Characteristics of the monkeypox virus isolate obtained from the first patient in Russia and its sensitivity to 7-[N-(4-trifluoromethylbenzoyl)-hydrazinocarbonyl]-tricyclo-[3.2.2.0^2,4]non-8-en-6-carboxylic acid
- Authors: Ovchinnikova A.S.1, Odnoshevsky D.A.1, Kabanov A.S.1, Bodnev S.A.1, Pyankov O.V.1, Os'kina O.P.1, Sivay M.V.1, Bespalov A.V.1, Tregubchak T.V.1, Shishkina L.N.1, Taranov O.S.1, Zolin V.V.1, Sergeev A.A.1, Agafonov A.P.1
-
Affiliations:
- State Scientific Center of Virology and Biotechnology "Vector"
- Issue: Vol 101, No 6 (2024)
- Pages: 748-757
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18661
- DOI: https://doi.org/10.36233/0372-9311-589
- EDN: https://elibrary.ru/yuugtk
- ID: 18661
Cite item
Abstract
Introduction. Since early May 2022, more than 90,000 cases of monkeypox virus infection have been reported in more than 70 countries around the World. This is the largest outbreak of monkeypox ever recorded outside of Africa.
The aim of the study is to confirm the first case of monkeypox in Russia, to isolate and sequence a new strain of monkeypox virus (MPXV), and to assess its sensitivity to the 7-[N-(4-trifluoromethylbenzoyl)-hydrazinocarbonyl]-tricyclo-[3.2.2.0^2,4]non-8-en-6-carboxylic acid (NIOCH-14) antipox drug.
Materials and methods. The biological materials obtained from the affected area of the skin (contents of vesicles), a nasopharyngeal smear, sputum and venous blood from a patient with suspected monkeypox were used. The disease was confirmed by PCR followed by determination of the nucleotide sequence of viral DNA by sequencing. Isolation of the new MPXV strain from clinical samples was carried out in Vero E6 cells. The antiviral effectiveness of NIOCH-14 against the new MPXV strain was assessed using an adapted spectrophotometric method.
Results. A diagnostic study of the biological samples of a patient who returned from a tourist trip to European countries with complaints of skin rashes all over the body revealed MPXV DNA. A new strain of MPXV was isolated from vesicles in Vero E6 cells, and the genomic sequence MPXV-pustule S45 was assembled using high-throughput parallel sequencing (NGS).
Discussion. The effectiveness of the finished dosage form of NIOCH-14 against the new strain of MPXV based on the results of determining the 50% virus inhibitory concentration (IC50) was 0.02 μg/mL, and the selectivity index (SI) was > 15,000.
Conclusion. In this study, the pathogen of monkeypox was detected and identified using real-time PCR, NGS and electron microscopy, and the first imported case of this disease in Russia was confirmed. It has been proven that the drug NIOCH-14 exhibits high antiviral activity in vitro against the new MPXV strain.
Full Text
Introduction
More than 60 years have passed since the discovery of the causative agent of a particularly dangerous zooanthroponous viral infection in humans, the monkeypox virus (MPXV), which belongs to the same Orthopoxvirus genus (Poxviridae family) as the smallpox virus and is highly lethal in humans (up to 17%) [1–3]. MPXV genomic sequencing has shown the existence of two branches of this virus: The West African clade and the Central African (Congo Basin) clade, each of which causes the MPXV disease, but the West African clade virus is considered less dangerous [4, 5]. Since its discovery, the disease has been endemic only to Central and West Africa [6–8]. However, since the beginning of May 2022, monkeypox has spread far beyond the African continent, prompting the World Health Organization to declare the 2022 outbreak a public health emergency of international concern1. Monkeypox is now spreading globally, with more than 90,000 infections already reported in more than 70 countries around the world. This is the largest outbreak of monkeypox ever seen in Africa and beyond.
Smallpox drugs play an important role in the control of monkeypox. A new specific drug for the treatment of monkeypox, Tecovirimat [9], an inhibitor of the viral protein VP37, has been approved in Europe, the United States, Canada, and several other countries. This drug is currently widely used for the therapy of this disease [10], but data on the identification of resistant MPXV variants to this drug have emerged [11–13]. The drug 7-[N-[N-(4-trifluoromethylbenzoyl)-hydrazinocarbonyl]-tricyclo-[3.2.2.0^2,4]non-8-en-6-carboxylic acid (NIOCH-14), which has a similar mechanism of action as Tecovirimat, has been registered in Russia [14].
