Hepatitis C virus care cascade for children in Moscow Region
- Authors: Meskina E.R.1, Galkina L.A.1, Tselipanova E.E.1, Odinaeva N.D.1,2
-
Affiliations:
- Moscow Regional Research and Clinical Institute
- Research Clinical Institute of Childhood
- Issue: Vol 99, No 5 (2022)
- Pages: 525-539
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/1276
- DOI: https://doi.org/10.36233/0372-9311-330
- ID: 1276
Cite item
Abstract
Background. Children and adolescents with infection caused by the hepatitis C virus (HCV) have not been given sufficient attention due to mild forms of HCV and delays in approval of antiviral treatment regimens. Omissions in the studies of pediatric cohorts and shortcomings of management policies aimed at children should be eliminated by improving screening coverage and access to treatment.
The aim of the study was to present the results of the cascade sequence of diagnostic testing, care and treatment of children with HCV in the Moscow Region (MR).
Materials and methods. The study included all HCV seropositive children of MR (n = 175), who underwent screening tests, and it did not include patients living with HIV/HCV coinfection. Children were observed from 2017 to 2022. The HCV RNA was detected in 164 children and HCV genotypes were identified in 99 children. The stage of liver fibrosis was assessed in 73 children by transient elastography and by FIB-4 index calculation.
Results. In MR, 93.7% of seropositive children were tested for HCV RNA; 71.2% of adolescents over 12 years of age received treatment. The prevalence of HCV seropositivity was estimated at 0.113/1,000 children population; the prevalence of chronic HCV infection was at least 0.059/1,000. The dominant HCV subtypes were GT 1b (43.4% [the 95% confidence interval, 33.5–53.8%]), GT 3a (23.2% [15.3–32.8%]) and GT 3a/3b (20.2% [12.8–29.5%]). The incidence of viremic HCV infection per 100,000 children was 3.3 among children under 3 years of age; 7.0 – among children aged 3–6 years; 7.7 – among children aged 7–11 years, 4.4 – among adolescents older than 12 years. Natural HCV clearance was reported at the frequency of 19.5% [13.8–26.4%]. Extrahepatic manifestations were of rare occasion – 2.9% [0.9–6.5%]. Vertical transmission was the primary route of HCV transmission (78.3% [71.4–84.2%]); infection is assumed to occur during medical invasive procedures — 7.4% [4.0–12.4%], drug using — 0.6% [0.01–3.10%], in the family household – 0.6% [0.01–3.10%]. New cases of HCV infection were more frequently detected during routine examination of children prior to hospitalization or children born to mothers with HCV. Viremic HCV was confirmed in 90.2% [84.6–94.3%], including HCV infection – in 53.4% [45.0–61.6%], chronic liver disease – in 35.8% [28.1–44.1%] having low activity and occasional consequences (the fibrosis METAVIR score of F1 and F1-2 – 17.8% [9.8–28.5%]). No significant clinical and epidemiological differences between the natural course of chronic HCV infection and the liver disease caused by HCV have been found. The burden of pediatric HCV in MR is aggravated by a significant proportion of socially vulnerable patients and patients with comorbid conditions.
Conclusion. One of the solutions for detection of new pediatric cases of HCV infection in MR can be offered by improvement of collaboration and continuity of care among healthcare organizations and early treatment of women of childbearing age. Further research is required to evaluate the effectiveness of routine testing of all socially vulnerable pediatric groups. Early application of pan-genotypic antiviral treatment regimens can contribute significantly to control of the HCV infection incidence in children.
Full Text
Chronic infection caused by the hepatitis C virus (HCV infection) poses a serious threat to public health, which is comparable with that of HIV and tuberculosis. The World Health Assembly defined HCV as a public health priority in 2016 and approved the Global Health Sector Strategy aimed to achieve a 90% reduction in new cases by 2030.1 Considering the scope of efforts required to achieve this ambitious goal, the concept of micro-elimination, as a stage of HCV global elimination, can be used in target groups of patients, including children and adolescents living in a specific region or country [1].
Until recently, children and adolescents have not been given sufficient attention, mainly, due to mild forms of HCV and delays in approval of antiviral treatment (AVT) regimens in pediatric healthcare [2]. The global prevalence of HCV in pediatric population varies widely across countries; it can reach 0.13% and tends to increase with age throughout the world [3]. Some countries report an increase in the HCV incidence among adolescents, which is associated with tattooing, body piercing and high-risk behavior that are becoming increasingly common [4]. According to some data, Russia represents the largest pool of HCV infected children in Europe (and ranks sixth globally) [3] with adolescents accounting for the highest percentage [5, 6].
The data show that in Russia, a quarter of children who are regular patients are infected during medical procedures; their average age is 2 years or younger [7]; these numbers differ significantly from the European statistics, demonstrating the insufficient attention to the early detection of HCV in adolescents. During 2016–2020, the incidence rates among children and adults in Russia decreased gradually [6], though the reported number of new cases may not represent the real picture [8], as many infected adolescents did not have indications for testing [9]. According to published data, half of the children with chronic HCV infection in Russia live with liver disease, and liver fibrosis (LF) progresses by the age of 15 [7].
The care cascade (CC) for HCV infection (routine testing, diagnostics, dynamic monitoring and the beginning of treatment) is not sufficiently effective both in Russia [10, 11] and other countries [9]. The presently available new technologies that reduce the complexity of the CC complex and facilitate its implementation can be essential for successful HCV elimination.
