Molecular genetic characteristics of Streptococcus pneumoniae serogroups 15 and 11 representatives circulating in Russia and their relationship with global genetic lineages
- Authors: Isaeva G.S.1,2, Tsvetkova I.A.3,4, Nikitina E.V.3, Zaripova A.Z.1,5, Bayazitova L.T.1,2, Isaeva R.A.1,2, Polev D.E.6, Saitova A.T.6, Kraeva L.A.6, Goncharov N.E.6, Kalinogorskaya O.S.3, Gordeeva S.A.7, Sidorenko S.V.3
-
Affiliations:
- Kazan State Medical University
- Kazan Research Institute of Epidemiology and Microbiology
- Pediatric Research and Clinical Center for Infectious Diseases
- St. Petersburg State Pediatric Medical University
- Center of Hygiene and Epidemiology in the Republic of Tatarstan (Tatarstan)
- Saint-Petersburg Pasteur Institute
- Clinical Infectious Diseases Hospital named after S.P. Botkin
- Issue: Vol 101, No 4 (2024)
- Pages: 483-501
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18539
- DOI: https://doi.org/10.36233/0372-9311-498
- EDN: https://elibrary.ru/gciets
- ID: 18539
Cite item
Abstract
Aim of the study. Genetic analysis of Streptococcus pneumoniae serogroups 15 and 11 circulating in Russia according to the following parameters: serotype affiliation; clonal complex (CC); presence of resistance and virulence determinants; relatedness to genetic lineages circulating in the world, and justification of inclusion of the actual serotypes of serogroups 15 and 11 in the future conjugate vaccine composition.
Materials and methods. The study included whole genome data of S. pneumoniae serogroups 11 and 15.
Results. Genomes of serogroup 15 strains from Russia are represented mainly by serotypes 15B and 15C, the majority of which belong to CC-1025 and CC-1262. CC-1025 is characterized by a more frequent association with invasive diseases. Representatives of CC-1025 and CC-1262 contain virulence determinants unique to these genetic lineages within the studied population of serogroup 15: oligopeptide transporters, fructose-specific PTS system, unique hydrolase variants, additional iron ion transporters, the gene of zinc metalloprotease ZmpC (activating human MMP9). The genomes of serogroup 11 are represented mainly by serotype 11A, the majority belong to CC-62 and CC-1012. The virulence determinants unique to CC-62 (within the studied serogroup 11) include bacteriocins, components of oligopeptide transport, flavin reductase-like protein (adhesin, also protects bacteria from oxidative stress), fucose processing operon, PsaA (adhesin, also a component of the ATP-binding cassette transporter that imports manganese ions).
Conclusion. In the Russian Federation, serogroups 15 and 11 are the most common non-vaccine serogroups. No antimicrobial resistance determinants have been identified in the genomes of representatives of these serogroups. For each of the genetic lineages prevalent in Russia and associated with serogroups 15 and 11, unique virulence determinants within the studied serogroup have been identified, which may contribute to the success of these lineages. It is advisable to include serotypes 15B and 11A in vaccines promising for the Russian Federation.
Full Text
Introduction
Invasive pneumococcal diseases (pneumonia, meningitis and sepsis) are the most common cause of mortality among children under 5 years of age and adults against the background of reduced immune defense [1, 2].
More than 100 serotypes of Streptococcus pneumoniae are known, some of which are highly virulent and capable of causing invasive pneumococcal infection. After the introduction of pneumococcal vaccination with conjugated polysaccharide vaccines into national childhood immunization programs, the previously widespread serotypes have been replaced by non-vaccine serotypes [3]. Two conjugated polysaccharide vaccines are approved for use in Russia: 10-valent (Synflorix, GlaxoSmithKline) and 13-valent (Prevenar 13, PCV13, Pfizer), as well as 23-valent polysaccharide vaccine (Pneumomax 23, Merk Sharp & Dohme). PCV13 is included in the national immunization schedule for vaccination of children.
Already early after the start of the national PCV13 vaccination program, a change in the serotype composition of the S. pneumoniae population among healthy children was observed, with the coverage of circulating serotypes by the PCV13 vaccine being about 50% [4]. Among the serotypes not covered by PCV13 vaccine, pneumococci of serogroups 15 and 11 predominate in vaccinated healthy children both in the early (2016-2018) [4] and late (2020–2022) periods after the start of vaccination [5–7]. It should be noted that serotypes 15BC and 11AD, which were not widespread in the pre-vaccination period, were found in children [8], as well as in adults [9, 10] with pneumococcal meningitis in the corresponding period [8].
In a pneumococcal population, there is often an association of a serotype with a particular genetic lineage - a group of closely related isolates belonging to one or more closely related clonal complexes (CC) or dominant sequencing types (ST). Populations of pneumococci of serogroups 15 and 11 have regional peculiarities. Thus, representatives of serogroup 15 are associated with genetic lineages CC-199 and CC-63 in the USA and Iceland, with CC-1025 and CC-1262 - in Russia (data from PubMLST database). Representatives of serogroup 11 are mainly associated with the ubiquitous genetic lineage CC-62, but the genetic lineage CC-1012 is also common in Russia. In some regions (Japan), an increase in the prevalence of multidrug-resistant strains of serotype 15A has been noted [11]. Thus, monitoring the antibiotic sensitivity of emerging epidemiologically significant genetic lineages is also important.
Due to the significant increase in the prevalence of serotypes of serogroups 15 and 11 among various population groups against the background of the widespread vaccination with PCV13, as well as due to their association with invasive diseases, the analysis of these strains is of fundamental and practical importance. In particular, identification of individual serotypes within these serogroups (since routine molecular typing methods do not allow differentiation of close serotypes), analysis of accumulated data on cross-immunogenicity of close serotypes, study of the invasive potential of genetic lineages associated with these serotypes — all this is important for determining the serotype composition of the future conjugated polysaccharide vaccine promising for Russia.
Objectives of the study — genetic analysis of S. pneumoniae serogroups 15 and 11 circulating in Russia according to the following parameters: serotype affiliation; clonal complex; presence of resistance and virulence determinants; relatedness to genetic lineages circulating in the world; presence of unique genes significant for virulence; justification of inclusion of the actual serotypes of serogroups 15 and 11 in the future conjugate vaccine composition.
Materials and methods
Sampling
The study included strains of serogroups 11 and 15 of S. pneumoniae from Russia for which full genomic data were available: isolates isolated at the Children's Research and Clinical Center for Infectious Diseases and the Botkin Clinical Infectious Diseases Hospital (St. Petersburg), Kazan Research Institute of Epidemiology and Microbiology (as part of the SAPIENS project). S.P. Botkin (St. Petersburg), Kazan Research Institute of Epidemiology and Microbiology (within the SAPIENS project), as well as full genomic data of isolates from different Russian cities obtained during the PEGAS study [10, 12].
The study was conducted with the voluntary informed consent of patients or their legal representatives. The study protocol was approved by the SAPIENS Ethical Committee (version 3.1 of 27.01.2020).
The choice of serotypes is explained by the significant spread of pneumococci belonging to these serotypes against the background of PCV13 vaccination, with only serotypes 11A and 15B included in the new PCV20 (Pfizer, currently not registered in Russia) and in Pneumomax 23. The selected isolates were isolated in different time periods (from 2001 to 2022) from carriers and patients with invasive diseases, from patients of different age groups. Two datasets were supplemented with full genomic data of S. pneumoniae strains isolated in different regions of the world — 23 strains for serogroup 11 dataset and 13 strains for serogroup 15 dataset. When selecting full-genomic data of S. pneumoniae from other regions of the world, the datasets included representatives of all available in the PubMLST database STs associated with the analyzed pneumococcal serotypes from different regions of the world with an interval of 1–4 years (depending on the prevalence).
Serogroup 15 dataset included genomes of 45 isolates: 32 from Russia and 13 from other regions of the world. The analysis included whole genome data from isolates obtained from various clinical samples: patients with meningitis (n = 11; source of isolation — liquor), pneumonia (n = 11; source of isolation: 10 — sputum, 1 — not specified), acute otitis media (n = 3; source of isolation: middle ear fluid), carriers (n = 20; source of isolation — nasopharynx).