Human monkeypox is no longer a rare disease and represents a public health problem, so it is important to have access to registered drugs that can effectively counteract this disease.
The aim of this study was to confirm the first reported case of MPXV in Russia, to isolate and sequencing an isolate of MPXV, and to assess its sensitivity to the domestic anti-monkeypox drug NIOCH-14.
Materials and methods
Cell culture
The Vero E6 cell culture obtained from the collection of cell cultures of the State Scientific Center of Virology and Biotechnology "Vector" was used. DMEM medium in the presence of 2% fetal bovine serum supplemented with penicillin (100 IU/mL) and streptomycin (100 µg/mL) was used as a maintenance medium for MPXV cultivation.
Patient, test samples
The patient was a male, 28 years old, with clinical signs of monkeypox, from whom material from the affected skin (contents of vesicles), nasopharyngeal swab and sputum, and venous blood was collected for the work. The study was conducted with voluntary informed consent of the patient. The study protocol was approved by the Ethical Committee of the State Scientific Center of Virology and Biotechnology "Vector" (protocol No. 5a dated 21.07.2022).
The work with MPXV was carried out at the Laboratory of Biosafety Level 4 of the State Scientific Center of Virology and Biotechnology "Vector".
Isolation, titration and culture of monkeypox virus
The sample with vesicle contents was diluted in 0.5 mL of DMEM medium supplemented with penicillin (100 IU/ml) and streptomycin (100 µg/mL) before addition to the Vero E6 cell culture monolayer. Venous blood, nasopharyngeal swab and sputum samples were used without additional dilution with medium before application to the cell monolayer. Supernatant of samples obtained after centrifugation at 700g for 10 min was added 50 μL to the wells of a 96-well plate with Vero E6 cell culture monolayer. The plates were incubated at 37ºC under 5% CO2 conditions and the appearance of cytopathic effect was monitored daily. Culture medium from the wells with the disrupted monolayer (passage 1) was collected and transferred to T-25 vials with the pre-grown Vero E6 cell culture monolayer for further incubation at 37ºC. When 80% cytopathic effect was reached in the cell monolayer, the vials were frozen/thawed 3 times and the resulting virus-containing suspension was clarified by centrifugation at 1200g for 10 min (passage 2). The virus-containing suspension of passage 2 was titrated by the plaque method in 24-well plates with Vero E6 cell culture monolayer and used for viral DNA extraction and subsequent analysis using high-throughput sequencing.
Obtaining whole genome nucleotide sequences
MPXV DNA was isolated from source material by phenol-chloroform method2.
The concentration of viral DNA was measured using the Qubit dsDNA HS Assay Kit (Qubit 3.0, Thermo Fisher Scientific), then the solution was used to prepare libraries for high-throughput sequencing on the Illumina platform. The Y-adapter ligation method (Illumina) was used to prepare libraries. Sequencing was performed on a MiSeq sequencer (Illumina) using the MiSeq Reagent Kit v2 (500-cycles) sequencing kit (Illumina).
Testing samples for the presence of MPXV DNA
Confirmatory diagnostic testing was performed using a reagent kit for detection of genetic markers (DNA) of orthopoxviruses, including MPXV, by real-time polymerase chain reaction (qPCR) using the Vector-MPCR rv-Ospa reagent kit for detection of DNA of smallpox, monkeypox, cowpox, and vaccinia viruses with hybridization-fluorescence detection in real time (Vector-MPCR rv-Ospa) (Vector, RZN No. 2016/3685).
Tested drug, inhibitor of orthopoxvirus replication
The domestic drug against MPXV NIOCH-14, series 010919, was used in the study [14].
Method for determination of antiviral activity of the preparation NIOCH-14 in vitro
Cytotoxicity and antiviral efficacy of NIOCH-14 (series 010919) were evaluated using the colorimetric method [15]. The wells of 96-well plates containing a monolayer of Vero E6 cells (~40 thousand cells per well) were firstly filled with 100 μL of serial dilutions of solutions prepared from the finished dosage form of NIOCH-14. Then 100 μL of MPXV dilution was added at a dose of 800 BOU/well (multiplicity of infection ~ 0.02 viral particles per cell), causing 100% cell destruction in the control monolayer without the drug, which occurs when cells are incubated with the virus 6 days after infection3. The cytotoxic activity of the drug was determined by its effect on cell destruction in the wells of the plate in which the virus was not introduced. Monolayers of cells in the wells of the plate, in which the virus was introduced without the drug (virus control) and monolayers of cells in the wells in which neither the virus nor the drug was introduced (cell control) were used as controls.