The principal measures aimed at elimination of existing omissions in the management of children and adolescents with HCV infection compared to adult management must include campaigns promoting the accessibility of tests and treatment intended for children and adolescents; fast evaluation of pan-genotypic treatment regimens and expedited approval of pediatric formulations of antivirals [4]. Special attention should be given to elimination of omissions in studies of pediatric cohorts and to studies of the prevalence of HCV viremia in age groups in priority countries, to the further validation of non-invasive tests for evaluation of the liver disease stage in children, to creating pediatric treatment registries and international consortiums for promotion of joint research programs.
As the reported HCV prevalence rates vary significantly across regions, an accurate clinical and epidemiological profile of HCV infection among children in the Moscow Region (MR) is required to assess its actual burden. The resulting data can be used for selecting priority methodological approaches and can be of critical importance for the public health system implementing its policy aimed at elimination of HCV among children and adolescents in MR.
The aim of the study is to present the results of the cascade sequence of diagnostic testing, care and treatment of children with HCV infection in MR.
Materials and methods
The type of study was a longitudinal observational clinical and epidemiological (non-interventional) study. The study was approved by the MR Health Ministry as the research within the program commissioned to the Vladimirsky Moscow Regional Research Clinical Institute by the Government for 2020–2024 as of 15/1/2020.
CC for patients with HCV includes routine testing of population and target cohorts to detect antibodies to HCV (anti-HCV), involvement of the centrally controlled healthcare system in MR, diagnostic tests for viremic infection and liver disease, monitoring for early detection of HCV consequences, complications, comorbidities and concurrent conditions, early treatment, monitoring of the response to treatment.
The anti-HCV tests were conducted in compliance with the requirements of epidemiological hepatitis B and C surveillance, which are specified in the Sanitary Regulations and Standards of the Russian Federation [12]. The study included results of routine and specific tests for HCV.
The study covered all tested HCV-seropositive children and adolescents of MR, including children who were perinatally exposed to HCV, who had confirmed viremic infection or had anti-HCV antibodies persisting for more than 18 months. During 2017–2022, a total of 175 children and adolescents with positive screening tests were qualified for observation. The information about patients was obtained from medical records of the patients who received medical treatment at outpatient clinics (Form No. 112/у), extracts from medical records of outpatients or inpatients (Form No. 027/у), from our own patient record database, and from the health information collected from lawful guardians of children. The standard parent or guardian interview procedure was used to collect data on perinatal exposure to HCV or any other routes of HCV transmission, time of infection, existing comorbidities, social factors (including any family members with HCV and coinfection), which could affect the HCV course in the patients under observation. The study included results of the anamnestic study conducted among the patients.
The study does not include patients living with human immunodeficiency virus (HIV) + HCV coinfection, who are assigned to the Center for Prevention and Control of AIDS and Infectious Diseases.
The quantification of HCV RNA (n = 164) and its genotyping (n = 99) were performed in children with the reverse transcriptase polymerase chain reaction using quantitative RT-HEPATOGEN-C real-time detection and RT- HEPATOGEN-C GENOTYPE kits (OOO NPO DNA-Technology) at the laboratory for AIDS and viral hepatitis of the Vladimirsky Moscow Regional Research Clinical Institute. Serum bilirubin levels as well as the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity were measured in 152 (86.9%) patients. Normal levels of ALT and AST are age dependent. To compare the results with the published data, the upper limit of the reference range equal to 40 IU/L was used.
Recommended non-invasive methods commonly used in pediatrics were used for assessment of consequences of HCV infection (the LF stage) [13]. Liver stiffness measurement by transient elastography was conducted in 43 (24.6%) patients, primarily in children with liver disease, using the FibroScan 502 TOUCH device with an ultrasound transducer (ECHOSENS) for non-invasive detection of LF. The results of the transient elastography were classified as normal (< 5.0 kPa, METAVIR score 0), mild scarring on the liver (from 5.0 to < 7.0 kPa, METAVIR score 1), moderate scarring (from 7.0 to < 9.0 kPa, METAVIR score 2), severe scarring (≥ 9.0 kPa); severe LF predictor (≥F3) (≥ 9 kPa) [13, 14]. In 30 (17.1%) patients, the LF stage was estimated using the FIB-4 index and the following formula: FIB-4 = age (years) × AST/(platelets (109/L) × sqrt(ALT). The FIB-4 value < 1.45 showed the absence of significant LF; the value of FIB-4 > 3.25 indicated the high probability of existence of LF with Ishak stage 4 to 6, demonstrating sufficient sensitivity and specificity [15]. As medically indicated, 55 (31.4%) patients were examined through abdominal ultrasound tests.
Lawful guardians of the children signed the informed consent permitting the personal data to be used for analysis, compilation and publishing of results, provided that confidentiality is observed.
The study report uses the following terms and definitions.
Chronic infection caused by hepatitis C virus (CHCV) was defined as HCV RNA detected in 2 or more blood samples spaced at least 6 months apart. For the children, who were not infected through a vertical route, the length of the diagnosed infection was calculated as the time from the date of the diagnosis verification.
Liver disease caused by HCV was diag- nosed by elevated ALT and AST levels in a child with detected HCV RNA and with or without LF.
