info@vnipchi.rospotrebnadzor.ru
ORCID iD: 0009-0008-4086-0327
Researcher, Laboratory of epidemiological analysis and epizootological monitoring
Russian Federation, VolgogradSvetlana K. Udovichenko
Volgograd Plague Control Research Institute
Email: info@vnipchi.rospotrebnadzor.ru
ORCID iD: 0000-0001-8682-1536
Cand. Sci. (Med.), leading researcher, Laboratory of epidemiological analysis and epizootological monitoring
Russian Federation, VolgogradNikolay A. Zarubin
Volgograd Plague Control Research Institute
Email: info@vnipchi.rospotrebnadzor.ru
ORCID iD: 0000-0001-7689-3421
Junior researcher, Laboratory of epidemiological analysis and epizootological monitoring
Russian Federation, VolgogradStanislav A. Kargashin
Volgograd Plague Control Research Institute
Email: info@vnipchi.rospotrebnadzor.ru
ORCID iD: 0000-0002-2498-9947
Junior researcher, Laboratory of epidemiological analysis and epizootological monitoring
Russian Federation, VolgogradYevgeny A. Gusev
Volgograd Plague Control Research Institute
Email: info@vnipchi.rospotrebnadzor.ru
ORCID iD: 0000-0002-9143-7907
Researcher, Laboratory of arboviral infections
Russian Federation, VolgogradArtem A. Baturin
Volgograd Plague Control Research Institute
Email: info@vnipchi.rospotrebnadzor.ru
ORCID iD: 0000-0001-9510-7246
Cand. Sci. (Biol.), Senior researcher, Laboratory of gene diagnostics of particularly dangerous infections
Russian Federation, VolgogradIrina A. Khabarova
Volgograd Plague Control Research Institute
Email: info@vnipchi.rospotrebnadzor.ru
ORCID iD: 0000-0003-1767-7790
Researcher, Laboratory of experimental biomodels
Russian Federation, VolgogradElena V. Putintseva
Volgograd Plague Control Research Institute
Email: info@vnipchi.rospotrebnadzor.ru
ORCID iD: 0000-0002-9368-6165
Cand. Sci. (Med.), leading researcher, Laboratory of epidemiological analysis and epizootological monitoring
Russian Federation, VolgogradAndrey V. Toporkov
Volgograd Plague Control Research Institute
Email: info@vnipchi.rospotrebnadzor.ru
ORCID iD: 0000-0002-3449-4657
Dr. Sci. (Med.), Associate Professor, Director
Russian Federation, VolgogradReferences
- Топорков А.В., ред. Лихорадка Западного Нила. Волгоград; 2017. Toporkov A.V., ed. West Nile Virus. Volgograd;2017.
- Захаров К.С., Магеррамов Ш.В., Матросов А.Н. Экологические аспекты районирования территории Саратовской области по уровню риска формирования очагов лихорадки Западного Нила. Поволжский экологический журнал. 2021;(1):3–15. Zakharov K.S., Magerramov Sh.V., Matrosov A.N. Ecological aspects of zoning the territory of the Saratov region by the risk level of formation of West Nile fever foci. Povolzhskiy Journal of Ecology. 2021;(1):3–15. DOI: https://doi.org/10.35885/1684-7318-2021-1-3-15 EDN: https://elibrary.ru/pafkqi
- Frasca F., Sorrentino L., Fracella M., et al. An update on the entomology, virology, pathogenesis, and epidemiology status of West Nile and Dengue viruses in Europe (2018–2023). Trop. Med. Infect. Dis. 2024;9(7):166. DOI: https://doi.org/10.3390/tropicalmed9070166
- Львов Д.К., ред. Вирусы и вирусные инфекции человека и животных. М.;2013. L'vov D.K., ed. Viruses and Viral Infections of Humans and Animals. Moscow;2013.
- Якименко В.В., Малькова М.Г., Тюлько Ж.С. и др. Трансмиссивные вирусные инфекции Западной Сибири (региональные аспекты эпидемиологии, экологии возбудителей и вопросы микроэволюции). Омск;2019. Yakimenko V.V., Malkova M.G., Tyulko J.S., et al. Transmissible Viral Infections of Western Siberia (Regional Aspects of Epidemiology, Ecology of Pathogens and Issues of Microevolution). Omsk;2019.
- Трифонов В.А., Бойко В.А., Потапов B.C. и др. Основные эпидемиологические закономерности заболеваемости некоторыми природно-очаговыми инфекциями в Республике Татарстан. Дезинфекционное дело. 2009;(3):39–42. Trifonov V.A., Boyko V.A., Potapov V.S., et al. Basic epidemiological patterns of incidence of some natural focal infections in the Republic of Tatarstan. Disinfection Affairs. 2009;(3):39–42. EDN: https://elibrary.ru/kwhowj
- Сычева К.А., Федорова М.В., Макенов М.Т. и др. Переносчики и резервуарные хозяева возбудителя лихорадки Западного Нила во время вспышки заболевания в Москве. В кн.: Материалы ХIV Ежегодного Всероссийского Конгресса по инфекционным болезням имени академика В.И. Покровского. Инфекционные болезни в современном мире: эволюция, текущие и будущие угрозы. М.;2022. Sycheva K.A., Fedorova M.V., Makenov M.T., et al. Vectors and reservoir hosts of the West Nile fever pathogen during the disease outbreak in Moscow. In: Proceedings of the XIV Annual All-Russian Congress on Infectious Diseases named after Academician V.I. Pokrovsky. Infectious Diseases in the Modern World: Evolution, Current and Future Threats. Moscow;2022. EDN: https://elibrary.ru/lguirc
- Львов Д.К., Альховский С.В., Жирнов О.П. 130 лет вирусологии. Вопросы вирусологии. 2022;67(5):357–84. L'vov D.K., Alkhovsky S.V., Zhirnov O.P. 130th anniversary of virology. Problems of Virology. 2022;67(5):357–84. DOI: https://doi.org/10.36233/0507-4088-140 EDN: https://elibrary.ru/qhembl
- Горностаева Р.М. Комары Москвы и Московской области. М.;1999. Gornostaeva R.M. Mosquitoes of Moscow and the Moscow Region. Moscow;1999.