The capsule of the finished dosage form of the preparation NIOCH-14 was opened, the contents were poured into a vial. 10 mL of dimethyl sulfoxide was added to the contents of the capsule. The resulting solution was mixed in equal volumes with the nutrient medium with antibiotic. To evaluate cytotoxicity against Vero E6 cell culture and antiviral activity against MPXV, serial 3-fold dilutions of the drug were prepared, 8 dilutions were used starting from a concentration of 600 µg/mL. To evaluate the antiviral activity of the drug, 8 dilutions starting from 2 µg/mL were used.
NIOCH-14 dilutions were added in the volume of 100 µL into the wells of 96-well plates with cell culture. After incubation at 37ºC for 2 h (prophylactic scheme), 100 μL of culture medium was added to the wells to assess cytotoxicity, and 100 μL of MPXV dilution was added to assess antiviral activity. As a result, the total volume of liquid in each well was 200 µL, the initial concentration of the drug in the wells for cytotoxicity was 300 µg/mL, and for antiviral activity was 1.00 µg/mL.
After 6 days of incubation at 37ºC, the cell monolayer was stained with neutral red dye for 2 hours. After removing the dye and washing the wells from its unbound fraction, 0.1 mL of lysing buffer was added to release the dye from the absorbed cells. The optical density of the resulting solution, which depends on the number of cells in the monolayer that were not destroyed by the drug or virus, was measured using an Emax spectrophotometer (Molecular Devices) at a wavelength of 490 nm. Using the SoftMax 4.0 program (Molecular Devices) we calculated the 50% toxic concentration (TC50, µg/mL) of the drug, at which 50% of cells in the uninfected monolayer are destroyed, and 50% inhibitory concentration (IC50, µg/mL) of the drug, at which 50% of cells in the infected monolayer are not destroyed (remain viable). Based on TC50 and IC50, the selectivity index (SI) of the drug was determined: SI = TC50/IC50.
Electron microscopic examination
MPXV-infected Vero E6 cells were separated using a rubber spatula and fixed in an equal volume of 8% paraformaldehyde solution for 1 day. After centrifugation (1500 rpm, 10 min) and washing three times, the precipitate was further fixed with 1% OsO4 solution. Dehydration, impregnation and casting in epon-araldite mixture were performed according to the generally accepted method. Ultrathin sections were prepared on a microtome (Reichert-Jung), stained with uranyl acetate and lead citrate. Sections were examined in a JEM 1400 electron microscope (Jeol). Photography and image analysis were performed using a Veleta digital camera and iTEM software package (SIS).
Statistical and bioinformatics analysis of data
MPXV titer data were presented as mean value and its standard deviation (n = 4).
Bioinformatics analysis of virus fragment sequences was performed using MIRA v. 4.9.6, BWA v. 0.7.15, IGV v. 4.9.6, BWA v. 0.7.15, IGV v. 2.3.78, Samtools v. 1.3.1, Bcftools v. 1.62, SnpEff v. 5.2. Whole-genome sequence alignment was performed using the MAFFT algorithm v. 7.505. The MPXV genome MPXV-M5312_HM12_Rivers (NC_063383.1), belonging to the West African clade, was used as a reference nucleotide sequence. A phylogenetic tree was constructed using the neighbor-joining method (IQ-Treev.2.1.4, GTR+G+I nucleotide substitution model) using Orthopoxvirus reference (RefSeq) sequences (n = 9). In addition, all MPXV sequences available by July 2024 from samples collected between January 2018 and August 2022 were downloaded from the GISAID database. The total number of sequences used for analysis was 2,289. Genome regions with missing positions in the sequence analyzed were excluded from the analysis.