Serogroup 11 dataset included genomes of 38 isolates: 15 from Russia and 23 from other regions of the world. The analysis included whole genome data from isolates obtained from various clinical samples: patients with meningitis (n = 3; source of isolation — liquor), pneumonia (n = 8; source of isolation — sputum), acute otitis media (n = 3; source of isolation — middle ear fluid), carriers (n = 20; source of isolation — nasopharynx), in 1 case there was no information about the diagnosis (source of isolation – blood). For 3 isolates there was no information about the diagnosis and source of isolation.
Whole genome sequencing
Whole genome sequencing (WGS) of pneumococcal isolates isolated in St. Petersburg or within the SAPIENS project was performed at the Pasteur Research Institute of Epidemiology and Microbiology. DNA was isolated from pure cultures of S. pneumoniae using the QIAamp DNA Mini Kit (Qiagen). WGS was performed on the DNBSEQ-G50 platform (MGI). Libraries for WGS were prepared using the MGIEasy Fast FS DNA Library Prep Set (MGI) according to the manufacturer's standard protocols. The median length of library fragments was 430 bp (identified using the QIAxcel Advanced system capillary gel electrophoresis system). Sequencing to obtain paired-end reads was performed on the DNBSEQ-G50 platform (MGI) using DNBSEQ-G50RS kits (FCL PE150/FCS PE150). Whole genome data of 11 S. pneumoniae isolates uploaded to GenBank (BioProject PRJNA971376, BioProject PRJNA1009429, BioProject PRJNA1076328, BioProject PRJNA1154393).
Bioinformatics analysis
For isolates sequenced at the Pasteur Research Institute of Epidemiology and Microbiology, the quality of the obtained nucleotide sequences was assessed using the program FastQC v. 0.11.8 (Babraham Bioinformatics). Quality filtering of reads and removal of PCR adapters and primers used in library preparation were performed using the program Cutadapt v. 1.15. For de novo genome assembly, we used the algorithm SPAdes v. 3.15.4. Final quality assessment was performed using the Quast v. 5.0.2 program. ST determination by MLST typing (Multilocus sequence typing) was performed using the MLST v. 2.0 program1. Genomes were annotated using RAST server (Rapid Annotations using Subsystems Technology). The serogroup and serotype affiliation of the strains were determined using the blastall program with an E-value threshold < 0.01. The obtained matches were filtered by bit-score and identity values. Searches were performed against a locally customized cps-locus sequence database of 90 serotypes. Genes and mutations associated with antibiotic resistance were identified against the CARD database [13]. Methods for nuclear genome and pan-genome analysis (R package micropan: Microbial Pan-Genome Analysis v. 2.1) were used to compare genomes [14]. Clusters of orthologs were identified based on distances calculated by pairwise comparison of amino acid sequences. The clustering was based on the complete-linkage clustering method, in which the distance between clusters is equal to the maximum distance between points from different clusters, with threshold distance criterion being 0.75. To identify associations of unique clusters of orthologs with genetic lineages, the presence/absence/variability statistics of genes in the genomes of the analyzed isolates were estimated using the Scoary v. 1.6.16 package2 [15].
Statistical analysis
For statistical processing we used the Scoary program, which allows us to obtain a list of genes significant for the corresponding trait, associated with the trait positively or negatively, sorted by p-values.
Results
To analyze the populations of S. pneumoniae serogroups 15 and 11 circulating in Russia and to characterize the genetic relationships between the genetic lines of serogroups 15 and 11 circulating in Russia and worldwide, pan-genome analysis was performed. For this purpose, two samples were formed, which included full genomic data of S. pneumoniae belonging to serogroups 15 and 11 from Russia and other regions of the world.
Analysis of S. pneumoniae serogroup 15
The study included full genomic data of 45 isolates of pneumococcus serogroup 15, including 32 isolates from different cities of Russia, as well as 13 isolates from other regions of the world (Table 1). Among the isolates of serogroup 15 isolated in Russia, 15 (46.9%) isolates belonged to serotype 15B, 12 (37.5%) to 15C, 3 (9.4%) to 15F, and 6 (6.3%) to 15A. Representatives of serotypes 15B/C were associated with 3 common STs (ST-1025, ST-199, ST-1262, of which only ST-199 is not found in Russia), as well as with rare STs. Serotypes 15A/F were associated predominantly with ST-63. ST-1025 isolates were isolated predominantly from sterile loci (isolation biomaterial — blood, liquor) and more frequently were associated with invasive diseases. Most isolates of this serogroup 15 were sensitive to antibiotics of different classes. Detailed characteristics of the analyzed isolates (ST, source of isolation, year of isolation, presence of antibiotic resistance determinants in the genomes, etc.) are presented in Table 1.
Table 1. Characteristics of serogroup 15 strains
Sample | РubMLST ID / ENA_accession | Country | Region | Year of isolation | Serotype | ST | Patient's age, years | Diagnosis | Source of isolation | Penicillin | Erythromycin | Tetracycline | Chloramphenicol | Co-trimoxazole |
PEGAS-5-1079 | R | Yaroslavl | 2016 | 15B | 1025 | 11 | MNG | CSF | S | S | S | S | R | |
PEGAS-5-1659 | R | Yaroslavl | 2017 | 15B | 1262 | 2 | MNG | CSF | S | S | S | S | S | |
PEGAS-2019-106 | R | Yaroslavl | 2019 | 15B | 1262 | 1 | MNG | CSF | S | S | S | S | S | |
PEGAS-2019-269 | R | Yaroslavl | 2019 | 15B | 1025 | 0,2 | MNG | CSF | S | S | S | S | R | |
PEGAS-2019-73 | R | Yaroslavl | 2019 | 15B | 1025 | 78 | PN | CSF | S | S | S | S | R | |
PEGAS-5-638 | R | Smolensk | 2016 | 15B | 1025 | 50 | MNG | CSF | S | S | S | S | R | |
PEGAS-2019-184 | R | Smolensk | 2019 | 15F | 6202 | 52 | MNG | CSF | S | S | S | S | S | |
PEGAS-2019-237 | R | Smolensk | 2019 | 15C | 1025 | 63 | PN | SP | S | S | S | S | R | |
PEGAS-2020-201 | R | Yuzhno-Sakhalinsk | 2020 | 15C | 1025 | 23 | PN | SP | S | S | S | S | R | |
PEGAS-2019-213 | R | Yuzhno-Sakhalinsk | 2019 | 15C | 16380 | 2 | PN | SP | R | S | S | S | R | |
PEGAS-2020-146 | R | Kirov | 2020 | 15C | 1262 | 1 | PN | SP | S | S | S | S | S | |
PEGAS-2019-343 | R | Seversk | 2019 | 15A | 12518 | 55 | PN | SP | S | S | S | S | S | |
PEGAS-2019-347 | R | Seversk | 2019 | 15C | 16349 | 70 | PN | SP | S | S | S | S | R | |
PEGAS-2019-373 | R | Tomsk | 2019 | 15C | 1262 | 3 | PN | SP | S | S | S | S | S | |
PEGAS-2019-375 | R | Tomsk | 2019 | 15B | 1262 | 86 | PN | SP | S | S | S | S | S | |
PEGAS-2019-390 | R | Tomsk | 2019 | 15C | 1262 | 61 | PN | SP | S | S | S | S | S | |
PEGAS-2020-229 | R | Tolyatti | 2020 | 15F | 16421 | 45 | PN | SP | S | S | S | S | S | |
ST_12518_2 | ERR1788193 | R | Moscow | 2014 | 15A | 12518 | 5 | PHR | NPS | S | S | S | S | S |
ST_3201_3 | ERR1788219 | R | Moscow | 2015 | 15B | 3201 | 2 | – | NPS. | R | S | S | S | R |
ST_1262_2 | ERR1788207 | R | Moscow | 2013 | 15B | 1262 | 5 | – | NPS | S | S | S | S | R |