Infection transmission routes: the vertical route of transmission (the mother is diagnosed with HCV), suspected infection associated with medical treatment (receiving blood products or invasive procedures at healthcare centers and absence of HCV in the mother and other family members), use of injecting drugs or the route is unknown.
The successful outcome of treatment with direct-acting antivirals (DAA) was defined as a sustained virologic response (absence of HCV RNA) 12 weeks (SVR12) and 24 weeks (SVR24) after completion of the treatment2.
The statistical analysis of the study results was performed with Microsoft Exсel 2011 and Statistica 6.0 (StatSoft Inc.). Discrete data are presented as frequency distribution expressed as percentage of the total number of patients in the group. The HCV prevalence was calculated as the number of cases per 1,000 children population, including respective age groups, and is expressed as permille. Incidence is calculated as the number of cases per 100,000 children population. Qualitative variables were compared using Pearson’s χ2 test or Fisher’s exact test when the number of observations in one of the cells of the four-fold table is less than 5, or χ2 for contingency tables. Differences at p < 0.05 were considered statistically significant. The significance of statistical differences was assessed using a 95% confidence interval (CI) for proportions.
To assess the effect size, we used criteria, risk ratios in the compared groups, reduction in the relative risk, reduction in the absolute risk, odds ratios (OR) representing the odds of outcome occurrence compared to the odds of its absence in compared groups, including calculation of confidence intervals. OR < 1 indicates risk reduction, OR = 1 – absence of effect, OR > 1 – increased risk.
Results
Testing and treatment coverage of children with HCV infection in Moscow Region
As of 1/6/2022, a total of 175 children who tested positive with an anti-HCV test were identified in MR; HCV RNA was detected in 164 (93.7%) patients. By the time of this publication, the HCV status had not been identified in 11 children, including 9 children under observation and 2 adolescents who were no longer observed when they turned 18. Viremic HCV was confirmed in 148 children (90.2% [84.6–94.3%] of the children tested for HCV RNA); HCV infection was initially confirmed in 16 children (9.8% [5.7–16.4%]) as the infection of their past medical history with spontaneous clearance of the virus (2 negative HCV RNA tests spaced 6 months apart). During 2017–2022, spontaneous clearance of the virus was confirmed in another 16 patients, and in the total population of children (before they were CC covered or during the observation) its frequency was 19.5% [13.8–26.4%].
Before their engagement in CC organized in MR, 3 adolescents had been receiving the dasabuvir + ombitasvir + paritaprevir + ritonavir (HCV genotype 1) combination treatment; sofosbuvir was administered to another 3 adolescents reaching SVR24, the pegylated interferon alpha-2b + ribavirin treatment was received by 8 children, including 4 children with SVR24 (50% of cases). Currently, CHCV treatment with pan-genotypic DAA regimens has been approved in Russia and is available to adolescents over the age of 12. During the observation period, 52 adolescents received treatment, and the treatment with glecaprevir + pibrentasvir was administered to 37 children (71.2%, including 2 patients treated unsuccessfully with interferons) with SVR24 reached in 97.3% of cases.
By the time of this publication, 91 children out of 100 children engaged in CC had CHCV infection (9 children were not tested for HCV RNA). The observation status for HCV infected children during 2017–2020 is presented in Table 1.
Table 1. Dynamics of the observation status for children with anti-HCV antibodies in MR during 2017–2022 (n = 175)
Year | Revealed | The observation was completed on 01.06.2022 | ||||||||||
total cases | including those identified for the first time | including previously registered | natural clearance | recovery after treatment | upon reaching the age of 18* | |||||||
n | % | n | % | n | % | n | % | n | % | n | % | |
2017 | 7 | 4,0 | 3 | 1,7 | 4 | 2,3 | 1 | 0,6 | 3 | 1,8 | 1 | 0,6 |
2018 | 25 | 14,3 | 7 | 4,0 | 18 | 10,3 | 3 | 1,8 | 8 | 4,6 | 1 | 0,6 |
2019 | 50 | 28,6 | 22 | 12,6 | 28 | 16,0 | 13 | 7,3 | 14 | 8,0 | 4 | 2,3 |
2020 | 21 | 12,0 | 12 | 6,9 | 9 | 5,2 | 4 | 2,3 | 7 | 4,0 | 2 | 1,1 |
2021 | 31 | 17,7 | 15 | 8,6 | 16 | 9,1 | 4 | 2,3 | 10 | 5,6 | 1 | 0,6 |
2022 | 41 | 23,4 | 6 | 3,3 | 35 | 20,0 | 7 | 4,0 | 1 | 0,6 | – | – |
Total | 175 | 100 | 65 | 37,1 | 110 | 62,9 | 32 | 18,3 | 43 | 24,6 | 9 | 5,2 |
Note. *All adolescents with CHCV who reached the age of 18, but did not receive any antiviral treatment.
As shown in Table 1, the largest number of new and previously confirmed cases of HCV infection was reported by the centrally-controlled pediatric MR CC during the third year of its operation. During 2020–2022, the proportion of newly detected cases monitored by CC gradually increased (42.9, 51.6 and 85.1%, respectively; p = 0.029), though, as previously, children with a long HCV history entered the annual lists to be monitored for 5 years.