Results
Detection of MPXV in clinical samples
The study was conducted with clinical specimens obtained from a citizen of the Russian Federation who returned from a tourist trip to European countries — Spain, Portugal, etc. (where at that time there was an increase in the incidence of monkeypox. A few days after his return from abroad (08.07.2022), the patient went to a medical institution with complaints of a rash all over his body. Based on clinical symptoms and epidemiologic analysis, the patient was suspected to have monkeypox. Nasopharyngeal swab and sputum, as well as the contents of vesicles collected from the patient upon his admission to the medical institution and transferred to the laboratory of the State Scientific Center of Virology and Biotechnology "Vector" for PCR examination were positive for MPXV DNA content, which confirmed the diagnosis of monkeypox. At the same time, MPXV DNA was not detected in the patient's venous blood sample also submitted for testing (Table).
Presence of MPXV DNA in samples tested with the Vector-MPCR rv-Ospa reagent kit
Sample type | Indicator | Study results |
Vesicle contents | MPXV DNA | Found, Ct = 19.72 |
Nasopharyngeal swab and sputum | MPXV DNA | Found, Ct = 16.18 |
Venous blood | MPXV DNA | Not found, Ct > 40* |
Note. *According to the manufacturer's instructions, a Ct value > 40 is considered a negative test result.
MPXV DNA from patient samples was subjected to high-throughput sequencing and subsequent bioinformatics analysis. The genomic sequence MPXV-pustule S45 (sequence number vect2SM413009in the VGARus database) was assembled based on the reads obtained from sequencing of MPXV DNA isolated from the patient's clinical material. The length of the obtained sequence was 197,203 bp (98.77%), the average depth of coverage was 11.72, the number of undecoded nucleotides was 3,346, and the total number of reads per target genome was 27,168.
Phylogenetic analysis of the MPXV-pustule_S45 sequence (Fig. 1) showed that the studied MPXV isolate belongs to genetic clade IIb, lineage B.1. The MPXV sequences isolated in 2022 from patients from the USA, Peru, and Western European countries (Germany, Portugal and Ireland) are the most genetically similar to the investigated sample.
Fig. 1. Phylogenetic tree constructed using the maximum likelihood method.
Mpox sequences loaded from GISAID are highlighted on the circular cladogram. The position of the investigated sequence MPXV-pustule_S45 and its closest sequences is circled. A detailed cladogram is given.
Analysis of the nucleotide sequences of MPXV-pustule_S45 in comparison with the MPXV reference sequence NC_063383.1 showed the presence of 66 nucleotide substitutions, 32 of which are missense mutations, and one substitution leads to a frameshift (gene OPG055). Furthermore, the MPXV-pustule_S45 sequence examined contains an E353K mutation in the F13L protein (OPG057 gene, genomic position 39139). However, no mutations causing resistance to Tecovirimat were detected when analyzing MPXV-pustule_S45.
Isolation of monkeypox virus in cell culture
To isolate viable MPXV from clinical samples, samples with the contents of vesicles from a diseased person were used. Two days after inoculation of Vero E6 cell culture, a slight change in cell morphology was observed in comparison with uninfected control cell culture, which became manifest as cytopathic action on the 5th day after infection. Two consecutive passages were carried out to develop a working virus stock and deposit it in the State Collection of Viral Infectious Diseases and Rickettsioses Pathogens, functioning at the State Scientific Center of Virology and Biotechnology "Vector", as St. Petersburg-22 MPXV strain. The efflux titer of the virus in the culture fluid was 5.9 ± 0.3 log10 BOU/mL.
Electron microscopic examination
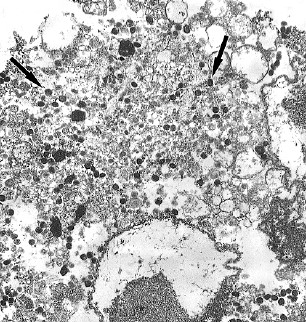
A sample of Vero E6 cells infected with the St. Petersburg-22 MPXV strain was examined using electron microscopy (Fig. 2).
Fig. 2. Vero E6 cells infected with a new St. Petersburg-22 MPXV strain.
a, b — 48 h after infection, the viral factory occupies most of the cell cytoplasm; viral particles are represented at all stages of the formation cycle (arrows); immature virions are prevalent (image b).
Images c–e — 72 h after infection, image c shows the prevalence of mature viral particles (arrows). In image d, the arrow indicates an immature viral particle. Image e shows the typical morphology of a mature orthopoxvirus virion.