ST_1262_3 | ERR1788225 | R | Moscow | 2015 | 15B | 1262 | 5 | PHR | NPS | S | S | S | S | R |
ST_1025_5 | ERR1788208 | R | Moscow | 2014 | 15C | 1025 | 5 | PHR | NPS | S | S | S | S | R |
ST_3557_1 | ERR1788206 | R | Moscow | 2013 | 15B | 3557 | 2 | PHR | NPS | R | S | R | S | R |
6_2F1 | PRJNA1154393 | R | Moscow | 2011 | 15F | 6202 | – | NPS | S | S | S | S | S | |
27_Kz | PRJNA971376 | R | Kazan | 2020 | 15C | 1025 | 3 | – | NPS | S | S | S | S | R |
12001 | PRJNA1076328 | R | Saint-Petersburg | 2016 | 15B | 1262 | 3 | – | NPS | S | S | S | S | S |
12456 | PRJNA1076328 | R | Saint-Petersburg | 2016 | 15B | 1025 | 5 | – | NPS | S | S | S | S | R |
108 | PRJNA1154393 | R | Saint-Petersburg | 2021 | 15C | 1349 | MNG | CSF | R | S | S | S | R | |
76_B | PRJNA1076328 | R | Saint-Petersburg | 2021 | 15B | 1025 | 44 | MNG | CSF | S | S | S | S | R |
137_B | PRJNA1076328 | R | Saint-Petersburg | 2022 | 15C | 1025 | 38 | MNG | CSF | S | S | S | S | R |
138_B | PRJNA1076328 | R | Saint-Petersburg | 2022 | 15C | 1025 | 38 | MNG | CSF | S | S | S | S | R |
336_B | PRJNA1076328 | R | Saint-Petersburg | 2022 | 15B | Unkn_21 | 64 | MNG | CSF | S | S | S | S | S |
ST_63_3 | ERR065297 | U | Massachusetts | 2004 | 15A | 63 | 6 | – | NPS | R | R | S | R | S |
ST_63_4 | ERR068032 | U | Massachusetts | 2004 | 15A | 63 | 6 | – | NPS | R | R | S | R | R |
ST_63_5 | ERR069724 | U | Massachusetts | 2004 | 15A | 63 | 6 | – | NPS | R | R | S | R | S |
ST_199_1 | ERR069751 | U | Massachusetts | 2001 | 15C | 199 | 2 | – | NPS | S | S | S | S | S |
ST_199_2 | ERR069691 | U | Massachusetts | 2004 | 15B | 199 | 2 | – | NPS | S | S | S | S | S |
ST_199_3 | ERR069774 | U | Massachusetts | 2001 | 15C | 199 | 2 | – | NPS | S | S | S | S | S |
ST_199_4 | ERR065975 | U | Massachusetts | 2001 | 15B | 199 | 2 | – | NPS | S | S | S | S | S |
ST_199_11 | ERR540653 | I | Reykjavik | 2010 | 15B | 199 | 2 | – | NPS | S | S | S | S | S |
ST_199_16 | ERR755466 | I | Reykjavik | 2013 | 15C | 199 | 2 | ОM | MEF | S | S | S | S | S |
ST_199_17 | ERR755326 | I | Reykjavik | 2013 | 15B | 199 | 3 | ОM | MEF | S | S | S | S | S |
ST_199_13 | ERR470151 | I | Koupavogur | 2009 | 15C | 199 | 4 | – | NPS | S | S | S | S | S |
ST_199_18 | ERR755336 | I | Habnarfjordur | 2013 | 15B | 199 | 2 | ОM | MEF | S | S | S | S | S |
ST_199_21 | ERR755384 | I | Habnarfjordur | 2014 | 15C | 199 | 4 | – | NPS | S | S | S | S | S |
Note. MNG — meningitis; PN — pneumonia; Phr — pharyngitis; OM — otitis media; CSF — cerebrospinal fluid; SP — sputum; NPS — nasopharyngeal smear; MEF — middle ear fluid; R/S — presence/absence of determinants of resistance (source: Prediction of antimicrobial resistance in PATRIC and RAST, URL: https://www.bv-brc.org/job).
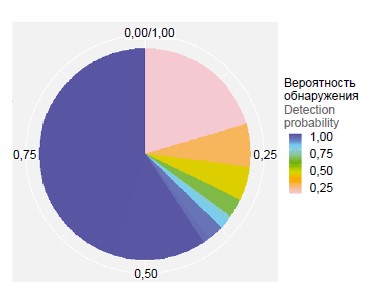
The pan-genome of S. pneumoniae isolates of serogroup 15 was characterized by comparing all proteins (blast-all-all). In representatives of serogroup 15 the share of the main (conserved) part of the genome was 59.8% — 1286 genes were present in all genomes of the analyzed sample (Fig. 1). In the population of serogroup 15, 2097 clusters of orthologs were identified, the most numerous cluster was represented by 296 proteins. The pan-genome of pneumococcus serogroup 15 isolates belongs to the closed pan-genome (alpha index value > 1), and its size approaches a constant as more genomes are used (Hipps' law) [14]. This may indicate that the genome diversity of serogroup 15 representatives has reached saturation, regardless of the time period and geographic region of isolates isolation, as well as their belonging to the genetic lineage.
Fig. 1. Distribution of gene families of the pan-genome of S. pneumoniae serogroup 15 strains. The color of the sector reflects the probability of identification of the gene family in the genomes of isolates. The blue color shows highly conservative («core genome») gene families. For a color version of the picture, see the journal’s website.
All representatives of the genetic lineage ST-1025 are associated with a homogeneous dendrogram cluster describing the relationship between strains based on pan-genome analysis and taking into account both the presence or absence and homology of available amino acid sequences (Fig. 2). All ST-1025 representatives contain in their genomes a unique operon encoding oligopeptide transporter components. Furthermore, ST-1025 representatives contain in their genomes a unique operon encoding components of the fructose-specific phosphotransferase transport system (PTS). ST-1025 isolates also contain unique variants of hydrolases, iron ion transporters, and the zinc metalloprotease gene ZmpC (Table 2).
Fig. 2. A dendrogram describing the clustering of S. pneumoniae isolates of serogroup 15 by pan-genome R micropan analysis (presence/absence and gene homology).
Table 2. Unique proteins of the СС-1025 genetic lineage representatives*
Sequence ID | Homology with known proteins, % | Protein name | Proposed function |
27_Kz_seq27 | 100 | ABC iron (III) transporter, permease | Transport of iron III+ ions |
27_Kz_seq161 | 96 | ABC transporter, permease | Transport of iron III+ ions |
27_Kz_seq266 | 97,9 | Membrane succinate permease DctA, sodium symporter | Transport of dicarboxylic acids |
27_Kz_seq792 | 100 | Component IIC of the phosphotransferase system (PTS) | Protein-N(PI)-phosphohistidine-fructose-PTS |
27_Kz_seq793 | 99 | Component IIB of the PTS | |
27_Kz_seq794 | 100 | Component IIA of the PTS | |
27_Kz_seq795 | 100 | Hypothetical nitrogen regulatory protein IIA of the PTS system | |
27_Kz_seq796 | 99,9 | A hypothetical transcription antiterminator of the BglG family | |
27_Kz_seq1007 | 100 | High affinity permease Fe2+/Pb2+ | Ferrum ions transport |
27_Kz_seq1008 | 99,7 | DyP-type peroxidase (IPR006314) | DyP proteins have characteristics that distinguish them from other peroxidases: broad substrate specificity, lack of homology with most other peroxidases, and the ability to function well under conditions of lower pH values |
27_Kz_seq1359 | 99,9 | Zinc-dependent metalloproteinase ZmpC | Cleaves and activates human matrix metalloproteinase-9. The role in the virulence and pathogenicity of pneumococcus in the lungs |
27_Kz_seq1361 | 100 | Hypothetical acetyltransferase | Unknown |
27_Kz_seq1489 | 100 | N-acetylneuramic acid epimerase | Mutarotation of sialic acids. The presence of sialic acids in the elements of the bacterial cell surface helps them evade the innate immune response of the host |
27_Kz_seq1490 | 100 | Substrate-binding subunit AppA, ABC component of the oligopeptide transporter | Transport of oligopeptides |
27_Kz_seq1494 | 99,8 | Hypothetical glycosylhydrolase family 32 | Unknown |
Note. *These proteins are encoded in the genomes of 13 isolates: 556_PEGAS_2019_269, 573_PEGAS_2019_73, 594_PEGAS_2019_237, 601_PEGAS_2019_347, 636_PEGAS_2020_201, 76_B, MiSeq_27_Kz, ST_1025_5, 12456, 137_B, 138_B, 521_PEGAS_5_1079, 526_PEGAS_5_638)
Along with ST-1025, the prevalence of ST-1262 may be associated with the presence in the genomes of its representatives of factors that provide higher adaptability to stress conditions (Table 3).