Epidemiology of HCV infection among pediatric population of MR
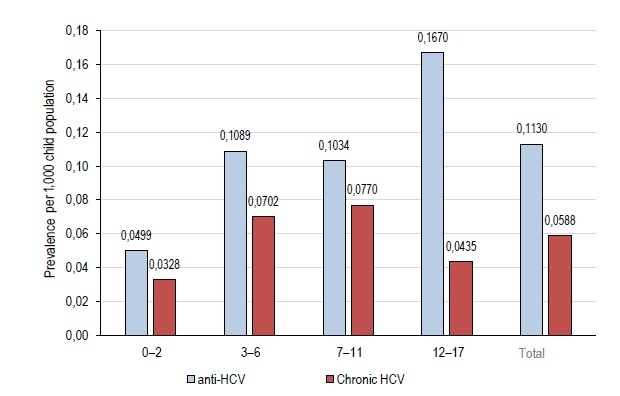
The prevalence of HCV infection (based on the available data) among children and adolescents in MR is not less than 0.113/1,000 children population; the prevalence of the confirmed pediatric CHCV in MR was around 0.059/1,000 by the time of this publication.
The prevalence rates of anti-HCV (virus exposed) and viremic CHCV among children aged 3–12 years are almost identical and are higher than the rates among children of the younger age (Fig. 1). The infection prevalence (based on the available data) among adolescents is twice as high as the prevalence among children of younger age groups. However, based on the data for 1/6/2022, the prevalence of CHCV infection among adolescents is significantly lower due to the treatment availability for this category of patients in Russia and the reached levels of treatment coverage.
Fig. 1. Age-specific prevalence of anti-HCV (over the entire observation period) and CHCV (as of 1/6/2022) per 1,000 children in the pediatric population of MR.
All the present and past HCV cases reported in 2017-2022 were analyzed for detailed assessment of the epidemiological profile of pediatric HCV infection in MR. Adolescents accounted for the largest proportion of the observed patients (Table 2), as DAA-based treatment of HCV was approved in Russia only in 2019. Girls made up the largest percentage. A quarter of patients with HCV (26.3%) were true orphans and social orphans living in institutions or with adoptive families, twice as often with guardians as with adoptive parents (Table 2).
Table 2. Some demographic and social factors typical of children with anti-HCV in MR (n = 175)
Indicator | Frequency of indicators | ||
n | % | 95% CI | |
Age, years: | |||
< 3 | 10 | 5,7 | 2,8–10,2 |
3–7 | 45 | 25,7 | 19,4–32,9 |
7–12 | 47 | 26,9 | 20,4–34,1 |
≥ 12 | 73 | 41,7 | 34,3–49,4 |
Gender: | |||
female | 104 | 59,4 | 51,8–66,8 |
male | 71 | 40,6 | 33,2–48,2 |
Рupils of social institutions | 7 | 4,0 | 1,6–8,4 |
Аccommodation: | |||
with parents | 129 | 73,7 | 66,5–80,1 |
with guardians | 26 | 14,9 | 9,9–21,0 |
with adoptive parents | 13 | 7,4 | 4,0–12,4 |
In most of the cases among the pediatric population, anti-HCV antibodies were detected during routine tests prior to the scheduled hospitalization, during hospitalization through the emergency department (one third of cases) or during examination of infants born to mothers with HCV (one third of cases; Table 3). The proportion of target groups with anti-HCV antibodies detected during scheduled tests was not large. Anti-HCV antibodies in one fifth (21.9%) of children among 137 children with perinatal exposure to HCV were detected late, during the examination for other reasons. The HCV transmission route was not identified in 5 parentless children, as there were not enough data (Table 3).
Table 3. Detection frequency (%) of anti-HCV antibodies among tested pediatric groups (n = 175)
Contingents | Detection frequency | ||
n | % | 95% CI | |
Routine testing: | |||
the entire population (planned hospitalization) | 52 | 29,7 | 23,1–37,1 |
including children born to mothers with HCV | 25 | 14,3 | 9,5–20,4 |
conscripts | 2 | 1,1 | 0,14–4,10 |
including children born to mothers with HCV | 1 | 0,6 | 0,01–3,10 |
upon admission to an educational institution | 9 | 5,1 | 2,4–9,5 |
including children born to mothers with HCV | 1 | 0,6 | 0,01–3,10 |
Vulnerable groups: | |||
children born to mothers with HCV | 66 | 37,7 | 30,5–45,3 |
including children born to mothers with HCV/HBV | 1 | 0,6 | 0,01–3,10 |
children born to mothers with HCV/HIV | 53 | 30,3 | 23,6–37,7 |
foster/adopted children* | 39 | 22,3 | 16,4–29,2 |
including children born to mothers with HCV | 39 | 22,3 | 16,4–29,2 |
children without parental care | 7 | 4,0 | 1,6–8,4 |
including children born to mothers with HCV | 2 | 1,1 | 0,14–4,10 |
Target contingents: | |||
patients with liver disease | – | – | – |
contact in HCV foci | 1 | 0,6 | 0,01–3,10 |
recipients of blood products | 6 | 3,4 | 1,3–7,3 |
oncological diseases | 4 | 2,3 | 0,63–5,80 |
including children born to mothers with HCV | 1 | 0,6 | 0,01–3,10 |
injecting drug users | 1 | 0,6 | 0,01–3,10 |
tuberculosis | 3 | 1,7 | 0,35–4,90 |
including children born to mothers with HCV | 2 | 1,1 | 0,14–4,10 |
Note. *Foster/adopted children were initially examined due to their perinatal exposure to HCV.