- Гуцевич А.В., Мончадский А.С., Штакельберг А.А. Фауна СССР. Насекомые двукрылые. Комары. Семейство Culicidae. Том 3. Ленинград;1970. Gutsevich A.V., Monchadsky A.S., Shtakelberg A.A. Fauna of the USSR. Diptera Insects. Mosquitoes. The Family Culicidae. Volume 3. Leningrad;1970.
- Филиппова Н.А. Иксодовые клещи подсем. Ixodinae. Фауна СССР. Паукообразные. Том 4. М.;1977. Filippova N.A. Ixodic Ticks of the Subfamily. Ixodinae. Fauna of the USSR. Arachnids. Volume 4. Moscow;1977.
- Федорова М.В., Сычева К.А. Кровососущие комары (Diptera:Culicidae) Краснодарского края и полуострова Крым: определитель. М.;2024. Fedorova M.V., Sycheva K.A. Bloodsucking Mosquitoes (Diptera:Culicidae) of the Krasnodar Territory and the Crimean Peninsula: Identification Guide. Moscow;2024.
- Беклемишев В.Н. К изучению зараженности клещей – переносчиков энцефалита методом биопробы. Вопросы вирусологии. 1963;8(2):240–2. Beklemishev V.N. On the study of infection of ticks – carriers of encephalitis by the bioprobe method. Problems of Virology. 1963;8(2):240–2.
- Антонов В.А., Смоленский В.Ю., Путинцева Е.В. и др. Эпидемиологическая ситуация по лихорадке Западного Нила в 2011 году на территории Российской Федерации и прогноз ее развития. Проблемы особо опасных инфекций. 2012;(1):17–21. Antonov V.A., Smolensky V.Yu., Putintseva E.V., et al. West Nile fever epidemic situation in the Russian Federation territory in 2011 and prognosis of its development. Problems of Particularly Dangerous Infections. 2012;(1):17–21. DOI: https://doi.org/10.21055/0370-1069-2012-1(111)-17-21 EDN: https://elibrary.ru/origtz
- Львов Д.К., Савченко С.Т., Алексеев В.В. и др. Эпидемиологическая ситуация и прогноз заболеваемости лихорадкой Западного Нила на территории Российской Федерации. Проблемы особо опасных инфекций. 2008;(1):10–2. L'vov D.K., Savchenko S.T., Alekseev V.V. et al. Epidemiological situation and prognostication of the West Nile fever morbidity in the territory of the Russian Federation. Problems of Particularly Dangerous Infections. 2008;(1):10–2. DOI: https://doi.org/10.21055/0370-1069-2008-1(95)-10-12 EDN: https://elibrary.ru/iqfwsf
- Федорова М.В., Бородай Н.В. О необходимости и путях совершенствования энтомологического мониторинга при эпидемиологическом надзоре за лихорадкой Западного Нила. Медицинская паразитология и паразитарные болезни. 2017;(2):37–42. Fedorova M.V., Borodai N.V. On the necessity and ways to improve entomological monitoring in the epidemiological surveillance of West Nile fever. Medical parasitology and parasitic diseases. 2017;(2):37–42. EDN: https://elibrary.ru/ysticd
- Квасов Д.А., Бородай Н.В., Гайдукова Е.П. и др. Результаты мониторинга за Лихорадкой Западного Нила в Воронежской области. В сб.: Состояние и проблемы экосистем Среднерусской лесостепи. Труды биологического центра ВГУ «Веневитиново», Том 34. Воронеж;2022:37–44. Kvasov D.A., Borodai N.V., Gaidukova E.P., et al. Monitoring results for West Nile fever in the Voronezh region. In: The State and Problems of Ecosystems of the Central Russian Forest Steppe. Proceedings of the Biological Center of VSU «Venevitinovo», Volume 34. Voronezh;2022:37–44. EDN: https://elibrary.ru/ocvpck
- Алексейчик И.О., Путинцева Е.В., Смелянский В.П. и др. Особенности эпидемической ситуации по лихорадке Западного Нила на территории Российской Федерации в 2018 г. и прогноз ее развития на 2019 г. Проблемы особо опасных инфекций. 2019;(1):17–25. Alekseichik I.O., Putintseva E.V., Smelyansky V.P., et al. Peculiarities of the epidemic situation on West Nile fever in the territory of the Russian Federation in 2018 and forecast of its development in 2019. Problems of Particularly Dangerous Infections. 2019;(1):17–25. DOI: https://doi.org/10.21055/0370-1069-2019-1-17-25 EDN: https://elibrary.ru/cgbjja
Supplementary files