Table 3. Unique proteins of the CC-1262 genetic lineage representatives*
Sequence ID | Homology with known proteins, % | Protein name | Proposed function |
552_PEGAS_2019_106_seq440 | 100 | Phage shock protein PspC | The integrity of the inner membrane in response to extracytoplasmic stress conditions |
552_PEGAS_2019_106_seq590 | 100 | Satellite phage hypothetical protein (Streptococcus satellite phage Javan725) | Prophage component |
552_PEGAS_2019_106_seq591 | 100 | Satellite phage hypothetical protein (Streptococcus satellite phage Javan296) | Prophage component |
552_PEGAS_2019_106_seq592 | 100 | Primase C-terminal 1 domain-containing protein | Prophage component |
552_PEGAS_2019_106_seq624 | 100 | Methionine tRNA ligase | The initiation of protein synthesis |
552_PEGAS_2019_106_seq686 | 98,6 | ABC transporter, ATP-binding subunit, GlnQ | Transport of glutamine |
552_PEGAS_2019_106_seq915 | 99 | Superfamily 2 helicase | Unknown |
552_PEGAS_2019_106_seq1038 | 99,4 | O-acetylhomoserine aminocarboxypropyltransferase | Synthesis of methionine |
552_PEGAS_2019_106_seq1080 | 91 | AAA ATPase | ATP hydrolysis |
552_PEGAS_2019_106_seq1081 | 85 | Serine protease | Possible signaling function |
552_PEGAS_2019_106_seq1112 | 100 | Hypothetical macrolide efflux transporter | Possible macrolide efflux |
552_PEGAS_2019_106_seq1113 | 100 | Hypothetical protein | Unknown |
552_PEGAS_2019_106_seq1114 | 100 | Group I pyridoxal-dependent decarboxylase (cleaves Orn/Lys/Arg and glycine) | Amino acid metabolism |
Note. *These proteins are encoded in the genomes of 10 isolates: PEGAS_2019_106, 605_PEGAS_2019_373, 607_PEGAS_2019_375, 609_PEGAS_2019_390, 12001, 625_PEGAS_2020_146, ST_1262_2, ST_1262_3, 534_PEGAS_5_1659, 552_PEGAS_2019_106
Analysis of S. pneumoniae serogroup 11
The sample of serogroup 11 representatives included full genomic data of 15 isolates from different cities of Russia, as well as 23 isolates from other regions of the world. Among the isolates of serogroup 11 isolated in Russia, 13 (86.7%) isolates belonged to serotype 11A and 2 (13.3%) to serotype 11D. Representatives of serogroup 11 were associated with two common genetic lineages: CC-62 (circulating ubiquitously) and CC-1012, as well as with rare STs. Isolates belonging to CC-62 were isolated predominantly from the nasopharynx. Isolates belonging to CC-1012 were frequently associated with invasive diseases (biomaterial of isolation was liquor). Most isolates of serogroup 11 were sensitive to antibiotics of different classes (Table 4).
Table 4. Characteristics of serogroup 11 strains
Sample | PubMLST / ENA_accession number | Сountry | Region | Isolation year | Serotype | ST | Patient's age, years | Diagnosis | Source of isolation | Penicillin | Erythromycin | Tetracycline | Chloramphenicol | Co-trimoxazole |
PEGAS-2019-401 | Russia | Krasnodar | 2019 | 11A | 1012 | 61 | MNG | CSF | S | S | S | S | S | |
PEGAS-2019-64 | Russia | Yaroslavl | 2019 | 11A | 156 | 66 | PN | SP | S | S | R | R | R | |
PEGAS-2019-113 | Russia | Smolensk | 2019 | 11A | 1012 | 57 | PN | SP | S | S | S | S | S | |
PEGAS-2019-344 | Russia | Seversk | 2019 | 11D | 62 | 67 | PN | SP | S | S | S | S | S | |
PEGAS-2019-349 | Russia | Seversk | 2019 | 11A | 1012 | 85 | PN | SP | S | S | S | S | S | |
PEGAS-2020-149 | Russia | Kirov | 2020 | 11A | 6191 | 62 | PN | SP | S | S | S | S | R | |
PEGAS-2020-150 | Russia | Kirov | 2020 | 11A | 62 | 1 | PN | SP | S | S | S | S | S | |
PEGAS-2020-226 | Russia | Tolyatti | 2020 | 11A | 62 | 34 | PN | SP | S | S | S | S | S | |
PEGAS-2019-114 | Russia | Moscow | 2019 | 11A | 1012 | 72 | PN | SP | S | S | S | S | S | |
ST_62_27 | ERR1788222 | Russia | Moscow | 2012 | 11A | 62 | 5 | – | NPS | S | S | S | S | S |
ST_62_28 | ERR1788215 | Russia | Moscow | 2014 | 11A | 62 | 5 | PhR | NPS | S | S | S | S | S |
ST_1012_3 | ERR1788171 | Russia | Moscow | 2013 | 11A | 1012 | 3 | MNG | CSF | S | S | S | S | S |
ST_1012_4 | ERR1788140 | Russia | Moscow | 2011 | 11A | 1012 | 3 | MNG | CSF | S | S | S | S | S |
105_Kz | PRJNA1009429 | Russia | Kazan | 2020 | 11D | 62 | 4 | – | NPS | S | S | S | S | S |
25_B | PRJNA1076328 | Russia | Saint Petersburg | 2021 | 11A | 1050 | 60 | – | BL | S | S | S | S | S |
ST_62_3 | ERR069801 | USA | Massachusetts | 2001 | 11A | 62 | 2 | – | NPS | S | S | S | S | S |
ST_62_4 | ERR069822 | USA | Massachusetts | 2001 | 11A | 62 | 3 | — | NPS | S | S | S | S | S |
ST_62_5 | ERR065964 | USA | Massachusetts | 2001 | 11A | 62 | 3 | — | NPS | S | S | S | S | S |
ST_62_6 | ERR069804 | USA | Massachusetts | 2001 | 11A | 62 | 6 | – | NPS | S | S | S | S | S |
ST_62_7 | ERR065326 | USA | Massachusetts | 2004 | 11A | 62 | 2 | — | NPS | S | S | S | S | S |
ST_62_8 | ERR069707 | USA | Massachusetts | 2004 | 11A | 62 | 2 | – | NPS | S | S | S | S | S |
ST_62_9 | ERR069727 | USA | Massachusetts | 2004 | 11A | 62 | 2 | – | NPS | S | S | S | S | S |
ST_62_10 | ERR065310 | USA | Massachusetts | 2004 | 11A | 62 | – | NPS | S | S | S | S | S | |
ST_62_11 | ERR124268 | USA | Massachusetts | 2007 | 11A | 62 | 6 | – | NPS | S | S | S | S | S |
ST_62_12 | ERR129079 | USA | Massachusetts | 2007 | 11A | 62 | 6 | – | NPS | S | S | S | S | S |
ST_62_13 | ERR129211 | USA | Massachusetts | 2007 | 11A | 62 | 6 | – | NPS | S | S | S | S | S |
ST_62_14 | ERR129131 | USA | Massachusetts | 2007 | 11A | 62 | 6 | – | NPS | S | S | S | S | S |
ST_62_15 | ERR470324 | Iceland | Reykjavik | 2009 | 11A | 62 | 3 | – | NPS | S | S | S | S | S |
ST_62_16 | ERR449847 | Iceland | Reykjavik | 2009 | 11A | 62 | 65 | PN | NA | S | S | NA | NA | NA |
ST_62_20 | ERR470201 | Iceland | Reykjavik | 2010 | 11A | 62 | 11 | OM | MEF | S | S | S | S | S |
ST_62_21 | ERR540645 | Iceland | Reykjavik | 2010 | 11A | 62 | 5 | – | NPS | S | S | S | S | S |
ST_62_22 | ERR540483 | Iceland | Reykjavik | 2010 | 11A | 62 | 60 | PN | NA | S | S | S | S | S |
ST_62_17 | ERR470261 | Iceland | Mosfellsbaer | 2009 | 11A | 62 | 17 | OM | MEF | S | S | S | S | S |
ST_62_18 | ERR449827 | Iceland | Mosfellsbaer | 2009 | 11A | 62 | 42 | PN | NA | S | S | S | S | S |
ST_62_19 | ERR470192 | Iceland | Selfoss | 2010 | 11A | 62 | 1 | OM | MEF | S | S | S | S | S |
ST_62_23 | ERR755493 | Iceland | Hafnarfjörður | 2014 | 11A | 62 | 5 | – | NPS | S | S | S | S | S |
ST_62_24 | ERR755501 | Iceland | Hafnarfjörður | 2014 | 11A | 62 | 5 | – | NPS | S | S | S | S | S |
ST_62_26 | ERR755548 | Iceland | Kopavogur | 2014 | 11A | 62 | 6 | – | NPS | S | S | S | S | S |
Note. MNG — meningitis; PN — pneumonia; Phr — pharyngitis; OM — otitis media; CSF — cerebrospinal fluid; SP — sputum; NPS — nasopharyngeal smear; MEF — middle ear fluid; R/S — presence/absence of determinants of resistance (source: Prediction of antimicrobial resistance in PATRIC and RAST. URL: https://www.bv-brc.org/job).