Ultrastructural analysis of infected cells showed the presence of orthopoxvirus-specific viral factories, which after 48 h occupied most of the cytoplasm of infected Vero E6 cells. Viral particles, typical for orthopoxviruses, 48 h after infection of Vero E6 cells, were present at all stages of their formation cycle, with mainly immature virions predominating. At the same time, 72 h after infection of Vero E6 cells, mature viral particles were predominantly observed.
Antiviral activity of NIOCH-14 in in vitro experiments
The St. Petersburg-22 strain of MPXV was used to evaluate the antiviral efficacy of NIOCH-14. The results are presented in Fig. 3.
Fig. 3. Diagram of cytotoxicity (TC50 > 300 µg/mL) and antiviral activity of the finished dosage form of NIOCH-14 against the St.Petersburg-22 MPXV strain (IC50 = 0.02 µg/mL) in Vero E6 cell culture (SoftMax 4.0 program).
On the X-axis — drug concentration (µg/mL) in logarithmic scale of measurement; on the Y-axis — optical density (optical density units) in linear scale of measurement.
The TC50 for NIOCH-14 was > 300 µg/mL (> 731 µM) and the IC50 of NIOCH-14 for the St. Petersburg-22 strain of MPXV was 0.016 ± 0.009 µg/mL (0.039 ± 0.009 µM), whereas the SI was > 18,750.
Discussion
The monkeypox outbreak, which has been declared a public health emergency of international concern by WHO, has been spreading globally since early May 2022, affecting predominantly men who have sex with men. Cases have been reported in more than 70 countries worldwide, posing a public health threat of international concern. According to WHO recommendations, laboratories use samples from skin lesions, pharyngeal and nasopharyngeal swabs and blood for diagnostic tests4. In this case, the virus is detected most often in samples from skin lesions and less often in blood, which is probably due to the fact that viremia occurs during a very early and short period of infection and usually blood contains less virus than skin lesions [16]. Examination of samples (nasopharyngeal swabs, sputum, and vesicle contents) obtained from the first monkeypox patient registered in Russia also allowed us to detect MPXV DNA in nasopharyngeal swabs, sputum and vesicle contents, and to isolate a viable virus culture only from vesicle contents, while no virus was detected in the blood sample.
Sequencing data analysis of MPXV-pustule S45 sequence of the St. Petersburg-22 MPXV strain showed that it belongs to genetic clade IIb, lineage B.1. The genome of the St. Petersburg-22 strain is closely related to 19 MPXV isolates detected in summer and fall of 2022 in different countries.
According to studies, it is known that a person usually becomes infectious after the onset of disease symptoms [17]. However, recent studies on the spread of MPXV have established cases of virus excretion by patients without symptoms of the disease [18]. Thus, the infection of the first monkeypox patient in Russia, given that the incubation period of the disease can be up to 3 weeks, could have occurred through contact with both an asymptomatic patient and a previously ill patient, since the virus can be excreted with seminal fluid, oropharyngeal and anogenital secretions of the patient for some time after the disappearance of symptoms [19]. This is supported by the fact that contact with a monkeypox patient with a pronounced clinical picture of the disease was hardly possible due to the usually pronounced pain syndrome.
The first symptoms of monkeypox in a patient from Russia were diagnosed on 06.07.2022. Given the incubation period, which can range from 3 days to 3 weeks [20], his probable infection occurred during the period from mid-June to early July 2022. According to GISAID data, among the phylogenetically close sequences identified during this time period, the epidemiologically related to the MPXV strain St. Petersburg-22 patients could be those with the sequences hMpxV/USA/CA-CDPH-000009/2022 (identified on 06/30/2024), hMpxV/Ireland/D-NVRL-Z22IRL00145/2022 (identified on 06/17/2024) if, for example, patients were asymptomatic, or even hMpxV/Portugal/INSA-PT0018/2022 (identified on 06/01/2024), if infection occurred from a recently recovered individual. However, as previously indicated, infection most likely occurred through contact with an asymptomatic Mpox carrier. It is also interesting to note that the genetically closest sequences hMpxV/Peru/LIM-INS-020/2022 and hMpxV/USA/CA-LACPHL-MA00050/2022 were identified around the same time as the MPXV sequence from the first patient in Russia, namely July 12 and 20, 2022, respectively. It is possible that the patients from whom viruses with these sequences were obtained are linked to the Russian patient by a common source of infection.