In MR, most of the children were infected perinatally (Table 4); children exposed to HIV + HCV coinfection accounted for 30% in the transmission route structure. Other routes of virus transmission included invasive medical procedures (surgeries, dental care), blood product transfusion, injecting drugs use, which were of rare occurrence. In one case, HCV was detected in a child having a household contact with the grandfather who had HCV, though the other family members tested negative for anti-HCV. In addition to the mothers, in 4 (2.3%) households, HCV was also diagnosed in the child’s father, and in 1 household, it was diagnosed in the grandfather (the vertical route of the child’s infection was confirmed in all the above cases). The transmission route remained unidentified in 13% of the observed cases. HCV seropositive children having tattoos or piercing were absent in MR.
Table 4. Structure of HCV transmission routes and the region of infection (n = 175)
Transmission paths | Number of cases | ||
n | % | 95% CI | |
Vertical: | 137 | 78,3 | 71,4–84,2 |
including perinatal contact with: | |||
HCV | 82 | 46,9 | 39,3–54,5 |
HCV + HIV | 53 | 30,3 | 23,6–37,7 |
HCV + HBV | 1 | 0,6 | 0,01–3,10 |
HCV + tuberculosis | 1 | 0,6 | 0,01–3,10 |
HCV + syphilis | 1 | 0,6 | 0,01–3,10 |
Invasive medical procedures | 7 | 4,0 | 1,6–8,4 |
Transfusion of blood products | 6 | 3,4 | 1,3–7,3 |
Injecting drug use | 1 | 0,6 | 0,01–3,1 |
Contact in the HCV focus | 1 | 0,6 | 0,01–3,1 |
Unknown | 23 | 13,1 | 8,5–19,1 |
The region of infection: | |||
Moscow region | 158 | 90,3 | 84,9–94,1 |
other regions of the Russian Federation | 12 | 6,9 | 3,6–11,7 |
neighboring countries | 5 | 2,9 | 0,93–6,50 |
The main shortcoming of the measures aimed at HCV detection results from the delayed testing in children born to mothers with HCV. According to the Federal Statistical Monitoring Form No. 32 “Data on Medical Assistance to Pregnant Women, Women in Labor and New Mothers for 2021” (Form No. 64 was approved by the Federal Statistics Service on 7/2/2019), a total of 66,241 pregnant women were examined at medical centers of MR in 2021, or 92.9% of the total number of deliveries in MR (starting from 22 weeks). Among them, 809 (1.2%) pregnant women were identified as seropositive for HCV. Based on the data from the MR Department of the Federal Service for Surveillance over Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor), in the same year, a total of 422 (52%) infants under 12 months of age who were born to seropositive women were examined, and 44 (10.4%) children were assigned to the centrally-controlled CC in MR. In 29.5% of the mothers of the observed infants, anti-HCV antibodies were detected for the first time during their pregnancy.
Nearly 10% of cases became infected in other regions of Russia (Nizhny Novgorod, Ekaterinburg, Orenburg, Bashkortostan, the Republic of Crimea) and neighboring countries (Uzbekistan, Turkmenistan).
HCV genotypes (GT) were identified in 99 (56.6%) patients. GT 1 (51.5%) and GT3 (45.5%) were dominant, though GT 1 was characterized by slightly more frequent occurrence (Fig. 2). GT 2 was of rare occurrence. The decreasing sequence of dominance was demonstrated by subtypes GT 1b, GT 3а and GT 3а/3b.
Fig. 2. Distribution of HCV genotypes in the pediatric population of MR (n = 99).
Clinical epidemiology of HCV infection in the pediatric population of MR
Among 148 children tested positive for HCV RNA, viremic CHCV infection was diagnosed in 79 (53.4% [45.0–61.6%]), chronic liver disease caused by HCV - in 53 (35.8% [28.1–44.1%]); moderately active hepatitis occurred rarely (in 5 (5.4%) out of 148 patients, while the other had mild hepatitis). Conjugation jaundice was diagnosed only in 3 children with Gilbert’s syndrome. During the 5-year period, 16 (10.8% [6.3–17.0%]) children had natural virus clearance.
Ultrasonography signs of changes in internal organs were detected in most of the observed patients (65.5% of the total number of patients), including enlarged liver (12.7%) and spleen (10.9%), diffuse changes in the liver parenchyma (12.7%) and pancreas (22.2%), deformation of the gallbladder (32.7%), enlarged mesenteric lymph nodes (1.9%). Combination of signs was found in 12 (21.8%) patients.
The LF stage was generally assessed at the age of 8.9 [1–16] years after viremic CHCV had been confirmed. In most of the cases, LF was absent (METAVIR score F-0, Fig. 3); mild LF (F1 and F1–2) was of rare occurrence (17.8% [9.8–28.5%] cases). Based on the available data, unfavorable consequences of CHCV (LF METAVIR score F1 and F1–2) were caused by infection with dominant subtypes GT 1b (n = 3), GT 3a (n = 2) and GT 3a/3b (n = 2).
Fig. 3. METAVIR scores for LF stages in the pediatric HCV population of MR (n = 73).