End of the Table 4
Pan-genome analysis of S. pneumoniae isolates of serogroup 11 showed a higher degree of genome heterogeneity in this group (Fig. 3). The share of the main (conserved) part of the genome was 36% — 820 genes were present in all genomes of the analyzed sample (Fig. 3). In the population of serogroup 11, 1864 clusters of orthologs were identified, the most numerous cluster was represented by 191 proteins. The pan-genome of the pneumococcal isolates of serogroup 11 serogroup 11 belonged to the open pan-genome — the alpha index value < 1 (0.82), i.e. the pan-genome size of this group should increase, as more genomes are included in analysis. This may indicate greater variability of genomes of this group and greater diversity of the additional part of the genome of representatives of serogroup 11 (Fig. 4), their potentially greater adaptability. This fact is consistent with the high prevalence of CC-62 in different regions of the world in different periods of time.
Fig. 3. Distribution of gene families of the pan-genome of S. pneumoniae serogroup 11 strains. The color of the sector reflects the probability of identification of the gene family in the genomes of isolates. The blue color shows highly conservative («core genome») gene families. For a color version of the picture, see the journal’s website.
Fig. 4. Dendrogram describing the clustering of S. pneumoniae serogroup 11 isolates by pan-genome R micropan analysis (presence/absence and gene homology).
SS-62 representatives contain in their genomes a unique operon encoding the synthesis of bacteriocin involved in interspecific competition, oligopeptide transporter components, and flavin reductase-like protein that promotes adhesion and protects the bacterium from oxidative stress, which increases the virulence of the microorganism (Table 5). Also, all representatives of SS-62 contain a fucose processing operon and PsaA (a component of the ATP-binding cassette transporter that imports manganese ions and is also an adhesin).
Table 5. Unique proteins of the serogroup 11 genetic lineages representatives
ID последовательности Sequence ID | Homology with known proteins, % | Protein name | Proposed function |
СС-62* — 29 isolates | |||
GID11_seq178 | 100 | Bacteriocin | Interspecific competition |
GID11_seq180 | 87,5 | Transposase ISSmu1 | Prophage component |
GID11_seq303 | 98,8 | O6-methylguanine DNA methyltransferase | DNA repair. Maintaining the stability of the genome |
GID11_seq357 | 100 | L-fuculose phosphate aldolase | Metabolism of fucose |
GID11_seq358 | 99,3 | RbsD/FucU family transport protein | |
GID11_seq359 | 98,6 | Enzyme IIA component of the phosphotransferase system (PTS) | |
GID11_seq363 | 99,6 | Hypothetical protein | Unknown |
GID11_seq364 | 99,8 | F5/8 type C domain-containing protein | It can act as a protective agent. Possibly, regulation of complement activation (lectin pathway) |
GID11_seq373 | 56 | Pneumococcal surface protein A-like protein | An adhesive and a component of an ATP-binding cassette conveyor importing manganese ions. It is possible that PsaA, like many other virulence factors, performs two functions during infection: direct adhesion and participation in the absorption of manganese |
GID11_seq740 | 97,7 | Hypothetical helicase | Unknown |
GID11_seq974 | 51,8 | ABC transporter, permease | Transport |
GID11_seq975 | 52,7 | ABC transporter, ATP-binding subunit | |
GID11_seq976 | 43,3 | ArsR family transcriptional regulator | |
GID11_seq1078 | 96,9 | Superfamily II group DNA or RNA helicases | Possible regulation of expression |
GID11_seq1083 | 100 | Flavin reductase-like domain-containing protein | Flavin reductase is present on the surface of pneumococci. It promotes virulence by protecting against oxidative stress and mediating adhesion |
GID11_seq1103 | 95,5 | Transcription regulator BlpS | The domain binding to DNA |
GID11_seq1185 | 28,8 | Component of the antimicrobial peptides ABC transport system | Interspecific competition |
GID11_seq1585 | 28 | HECT domain containing protein | Ubiquitin-protein ligases — protein utilization |
CC-1012** — 6 isolates | |||
GID12_seq99 | 100 | Guanosine triphosphate cyclohydrolase | The opening of the imidazole ring of guanosine triphosphate is catalyzed. An obligatory stage of biosynthesis of a variety of coenzymes (riboflavin and folate), tRNA bases |
GID12_seq198 | 100 | Hypothetical macrolide efflux protein | Possible macrolide efflux |
GID12_seq199 | 99,8 | Hypothetical protein | Unknown |
GID12_seq200 | 100 | Group I pyridoxal-dependent decarboxylase (cleaves Orn/Lys/Arg and glycine) | Amino acid metabolism |
GID12_seq887 | 98,3 | Competence system transport protein | Natural competence system |
GID12_seq1238 | 87,9 | DNA-binding protein of the satellite phage Streptococcus satellite phage Javan359 | Prophage component |
GID12_seq1240 | 100 | Hypothetical satellite prophage protein Streptococcus satellite phage Javan735 | Prophage component |
GID12_seq1279 | 91,4 | Argininosuccinate synthetase, rgG | Amino acid biosynthesis; L-arginine biosynthesis (L-arginine from L-ornithine and carbamoyl phosphate |
GID12_seq1281 | 98,4 | Bacteriocin-like peptide | |
Note. *The ST62 group: 642_PEGAS_2020_226, MiSeq_105_Kz, ST_62_10, ST_62_11, ST_62_12, ST_62_13, ST_62_14, ST_62_15, ST_62_16, ST_62_17, ST_62_18, ST_62_19, ST_62_20, ST_62_21, ST_62_22, ST_62_23, ST_62_24, ST_62_26, ST_62_27, ST_62_28, ST_62_3, ST_62_4, ST_62_5, ST_62_6, ST_62_7, ST_62_8, ST_62_9, 600_PEGAS_2019_344, 629_PEGAS_2020_150.
**The ST1012 group: ST_1012_3, ST_1012_4, 561_PEGAS_2019_401, 581_PEGAS_2019_114, 589_PEGAS_2019_113, 602_PEGAS_2019_349.
End of the Table 5
Representatives of the SS-1012 genetic lineage are less common, also mostly associated with serotype 11A, but isolated mainly from liquor and sputum. The unique features of this genetic lineage include the presence of the Streptococcus satellite phage Javan359. Representatives of SS-1012 have a bacteriocin unique to this genetic lineage. Also, SS-1012 isolates may have peculiarities of amino acid synthesis and riboflavin biosynthesis, which may be related to virulence, but this assumption needs to be verified in additional studies.