To isolate a live culture of MPXV, Vero cell culture is traditionally used, which is highly sensitive to orthopoxviruses. The virus titers reach the level of 5 log10 TCID50/mL when cultured for 6 days [21]. MPXV isolated from biological samples obtained from the first Russian patient with monkeypox, when cultured in Vero cell culture, reached a titer of 5.9 log10 CFU/mL in the culture fluid already 5 days after infection of the cell monolayer. At the same time, ultrastructural analysis of the infected monolayer of Vero E6 cell culture demonstrated the presence of characteristic signs of virus multiplication in cells and the presence of viral particles of classical type, characteristic of MPXV, similar to the ultrastructural picture in infected cells detected by other researchers [21].
The widespread use of Tecovirimat for the treatment of monkeypox patients led to the emergence of information about the discovery of MPXV variants with drug resistance to this drug [13, 22]. Mutations in the F13L gene, homologous in orthopoxviruses, have been found to reduce the sensitivity of the virus to Tecovirimat [11]. The study of the genetic structure of the Mpox strain isolated by us did not establish the presence of known resistance mutations to Tecovirimat, and the evaluation of the strain's sensitivity to the domestic drug NIOCH-14 showed high inhibitory activity of this drug, comparable to that previously estimated by us for the reference MPXV strain (TC50 > 100 μg/mL, IC50 = 0.013 μg/mL, SI > 7700) [15]. This proves that the preparation NIOCH-14 has high antiviral activity in vitro against the St. Petersburg-22 MPXV strain detected in the first patient in Russia.
Conclusion
The approaches used in this study made it possible to confirm the first imported case of monkeypox in Russia, to isolate virus from biological samples, to characterize the culture and biological properties of the St. Petersburg-22 MPXV strain, and to deposit the strain in the State Collection of Viral Infectious Pathogens and Rickettsioses of the State Scientific Center of Virology and Biotechnology "Vector". Furthermore, studies of the antipox activity of the domestic drug NIOCH-14 showed that it exhibits high antiviral efficacy in vitro against the first MPXV strain identified in Russia and can be used for the treatment of monkeypox patients.
1 Multi-country monkeypox outbreak: situation update. World Health Organization, June 17, 2022. URL: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON393
Multi-country monkeypox outbreak: situation update. World Health Organization, June 27, 2022. URL: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON396
2 Organization of laboratory operations using nucleic acid amplification methods when working with materials containing microorganisms of pathogenicity groups I–IV: methodological guidelines. Moscow; 2010. 51 p.
3 Sergeev A.A., Kabanov A.S., Bulychev L.E., et al. Method for evaluating the activity of therapeutic and prophylactic agents against the smallpox virus. Patent 2522483 RF (A61K 35/76, A61P 31/12, C12N 7/00).
4 World Health Organization. Laboratory testing for the monkeypox virus. Interim guidance. URL: https://apps.who.int/iris/rest/bitstreams/1425052/retrieve (data of acсess: 23.05.2022).
About the authors
Alena S. Ovchinnikova
State Scientific Center of Virology and Biotechnology "Vector"
Author for correspondence.