Diseases seen as extrahepatic manifestations of HCV infection [16, 17] were diagnosed in 2.9% [0.9–6.5%] of patients (polyneuropathy, thyroiditis, obesity of the 2nd degree, hemorrhagic vasculitis and iridocyclitis), including 3 children with a vertical route of HCV infection.
Comorbidities were diagnosed nearly in half of the cases (70–40.0%), including combination of 2 or 3 diagnoses in 8 (10.1%) cases. The most frequently occurred diseases were gastrointestinal diseases (Table 5). Allergic diseases (atopic dermatitis – in 7 children, allergic rhinitis – in 5 children, reported hives caused by an allergic reaction to food allergens – in 2 children) and neurological disorders (psychomotor retardation – in 4 children, infantile cerebral palsy, Down syndrome, functional disorders of the autonomic nervous system, epilepsy, autism) had an identical frequency of occurrence. Neoplasms (acute lymphoblastic leukemia, osteosarcoma, nephroblastoma, pancreatic tumor) were diagnosed in 5.1% of patients, and one child with vertical HCV infection had surgery for congenital nephroblastoma. Type 1 diabetes and unspecified hypoglycemia were diagnosed prior to the diagnosis of HCV infection. Four children had congenital anomalies, 3 of them had vertically transmitted HCV.
Table 5. Distribution of comorbidities in children with HCV in MR (n = 175)
Diseases | Number of cases | ||
n | % | 95% CI | |
Total | 70 | 40,0 | 32,7–47,7 |
Diseases of the digestive system | 25 | 14,3 | 9,5–20,4 |
including: | |||
functional disorders of the gastrointestinal tract | 10 | 5,7 | 2,8–10,3 |
biliary tract dysfunction | 5 | 2,9 | 0,93–6,50 |
chronic gastritis and gastroduodenitis | 10 | 5,7 | 2,8–10,3 |
Allergic diseases | 14 | 8,0 | 4,4–13,1 |
Diseases of the nervous system | 12 | 6,9 | 3,6–11,7 |
Neoplasms | 4 | 2,3 | 0,63–5,8 |
Diseases of the endocrine system and metabolism | 6 | 3,4 | 1,3–7,3 |
including: | |||
type 1 diabetes mellitus | 1 | 0,6 | 0,01–3,10 |
mucopolysaccharidosis type 2 | 1 | 0,6 | 0,01–3,10 |
Gilbert 's syndrome | 3 | 1,7 | 0,35–4,90 |
hypoglycemia unidentified | 1 | 0,6 | 0,01–3,10 |
Tuberculosis of the lungs | 3 | 1,7 | 0,35–4,9 |
Congenital cytomegalovirus infection | 2 | 1,1 | 0,14–4,1 |
Congenital malformations | 4 | 2,3 | 0,63–5,80 |
including: | |||
esophagus | 1 | 0,6 | 0,01–3,10 |
hearts | 2 | 1,1 | 0,14–4,10 |
cleft of hard and soft palate | 1 | 0,6 | 0,01–3,10 |
The comparison of symptoms and signs of HCV liver disease and CHCV infection showed that abdominal pain, enlarged liver (for those who received AVT, the analysis included only the pre-treatment data), enlarged spleen and Gilbert’s syndrome were observed only in children having liver disease (Table 6). However, the qualitative assessment of the variables did not reveal any significant difference in the frequency of symptoms and clinical signs between children with viremic CHCV, having or not having liver disease, except for abdominal pain. The frequency of intermittent moderate abdominal pain was higher by 11.9% [7.0–23.1%] in patients with liver disease; the relative risk was 3.35 [1.09–10.33], relative risk reduction was 2.35 [0.09–9.35] and odds ratio (OR) was 3.84 [1.12–13.21], sensitivity was 69.2%, specificity was 63.0%. Based on other variables, the confidence interval for the difference in proportions contains zero, and no differences were found, though the analysis revealed the tendency toward more frequent diffuse liver changes and active hepatocytic cytolysis. No significant effect of the age or HCV genotype on viremic HCV development was found. The frequency of extrahepatic manifestations of CHCV also did not depend on the activity of the inflammatory process in the liver.