Discussion
Since the introduction of PCV-13 into national immunization schedules, reports of increased circulation of S. pneumoniae serogroup 15, which is not covered by PCV13, have begun to appear [16–18]. 15B is one of the serotypes currently associated with relatively high mortality rates [19–22], development of invasive forms, particularly meningitis [23, 24]. According to recently published results of Chinese researchers, the most common circulating among children in China is pneumococcal serogroup 15 [25]. In Russia there is also a tendency of expansion of this serogroup [5, 6]. According to the results of our analysis, the two most common genetic lineages of serogroup 15 circulating in Russia, CC-1025 and CC-1262, are often associated with invasive diseases. Isolates of CC-1025 and CC-1262 are represented by serotypes 15B/C and have genetic determinants that may contribute to better adaptation and success of these genetic lineages and may potentially be associated with virulence (Tables 2, 3). In particular, oligopeptide transporters, in addition to transporting bacteriocins and chemokines, may be associated with the regulation of the expression of choline-binding proteins [26, 27]. A unique variant of fructose-specific PTS may also contribute to the selection of ST-1025 representatives in carriers on the background of vaccination due to energetic advantages. The zinc metalloprotease ZmpC specifically cleaves and activates human matrix metalloproteinase-9, which in turn degrades components of the extracellular matrix [28]. All ST-1262 strains contain a gene encoding a peptide that accounts for resistance to abortive phage infection (Table 3). As part of the satellite prophage, all representatives of ST-1262 have a gene encoding a phage shock protein that ensures the integrity of the cell inner membrane in response to extracytoplasmic stress conditions. It is possible that ST-1262 representatives have peculiarities of amino acid metabolism (Table 3), but this assumption needs to be verified.
Thus, potentially virulent pneumococci of serotypes 15B and 15C are circulating in Russia. It was previously established that the structural difference between these serotypes is based on variations in the short tandem repeat of thymine-adenine nucleotides in the wciZ O-acetyltransferase gene, which ensure mutual switching of serotypes 15B and 15C [29, 30]. The cross-immunogenicity of serotypes 15B/C with the formation of stable antibody titers was confirmed in earlier studies [30, 31]. Thus, vaccines containing serotype 15B could potentially limit the spread of virulent genetic lineages associated with serotypes 15B/C in the pneumococcal population.
According to the results of various studies, serotype 11A is currently spreading worldwide [32], both in pneumococcal carriers [33] and in invasive diseases [34]. According to A.B. Brueggemann et al, serotype 11A is more associated with asymptomatic carriers than with invasive disease, indicating a relatively low virulence potential [35]. However, some ST-62 strains of serotype 11A are capable of causing invasive diseases with high lethality [36]. According to the results of our study, ST-62 representatives contain in their genomes loci potentially capable of increasing the adaptability and virulence of the microorganism: loci encoding the synthesis of bacteriocins, transporters, including oligopeptides, adhesion proteins, flavin reductase, oxidative stress defense factors, complement activation regulators, and transcription regulators (Table 5). Our results are confirmed by the data of previous studies [37]. Thus, the research group of M.A. Higgins et al. previously showed the inability of S. pneumoniae to grow on fucose, despite the presence of regulatory and biochemical mechanisms of fucose metabolism [38]. It is assumed that the fucose processing pathway of S. pneumoniae plays a non-metabolic role in the interaction of this bacterium with the human host. Pneumococcal surface adhesin A (PspA) prevents activation of both classical and alternative complement pathways through its interaction with the C3b component [39]. PspA also interacts with human lactoferrin, inhibiting its bactericidal action [39]. Flavin reductase is present on the surface of pneumococci and promotes virulence by protecting against oxidative stress and mediating adhesion, and provides protection against pneumococcal infection [40]. The immune response to this protein increases with age [40]. SS-62 representatives contain other hypothetical regulators of complement activation, ABC-transporters and transcription regulators. Probably, the presence of a large number of adaptive factors allowed the genetic lineage ST-62, associated mainly with serotype 11A, to spread widely throughout the world.
Serogroup 11 includes 6 antigenically different serotypes (11A-11F) with highly homologous cps loci. The structural difference between the serotypes is due to either the mutations in the wcjE gene (manifested in serotypes 11A and 11E by differences in the degree of β-galactose-6-O-acylation) [41], or the N112S mutation in the wcrL glycosyltransferase gene (manifested by the addition of an additional carbohydrate residue to the repeating unit of the carbohydrate chain of the capsule in serotype 11D) [42]. Studies have shown that vaccines containing serotype 11A are very likely to limit the spread of serotype 11E, but not serotypes 11B, 11C, 11F, nor 11D (due to the presence of 2 types of carbohydrate chain structural units in its capsule) [43]. However, all serotypes except 11A are not widely distributed, and their inclusion in a future vaccine is not yet necessary.
There is no doubt that specific prophylaxis with pneumococcal vaccines plays a huge role in reducing invasive forms of pneumococcal infections both among children and adults, as evidenced by numerous publications from various countries that have introduced this vaccination into national calendars. However, the undeniable fact is the increased prevalence of non-vaccine serotypes of pneumococci, the invasive potential of which still requires clarification and additional research. One of the ways to further improve specific prophylaxis, some authors suggest the development of new vaccines with high valence. But it should also be taken into account that structural similarity between capsular polysaccharides of closely related serotypes of pneumococci may lead to induction of cross-reacting antibodies against serotype not covered by PCV, which may provide additional protective clinical effect.
Conclusion
Vaccination against invasive variants of pneumococci has played an important role in the spread of non-vaccine serotypes, and the epidemic processes associated with their expansion are a consequence and evidence of the effectiveness of vaccination. Serotype-specific vaccination leads to the spread of serotypes not covered by vaccines, some of which may exhibit increased virulence and/or antimicrobial resistance. In Russia, serogroups 15 and 11 are common among non-vaccine serogroups. No antimicrobial resistance determinants have been identified in the genomes of representatives of these serogroups. For each of the genetic lineages associated with serogroups 15 and 11 common in Russia, virulence determinants unique within the serogroup under study have been identified, which may contribute to the success of these lineages. Given the high virulence potential and prevalence, we can predict an increase in the epidemiologic importance of these genetic lineages in Russia. Inclusion of serotypes 15B and 11A in vaccines for use in Russia is advisable.
1 Center for Genomic Epidemiology.
URL: https://cge.food.dtu.dk/services/MLST/
2 URL: https://github.com/AdmiralenOla/Scoary
About the authors
Guzel Sh. Isaeva
Kazan State Medical University; Kazan Research Institute of Epidemiology and Microbiology
Author for correspondence.
Email: guisaeva@rambler.ru
ORCID iD: 0000-0002-1462-8734
D. Sci. (Med.), Deputy Director, Head, Department of microbiology named after Academician V.M. Aristovsky
Russian Federation, Kazan; KazanIrina A. Tsvetkova