Email: ovchinnikova_as@vector.nsc.ru
ORCID iD: 0000-0002-1745-7643
Cand. Sci. (Biol.), senior researcher, Department "Collection of Microorganisms"
Russian Federation, KoltsovoDmitrii A. Odnoshevsky
State Scientific Center of Virology and Biotechnology "Vector"
Email: ovchinnikova_as@vector.nsc.ru
ORCID iD: 0000-0003-0616-7488
researcher, Department "Collection of Microorganisms"
Russian Federation, KoltsovoAlexey S. Kabanov
State Scientific Center of Virology and Biotechnology "Vector"
Email: ovchinnikova_as@vector.nsc.ru
ORCID iD: 0000-0002-6287-0912
Cand. Sci. (Biol.), senior researcher, Department "Collection of Microorganisms"
Russian Federation, KoltsovoSergey A. Bodnev
State Scientific Center of Virology and Biotechnology "Vector"
Email: ovchinnikova_as@vector.nsc.ru
ORCID iD: 0000-0003-0599-3817
Cand. Sci. (Med.), leading researcher, Department "Collection of Microorganisms"
Russian Federation, KoltsovoOleg V. Pyankov
State Scientific Center of Virology and Biotechnology "Vector"
Email: ovchinnikova_as@vector.nsc.ru
ORCID iD: 0000-0003-3340-8750
Cand. Sci. (Biol.), Head, Department "Collection of Microorganisms"
Russian Federation, KoltsovoOksana P. Os'kina
State Scientific Center of Virology and Biotechnology "Vector"
Email: ovchinnikova_as@vector.nsc.ru
ORCID iD: 0000-0002-9165-1524
senior researcherm Biosafety department
Russian Federation, KoltsovoMaria V. Sivay
State Scientific Center of Virology and Biotechnology "Vector"
Email: ovchinnikova_as@vector.nsc.ru
ORCID iD: 0000-0002-4193-0779
Cand. Sci. (Biol.), senior researcher, Theoretical department
Russian Federation, KoltsovoAndrey V. Bespalov
State Scientific Center of Virology and Biotechnology "Vector"
Email: ovchinnikova_as@vector.nsc.ru
ORCID iD: 0000-0002-0281-3414
intern researcher, Theoretical department
Russian Federation, KoltsovoTatyana V. Tregubchak
State Scientific Center of Virology and Biotechnology "Vector"
Email: ovchinnikova_as@vector.nsc.ru
ORCID iD: 0000-0001-9608-2044
leading researcher, of the Department of genomic research
Russian Federation, KoltsovoLarisa N. Shishkina
State Scientific Center of Virology and Biotechnology "Vector"
Email: ovchinnikova_as@vector.nsc.ru
ORCID iD: 0000-0002-8264-0217
D. Sci. (Biol.), Head, Department of prevention and treatment of particularly dangerous infections
Russian Federation, KoltsovoOleg S. Taranov
State Scientific Center of Virology and Biotechnology "Vector"
Email: ovchinnikova_as@vector.nsc.ru
ORCID iD: 0000-0002-6746-8092
Head, Microscopic research department
Russian Federation, KoltsovoVladimir V. Zolin
State Scientific Center of Virology and Biotechnology "Vector"
Email: ovchinnikova_as@vector.nsc.ru
ORCID iD: 0000-0003-4120-1178
Cand. Sci. (Med.), Head, Biosafety department
Russian Federation, KoltsovoArtemiy A. Sergeev
State Scientific Center of Virology and Biotechnology "Vector"
Email: ovchinnikova_as@vector.nsc.ru
ORCID iD: 0000-0002-3591-1571
D. Sci. (Med.), Deputy Director General for scientific and epidemiological work
Russian Federation, KoltsovoAlexander P. Agafonov
State Scientific Center of Virology and Biotechnology "Vector"
Email: ovchinnikova_as@vector.nsc.ru
ORCID iD: 0000-0003-2577-0434
D. Sci. (Biol.), Director General
Russian Federation, KoltsovoReferences
- Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect. Dis. 2004;4(1):15–25. DOI: https://doi.org/10.1016/s1473-3099(03)00856-9
- Gispen R. Relevance of some poxvirus infections in monkeys to smallpox eradication. Trans. R. Soc. Trop. Med. Hyg. 1975;69(3):299–302. DOI: https://doi.org/10.1016/0035-9203(75)90122-4
- Jezek Z., Szczeniowski M., Paluku K.M., Mutombo M. Human monkeypox: clinical features of 282 patients. J. Infect. Dis. 1987;156(2):293–8. DOI: https://doi.org/10.1093/infdis/156.2.293
- Durski K.N., McCollum A.M., Nakazawa Y., et al. Emergence of monkeypox – West and Central Africa, 1970–2017. MMWR Morb. Mortal. Wkly Rep. 2018;67(10):306–10. DOI: https://doi.org/10.15585/mmwr.mm6710a5
- Hutin Y.J., Williams R.J., Malfait P., et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001;7(3):434–8. DOI: https://doi.org/10.3201/eid0703.010311