Table 6. Characteristics of HCV liver disease and CHCV infection
Symptoms and signs | HCV liver disease (n = 53) | Сhronic hepatitis C virus infection (n = 79) | р χ2 | ||
n | % | n | % | ||
Age, years: | 0,119 df = 3 | ||||
< 3 | 4 | 7,5 | 4 | 5,1 | |
3–7 | 18 | 34,0 | 15 | 19,0 | |
7–12 | 10 | 18,9 | 27 | 34,2 | |
≥ 12 | 21 | 39,6 | 33 | 41,8 | |
Abdominal pain | 9 | 17,0 | 4 | 5,1 | 0,024 |
Fatigue | 1 | 1,9 | – | – | – |
An increase in the size of the liver | 7 | 13,2 | 3 | 3,8 | 0,045 |
Increase in the size of the spleen | 4 | 7,5 | – | – | – |
Extrahepatic manifestations of HCV | 3 | 5,7 | 2 | 2,5 | 0,356 |
Diseases of the digestive system | 9 | 17,0 | 10 | 12,7 | 0,488 |
Gilbert 's syndrome | 3 | 5,7 | – | – | – |
The stage of fibrosis according to the METAVIR scale F1–2 | 5 | 17,2 | 2 | 5,4 | 0,122 |
Ultrasonography signs of changes from the abdominal organs | 16 | 80,0 | 11 | 55,0 | 0,092 |
including: | |||||
an increase in the size of the liver | 3 | 15 | 2 | 10 | 0,633 |
an increase in the size of the spleen | 4 | 20 | 1 | 2 | 0,152 |
diffuse changes in the liver | 5 | 25 | 1 | 2 | 0,077 |
diffuse changes in the pancreas | 5 | 25 | 4 | 20 | 0,705 |
deformation of the gallbladder | 5 | 25 | 10 | 50 | 0,103 |
GТ1а | 3 | 7,1 | 5 | 10 | р > 0,05 df = 5 |
GТ1b | 19 | 45,2 | 19 | 38 | |
GТ2 | 2 | 4,8 | 1 | 2 | |
GТ3а | 9 | 21,4 | 14 | 28 | |
GТ3b | 1 | 2,4 | 1 | 2 | |
GТ3а/3b | 8 | 19,0 | 10 | 20 | |
Note. Distribution of LF stages is given for n = 29 and n = 37; ultrasonography signs of changes in abdominal organs — for n = 20 and n = 20; HCV genotypes — for n = 42 and n = 50 in the compared groups, respectively; patients with natural virus clearance are not included in the table.
Discussion
For the first time, clinical and epidemiological data have been summarized to give a clear picture of the pediatric HCV infection in MR. One of the most likely reasons for insufficient or low frequency of HCV diagnostic tests among children and adolescents is the absence of approved treatment for children under 18 years of age before 2017; only few countries have recommendations for systematic testing and treatment of adolescents [2, 8].
Cohorts that are subject to testing are clearly defined in Russia, though up-to-date and objective information about the situation in different regions is critically important.
The global burden of HCV infection among children and adolescents is estimated at 0.15% [4]. In Russia, the prevalence of HCV infection among children has not been studied thoroughly enough. It is assumed that for children and adolescents aged 1–14 years and 15–19 years it can reach 14 and 80 per 100,000 children population, respectively [5] or its rates can be slightly lower [6]. Based on the previously published data, the frequency of seropositive samples among children in MR reaches 0.4–2.1% depending on the age [18]. Our own results demonstrate lower rates – 5.88 per 100,000 (0.0588‰) compared to the estimate given by Indolfi еt al. [4] or S. Mukomolov et al. [5] or show higher levels compared to the statistical data collected across Russia [6]. The pooled prevalence of previous or current infection in MR is comparable with the projected rate – 11.3 per 100,000 (0.113‰). N. V. Soboleva et al. who detected high prevalence of anti-HCV antibodies in MR [18] do not rule out the possibility of statistical bias associated with the methods used for collection of biological samples for their further testing and analysis. The lower rates of infection prevalence compared to the projected rates may have been associated with the decreasing incidence rates observed lately in Russia, fluctuations in the rates in different regions [6], and the launch of active DAA treatment administered to adolescents.
Indeed, the prevalence of HCV infection increases with increasing age [19], and higher incidence rates among adolescents are reported everywhere [6, 8, 11]. Adolescents prevailed in the studied cohort (41.7% [34.3–49.4%]), but by the time of this publication, the prevalence of viremic HCV among adolescents (0.0435‰) was significantly lower than the prevalence among children aged 3–6 years or 7–11 years (0.070–0.077‰, respectively) due to AVT.
Most frequently, anti-HCV antibodies were detected by routine screening tests or during examination of children born to mothers with HCV. However, in 21.9% of children having perinatal exposure to HCV, viremic infection was detected late, when they were examined for other reasons.
In MR, most of the cases have a vertical route of HCV transmission — 78.3% [71.4–84.2%], showing higher numbers compared to the published Russian data [5, 7, 11] and being almost approximate to European rates (90%) [20]. The main shortcoming of imperfect verification of HCV infection among children in MR can result from incomplete or delayed testing coverage of children born to infected mothers and can be also associated with insufficiency of the data provided by patients. The improved collaboration and continuity of care among healthcare organizations should be seen as an option in solving the problem. Another problem in implementing measures aimed at HCV detection is posed by frequently absent clinical and epidemiological data, which are important for identification of infection transmission routes in parentless children. The problem of routine examination of children from dysfunctional families has not been addressed by laws and regulations of Russia. Further studies are required to evaluate the efficiency of their routine testing, though they can be difficult to perform. Although the detection frequency of the assumed route of HCV transition during medical procedures was low, the detection of HCV infection in each oncological patient or blood product recipient has not only clinical, but also social significance. We have not found any tattooing or body piercing habits among adolescents of MR; the routine testing of this pediatric category in MR may not be as urgent as it is currently in Europe [4].
Therefore, the existing capabilities of HCV infection detection in children and adolescents of MR can be seen as limitations of the study. Further studies are required in this field.