Pediatric Research and Clinical Center for Infectious Diseases; St. Petersburg State Pediatric Medical University
Email: guisaeva@rambler.ru
ORCID iD: 0000-0002-0170-6975
Cand. Sci. (Biol.), junior researcher, Research department of medical microbiology and molecular epidemiology, assistant, Department of microbiology, virology and immunology
Russian Federation, St. Petersburg; St. PetersburgEkaterina V. Nikitina
Pediatric Research and Clinical Center for Infectious Diseases
Email: guisaeva@rambler.ru
ORCID iD: 0000-0002-9737-9496
Cand. Sci. (Biol.), researcher, Research department of medical microbiology and molecular epidemiology
Russian Federation, St. PetersburgAlbina Z. Zaripova
Kazan State Medical University; Center of Hygiene and Epidemiology in the Republic of Tatarstan (Tatarstan)
Email: guisaeva@rambler.ru
ORCID iD: 0000-0001-6790-0538
assistant, Department of microbiology named after Academician V.M. Aristovsky, Head, Personnel department
Russian Federation, Kazan; KazanLira T. Bayazitova
Kazan State Medical University; Kazan Research Institute of Epidemiology and Microbiology
Email: guisaeva@rambler.ru
ORCID iD: 0000-0002-2142-7682
Cand. Sci. (Med.), Head, Research laboratory of microbiology, Associate Professor, Department of microbiology named after Academician V.M. Aristovsky
Russian Federation, Kazan; KazanRegina A. Isaeva
Kazan State Medical University; Kazan Research Institute of Epidemiology and Microbiology
Email: guisaeva@rambler.ru
ORCID iD: 0000-0003-4366-6315
epidemiologist, resident
Russian Federation, Kazan; KazanDmitry E. Polev
Saint-Petersburg Pasteur Institute
Email: guisaeva@rambler.ru
ORCID iD: 0000-0001-9679-2791
Cand. Sci. (Biol.), senior researcher, Metagenomic research group, Department of epidemiology
Russian Federation, St. PetersburgAlina T. Saitova
Saint-Petersburg Pasteur Institute
Email: guisaeva@rambler.ru
ORCID iD: 0000-0002-5921-0745
laboratory assistant-researcher, Metagenomic research group, Department of epidemiology
Russian Federation, St. PetersburgLyudmila A. Kraeva
Saint-Petersburg Pasteur Institute
Email: guisaeva@rambler.ru
ORCID iD: 0000-0002-9115-3250
D. Sci. (Med.), Professor, Head, Laboratory of medical bacteriology
Russian Federation, St. PetersburgNikita E. Goncharov
Saint-Petersburg Pasteur Institute
Email: guisaeva@rambler.ru
ORCID iD: 0000-0002-6097-5091
junior researcher, Laboratory of medical bacteriology
Russian Federation, St. PetersburgOlga S. Kalinogorskaya
Pediatric Research and Clinical Center for Infectious Diseases
Email: guisaeva@rambler.ru
researcher, Research department of medical microbiology and molecular epidemiology
Russian Federation, St. PetersburgSvetlana A. Gordeeva
Clinical Infectious Diseases Hospital named after S.P. Botkin
Email: guisaeva@rambler.ru
ORCID iD: 0000-0003-0370-9624
Head, Centralized bacteriological laboratory
Russian Federation, St. PetersburgSergey V. Sidorenko
Pediatric Research and Clinical Center for Infectious Diseases
Email: guisaeva@rambler.ru
ORCID iD: 0000-0003-3550-7875
D. Sci. (Med.), Professor, Head, Research department of medical microbiology and molecular epidemiology
Russian Federation, St. PetersburgReferences
- Белозеров Е.С., Буланьков Ю.И., Васильев В.В. и др. Руководство по инфекционным болезням: Книга 2. СПб.; 2011. Belozerov E.S., Bulan'kov Yu.I., Vasil'ev V.V., et al. Handbook of Infectious Diseases: Book 2. St. Petersburg; 2011. EDN: https://elibrary.ru/zfzlej
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study. Lancet Infect. Dis. 2018;18(11):1191–210. DOI: https://doi.org/10.1016/S1473-3099(18)30310-4
- Daningrat W.O.D., Hafsah A., Ayu I.M., et al. Carriage of Streptococcus pneumoniae in children under five years of age prior to pneumococcal vaccine introduction in Southeast Asia: A systematic review and meta-analysis (2001–2019). J. Microbiol. Immunol. Infect. 2022;55(1):6–17. DOI: https://doi.org/10.1016/j.jmii.2021.08.002
- Sidorenko S., Rennert W., Lobzin Y., et al. Multicenter study of serotype distribution of Streptococcus pneumoniae nasopharyngeal isolates from healthy children in the Russian Federation after introduction of PCV13 into the National Vaccination Calendar. Diagn. Microbiol. Infect. Dis. 2020;96(1):114914. DOI: https://doi.org/10.1016/j.diagmicrobio.2019.114914
- Сидоренко С.В., Лобзин Ю.В., Реннерт В. и др. Изменения в серотиповом составе Streptococcus pneumoniae, циркулирующих среди детей в Российской Федерации, после внедрения 13-валентной пневмококковой конъюгированной вакцины. Журнал инфектологии. 2023;15(2):6–13. Sidorenko S.V., Lobzin Yu.V., Rennert V., et al. Changes in the serotype composition of Streptococcus pneumoniae circulating among children in the Russian Federation after the introduction of a 13-valent pneumococcal conjugate vaccine. Journal of Infectology. 2023;15(2):6–13. DOI: https://doi.org/10.22625/2072-6732-2023-15-2-6-13 EDN: https://elibrary.ru/qjgmps
- Исаева Г.Ш., Баязитова Л.Т., Зарипова А.З. и др. Региональные особенности серотипового состава Streptococcus pneumoniae, выделенных от детей-бактерионосителей дошкольного возраста в Республике Татарстан. Эпидемиология и вакцинопрофилактика. 2023;22(3):26–35. Isaeva G.Sh., Bayazitova L.T., Zaripova A.Z., et al. Regional features of the serotype composition of Streptococcus pneumoniae isolated from bacterial carriers of preschool age in the Republic of Tatarstan. Epidemiology and Vaccine Prevention. 2023;22(3):26–35. DOI: https://doi.org/10.31631/2073-3046-2023-22-3-26-35 EDN: https://elibrary.ru/avelpt
- Исаева Г.Ш., Зарипова А.З., Баязитова Л.Т. и др. Характеристика бактерионосительства S. pneumoniae в детской популяции. Журнал микробиологии, эпидемиологии и иммунобиологии. 2024;101(1):89–99. Isaeva G.Sh., Zaripova AZ., Bayazitova L.T., et al. Characteristics of bacterial transmission of S. pneumoniae in the pediatric population. Journal of Microbiology, Epidemiology and Immunobiology. 2024;101(1):89–99. DOI: https://doi.org/10.36233/0372-9311-445 EDN: https://elibrary.ru/wqbjrf
- Оганесян А.Н. Молекулярно-генетическая характеристика Streptococcus pneumoniae и эпидемиологические аспекты пневмококковых менингитов у детей: Автореф. дисс. М.; 2019. Oganesyan A.N. Molecular genetic characteristics of Streptococcus pneumoniae and epidemiological aspects of pneumococcal meningitis in children: Diss. Moscow; 2019.
- Муравьев А.А., Чагарян А.Н., Иванчик Н.В. и др. Эпидемиология серотипов S. pneumoniae, выделенных у лиц старше 18 лет: здоровых носителей, пациентов с острым средним отитом, внебольничной пневмонией и инвазивной пневмококковой инфекцией (исследование «SPECTRUM»). Клиническая микробиология и антимикробная химиотерапия. 2019;21(4):275–81. Muraviov A.A., Chagaryan A.N., Ivanchik N.V., et al. The prevalence of circulating S. pneumoniae serotypes in people older than 18 years: healthy carriers, patients with acute otitis media, community-acquired pneumonia, and invasive pneumococcal infections (epidemiological study «Spectrum»). Clinical Microbiology and Antimicrobial Chemotherapy. 2019;21(4):275–81. DOI: https://doi.org/10.36488/cmac.2019.4.275-281 EDN: https://elibrary.ru/oshtrt
- Миронов К.О., Корчагин В.И., Михайлова Ю.В. и др. Характеристика штаммов Streptococcus pneumoniae, выделенных от больных инвазивными пневмококковыми инфекциями, с использованием высокопроизводительного секвенирования. Журнал микробиологии, эпидемиологии и иммунобиологии. 2020;97(2):113–8. Mironov K.O., Korchagin V.I., Mikhailova Yu.V. et al. Characterization of Streptococcus pneumoniae strains isolated from patients with invasive pneumococcal infections using high-throughput sequencing. Journal of Microbiology, Epidemiology and Immunobiology. 2020;97(2):113–8. DOI: https://doi.org/10.36233/0372-9311-2020-97-2-113-118 EDN: https://elibrary.ru/lnxmqy
- Ono T., Watanabe M., Hashimoto K., et al. Serotypes and antibiotic resistance of Streptococcus pneumoniae before and after the introduction of the 13-valent pneumococcal conjugate vaccine for adults and children in a rural area in Japan. Pathogens. 2023 21;12(3):493. DOI: https://doi.org/10.3390/pathogens12030493