- Learned L.A., Reynolds M.G., Wassa D.W., et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am. J. Trop. Med. Hyg. 2005;73(2):428–34. DOI: https://doi.org/10.4269/ajtmh.2005.73.428
- Reynolds M.G., Emerson G.L., Pukuta E., et al. Detection of human monkeypox in the Republic of the Congo following intensive community education. Am. J. Trop. Med. Hyg. 2013;88(5):982–5. DOI: https://doi.org/10.4269/ajtmh.12-0758
- Sklenovská N., Van Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front. Public Health. 2018;6:241. DOI: https://doi.org/10.3389/fpubh.2018.00241
- Grosenbach D.W., Honeychurch K., Rose E.A., et al. Oral tecovirimat for the treatment of smallpox. N. Engl. J. Med. 2018;379(1):44–53. DOI: https://doi.org/10.1056/nejmoa1705688
- Hermanussen L., Brehm T.T., Wolf T., et al. Tecovirimat for the treatment of severe Mpox in Germany. Infection. 2023;51(5):1563–8. DOI: https://doi.org/10.1007/s15010-023-02049-0
- Smith T.G., Gigante C.M., Wynn N.T., et al. Tecovirimat resistance in mpox patients, United States, 2022–2023. Emerg. Infect. Dis. 2023;29(12):2426–32. DOI: https://doi.org/10.3201/eid2912.231146
- Mertes H., Rezende A.M., Brosius I., et al. tecovirimat resistance in an immunocompromised patient with mpox and prolonged viral shedding. Ann. Intern. Med. 2023;176(8):1141–3. DOI: https://doi.org/10.7326/l23-0131
- Garrigues J.M., Hemarajata P., Karan A., et al. Identification of tecovirimat resistance-associated mutations in human monkeypox virus – Los Angeles County. Antimicrob. Agents Chemother. 2023;67(7):e0056823. DOI: https://doi.org/10.1128/aac.00568-23
- Шишкина Л.Н., Шеремет О.П., Мадонов П.Г. и др. Пероральная лекарственная форма препарата в капсулах для лечения и профилактики заболеваний, вызываемых ортопоксвирусами. Патент РФ №2716709;2020. Shishkina L.N., Sheremet O.P., Madonov P.G., et al. Oral dosage form of the preparation in capsules for treating and preventing Orthopoxvirus-related diseases. Patent RF № 2716709; 2020. EDN: https://elibrary.ru/pkmxhn
- Кабанов А.С., Сергеев Ал.А., Булычев Л.Е. и др. Изучение противовирусной активности химически синтезированных соединений в отношении ортопоксвирусов в экспериментах in vitro. Проблемы особо опасных инфекций. 2013;(2):54–9. Kabanov A.S., Sergeev A.A., Bulychev L.E., et al. Studies of anti-viral activity of chemically synthesized compounds against Orthopoxviruses in vitro. Problemy osobo opasnykh infektsii. 2013;(2):54–9. EDN: https://elibrary.ru/qbzekx
- Álvarez Argüelles M.E., Martínez Z.P., Alba S.R., et al. Detecting, quantifying, and isolating monkeypox virus in suspected cases, Spain. Emerg. Infect. Dis. 2023;29(7):1465–9. DOI: https://doi.org/10.3201/eid2907.221229
- Yuan S., Jiang S.C., Zhang Z.W., et al. How and when does monkeypox (mpox) transmit: Implications for prevention and treatments. Front. Pharmacol. 2023;13:1109928. DOI: https://doi.org/10.3389/fphar.2022.1109928
- Brosius I., Van Dijck C., Coppens J., et al. Presymptomatic viral shedding in high-risk mpox contacts: A prospective cohort study. J. Med. Virol. 2023;95(5):e28769. DOI: https://doi.org/10.1002/jmv.28769
- Suñer C., Ubals M., Tarín-Vicente E.J., et al. Viral dynamics in patients with monkeypox infection: a prospective cohort study in Spain. Lancet Infect. Dis. 2023;23(4):445–53. DOI: https://doi.org/10.1016/s1473-3099(22)00794-0
- Thornhill J.P., Barkati S., Walmsley S., et al. Monkeypox virus infection in humans across 16 countries — April–June 2022. N. Engl. J. Med. 2022;387(8):679–91. DOI: https://doi.org/10.1056/nejmoa2207323
- Miranda M.D., Caldas G.C., Ferreira V.N., et al. Monkeypox (mpox) virus isolation and ultrastructural characterisation from a Brazilian human sample case. Mem. Inst. Oswaldo Cruz. 2023;118:e230090. DOI: https://doi.org/10.1590/0074-02760230090
- Lederman E.R., Davidson W., Groff H.L., et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J. Infect. Dis. 2012;206(9):1372–85. DOI: https://doi.org/10.1093/infdis/jis510
Supplementary files