- Миронов К.О., Гапонова И.И., Корчагин В.И. и др. Антигенная и генетическая характеристика штаммов Streptococcus pneumoniae, выделенных от больных инвазивными и неинвазивными пневмококковыми инфекциями, с использованием высокопроизводительного секвенирования. Журнал микробиологии, эпидемиологии и иммунобиологии. 2021;98(5):512–8. Mironov K.O., Gaponova I.I., Korchagin V.I., et al. Antigenic and genetic characterization of streptococcus pneumoniae strains isolated from patients with invasive and non-invasive pneumococcal infections by using high-throughput sequencing. Journal of Microbiology, Epidemiology and Immunobiology. 2021;98(5):512–8. DOI: https://doi.org/10.36233/0372-9311-144 EDN: https://elibrary.ru/kvjhkq
- Alcock B.P., Huynh W., Chalil R, et al. CARD 2023: expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023;51(D1):D690–9. DOI: https://doi.org/10.1093/nar/gkac920
- Snipen L., Liland K.H. Micropan: an R-package for microbial pan-genomics. BMC Bioinformatics. 2015;16:79. DOI: https://doi.org/10.1186/s12859-015-0517-0
- Brynildsrud O., Bohlin J., Scheffer L., et al. Rapid scoring of genes in microbial pan-genome-wide association studies with Scoary. Genome Biol. 2016;17(1):238. DOI: https://doi.org/10.1186/s13059-016-1108-8
- van der Linden M., Perniciaro S., Imöhl M. Increase of serotypes 15A and 23B in IPD in Germany in the PCV13 vaccination era. BMC Infect. Dis. 2015;15:207. DOI: https://doi.org/10.1186/s12879-015-0941-9
- Sheppard C, Fry N.K., Mushtaq S., et al. Rise of multidrug-resistant non-vaccine serotype 15A Streptococcus pneumoniae in the United Kingdom, 2001 to 2014. Euro Surveill. 2016;21(50):30423. DOI: https://doi.org/10.2807/1560-7917.es.2016.21.50.30423
- Nakano S., Fujisawa T., Ito Y., et al. Spread of meropenem-resistant Streptococcus pneumoniae serotype 15A-ST63 clone in Japan, 2012–2014. Emerg. Infect. Dis. 2018;24(2):275–83. DOI: https://doi.org/10.3201/eid2402.171268
- Harboe Z.B., Thomsen R., Riis A., et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 2009;6(5):e1000081. DOI: https://doi.org/10.1371/journal.pmed.1000081
- Oligbu G., Collins S., Sheppard C.L., et al. Childhood deaths attributable to invasive pneumococcal disease in England and Wales, 2006–2014. Clin. Infect. Dis. 2017;65(2):308–14. DOI: https://doi.org/10.1093/cid/cix310
- Stanek R.J., Norton N., Mufson M.A. A 32-year study of the effect of pneumococcal vaccines on invasive Streptococcus pneumoniae disease. Am. J. Med. Sci. 2016;352(6):563–73. DOI: https://doi.org/10.1016/j.amjms.2016.09.002
- van Hoek A.J., Andrews N., Waight P.A., et al. Effect of serotype on focus and mortality of invasive pneumococcal disease: coverage of different vaccines and insight into non-vaccine serotypes. PLoS One. 2012;7(7):e39150. DOI: https://doi.org/10.1371/journal.pone.0039150
- Olarte L., Barson W.J., Barson R.M., et al. Impact of the 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis in US Children. Clin. Infect. Dis. 2015;61(5):767–75. DOI: https://doi.org/10.1093/cid/civ368
- Thigpen M.C., Whitney C.G., Messonnier N.E., et al. Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N. Engl. J. Med. 2011;364(21):2016–25. DOI: https://doi.org/10.1056/NEJMoa1005384
- Shi W., Du Q., Yuan L., et al. Antibiotic resistance and molecular biological characteristics of non-13-valent-pneumococcal conjugate vaccine serogroup 15 Streptococcus pneumoniae isolated from children in China. Front. Microbiol. 2022;12:778985. DOI: https://doi.org/10.3389/fmicb.2021.778985
- Bruce K.E., Rued B., Tsui H.T., Winkler M.E. The Opp (AmiACDEF) oligopeptide transporter mediates resistance of serotype 2 Streptococcus pneumoniae D39 to killing by chemokine CXCL10 and other antimicrobial peptides. J. Bacteriol. 2018;200(11):e00745-17. DOI: https://doi.org/10.1128/JB.00745-17
- Thompson C.D., Bradshaw J., Miller W.S., et al. Oligopeptide transporters of nonencapsulated Streptococcus pneumoniae regulate CbpAC and PspA expression and reduce complement-mediated clearance. mBio. 2023;14(1):e0332522. DOI: https://doi.org/10.1128/mbio.03325-22
- Oggioni M.R., Memmi G., Maggi T., et al. Pneumococcal zinc metalloproteinase ZmpC cleaves human matrix metalloproteinase 9 and is a virulence factor in experimental pneumonia. Mol. Microbiol. 2003;49(3):795–805. DOI: https://doi.org/10.1046/j.1365-2958.2003.03596.x
- van Selm S., van Cann L., Kolkman M.A., et al. Genetic basis for the structural difference between Streptococcus pneumoniae serotype 15B and 15C capsular polysaccharides. Infect. Immun. 2003;71(11):6192–8. DOI: https://doi.org/10.1128/IAI.71.11.6192-6198.2003
- Spencer B.L., Shenoy A.T., Orihuela C.J., Nahm M.H. The pneumococcal serotype 15C capsule is partially o-acetylated and allows for limited evasion of 23-valent pneumococcal polysaccharide vaccine-elicited anti-serotype 15B antibodies. Clin. Vaccine Immunol. 2017;24(8):e00099-17. DOI: https://doi.org/10.1128/CVI.00099-17
- Hao L., Kuttel M.M., Ravenscroft N., et al. Streptococcus pneumoniae serotype 15B polysaccharide conjugate elicits a cross-functional immune response against serotype 15C but not 15A. Vaccine. 2022;40(33):4872–80. DOI: https://doi.org/10.1016/j.vaccine.2022.06.041
- Abdoli S., Safamanesh S., Khosrojerdi M., Azimian A. Molecular detection and serotyping of Streptococcus pneumoniae in children with suspected meningitis in Northeast Iran. Iran. J. Med. Sci. 2020;45(2):125–33. DOI: https://doi.org/10.30476/IJMS.2019.45423
- Kellner J.D., Vanderkooi O.G., Macdonald J., et al. Effects of routine infant vaccination with the 7-valent pneumococcal conjugate vaccine on nasopharyngeal colonization with streptococcus pneumoniae in children in Calgary, Canada. Pediatr. Infect. Dis. J. 2008;27(6):526–32. DOI: https://doi.org/10.1097/INF.0b013e3181658c5c
- Richter S.S., Dohrn C.L., Riahi F., et al. Changing epidemiology of antimicrobial-resistant Streptococcus pneumoniae in the United States, 2004-2005. Clin. Infect. Dis. 2009;48(3):e23–33. DOI: https://doi.org/10.1086/595857
- Brueggemann A.B., Meats E., Peto T., et al. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J. Infect. Dis. 2003;187(9):1424–32. DOI: https://doi.org/10.1086/374624
- Sjöström K., Spindler C., Ortqvist A., et al. Clonal and capsular types decide whether pneumococci will act as a primary or opportunistic pathogen. Clin. Infect. Dis. 2006;42(4):451–9. DOI: https://doi.org/10.1086/499242
- Camilli R., Bonnal R., Del Grosso M., et al. Complete genome sequence of a serotype 11A, ST62 Streptococcus pneumoniae invasive isolate. BMC Microbiol. 2011;11:25. DOI: https://doi.org/10.1186/1471-2180-11-25
- Higgins M.A., Suits M.D., Marsters C., Boraston A.B. Structural and functional analysis of fucose-processing enzymes from Streptococcus pneumoniae. J. Mol. Biol. 2014;426(7):1469–1482. DOI: https://doi.org/10.1016/j.jmb.2013.12.006
- Brown J., Hammerschmidt S., Orihuela C., eds. Streptococcus pneumoniae: molecular mechanisms of host-pathogen interactions. Elsevier;2015. DOI: https://doi.org/10.1016/C2012-0-00722-3
- Morozov G.I., Porat N., Kushnir T., et al. Flavin reductase contributes to pneumococcal virulence by protecting from oxidative stress and mediating adhesion and elicits protection against pneumococcal challenge. Sci. Rep. 2018;8(1):314. DOI: https://doi.org/10.1038/s41598-017-18645-8
- Calix J.J., Brady A., Du V.Y., et al. Spectrum of pneumococcal serotype 11A variants results from incomplete loss of capsule O-acetylation. J. Clin. Microbiol. 2014;52(3):758–65. DOI: https://doi.org/10.1128/JCM.02695-13
- Oliver M.B., Jones C., Larson T.R., et al. Streptococcus pneumoniae serotype 11D has a bispecific glycosyltransferase and expresses two different capsular polysaccharide repeating units. J. Biol. Chem. 2013;288(30):21945–54. DOI: https://doi.org/10.1074/jbc.M113.488528
- Calix J.J., Nahm M., Zartler E.R. Elucidation of structural and antigenic properties of pneumococcal serotype 11A, 11B, 11C, and 11F polysaccharide capsules. J. Bacteriol. 2011;193(19):5271–8. DOI: https://doi.org/10.1128/JB.05034-11
Supplementary files