Among 164 children tested for HCV RNA during the five-year period, the ongoing viremic HCV was confirmed in 90.2% [84.6–94.3%]. CHCV infection was diagnosed in half of the children, and chronic liver disease caused by HCV, being usually mild, was diagnosed in more than one third of patients, demonstrating slightly lower rates compared to the published Russian data [7]. During its natural course, liver disease slowly progresses by the age of adolescence, and a quarter of patients who already received treatment or started it have different LF stages depending on the length of infection [21]. However, we have not found any apparent relationship between the age and the frequency of liver disease or the LF stage, though LF was not confirmed in any child under 7 years of age. The low detection frequency of LF in the examined population may have affected the results. The obtained data can be seen as trustworthy, as the used diagnostic methods are reliable enough to exclude severe LF [15]. In total (before and during the observation), the spontaneous virus clearance was identified in 19.5% [13.8–26.4%]) of children. Its frequency is comparable to the reported international frequency rates [22, 23]. The distribution of HCV genotypes circulating in MR was similar to the previously published data on genotypes; subtype GT 1b dominated significantly, though GT 3 occurred fairly often [6, 7, 11]. Both in MR and other regions [23], extrahepatic manifestations have been rarely diagnosed.
Although mild HCV infection is of common occurrence in pediatric population, its social aspect cannot be neglected. One quarter of the children were under guardianship/adopted or left without parental support; one third of children were born to mothers with coinfection (HIV/tuberculosis/syphilis); 15.4% of children had significant comorbidities (oncological diseases, endocrine and metabolic disorders, including genetic disorders or congenital anomalies, congenital cytomegalovirus infection, tuberculosis). In addition, HCV infection is associated with social stigma in pediatrics [4].
Therefore, the problem of effective detection of HCV and early treatment acquires not only medical, but also social significance. At present, treatment of HCV infection is recommended regardless of the level of hepatocytic cytolysis. However, at the local level, recommendations for universal treatment of adolescents with HCV infection tend to lag behind3. Adolescents receive attention only in 20 out of 122 countries maintaining a national policy addressing HCV treatment using pan-genotypic DAA regimens [25]. Pan-genotypic AVT regimens have been recently approved in Russia [11]. Based on the published data, in the United States, only 1.6% of children with confirmed CHC received treatment [25]. Until recently, around 30–40% of European children have had access to treatment [4].
The treatment coverage of the target cohort (71.2%) in MR during the five-year period resulted in a twofold reduction in the prevalence of HCV infection among adolescents of MR compared to children of other age groups.
The main advantage of the study is its medical and social practical significance for MR, as it addresses most urgent challenges and tasks aimed at provision of access to medical care and treatment as well as at achievement of the long-range goal — elimination of HCV among children in MR. The representative selection was used to identify main characteristics of HCV infection in children and adolescents. The quality of medical services provided to children with HCV has improved significantly.
Conclusions
- Among the children with HCV infection, who were engaged in CC in MR, the testing coverage was 93.7% and the treatment coverage in the target cohort (adolescents over 12 years of age) was 71.2%, demonstrating higher levels compared to the international levels.
- The prevalence of seropositivity for HCV in the pediatric population of MR is estimated at 0.113 per 1,000 children; the prevalence of confirmed CHCV is estimated at 0.059. The dominant subtypes were GT 1b (43.4% [33.5–53.8%]), GT 3a (23.2% [15.3–32.8%]) and GT 3a/3b (20.2% [12.8–29.5%]) shown in descending order.
- In MR, on 01.06.2022, the CHCV infection incidence per 100,000 children of the respective age is 3.3 among children under 3 years of age; 7.0 — among children aged 3–6 years; 7.7 — among children aged 7–11 years; 4.4 — among adolescents over 12 years of age.
- Viremic HCV was confirmed in 90.2% [84.6–94.3%] of the examined children, including CHCV infection – in 53.4% [45.0–61.6%], chronic liver disease caused by HCV was confirmed in 35.8% [28.1–44.1%], being mostly mild and having occasional consequences caused by infection with dominant genotypes (LF METAVIR score F1 and F1–2 — in 17.2% [9.8–28.5%]). No significant clinical and epidemiological differences between the natural course of CHCV infection and liver disease were found.
- The frequency of spontaneous clearance of HCV (at the time of the clinical encounter or during the observation) was 19.5% [13.8–26.4%].
- Extrahepatic manifestations of HCV infection were diagnosed at a frequency rate of 2.9% [0.9–6.5%].
- The provisional data demonstrate that the spread of CHCV infection can be reduced through improved treatment coverage (71.2%). Although adolescents prevailed in the infected pediatric population (41.7% [34.3–49.4%]), the prevalence of viremic HCV among them in 2021–2022 (0.0435‰) was significantly lower than among children aged 3–6 years or 7–11 years (0.070–0.077‰, respectively) due to AVT.
- The burden of pediatric HCV infection in MR is aggravated by a significant proportion of socially vulnerable patients and patients with comorbidities.
- The vertical HCV transmission route was identified in 78.3% [71.4–84.2%] of patients; the presumed infection acquired during medical invasive procedures was identified in 7.4% [4,0–12,4%]; infection associated with drug use was identified in 0.6% [0.01–3.10%]; infection acquired through HCV household contacts was identified in 0.6% [0.01–3.10%] of patients.
- New cases of HCV infection were detected most frequently during routine examination prior to hospitalization or during examination of children perinatally exposed to HCV.
- A solution for effective detection of new cases of HCV infection can be found in measures aimed at improvement of collaboration and continuity of care among healthcare organizations and early treatment of women of childbearing age. Further studies are required to evaluate the efficiency of routine tests involving all socially vulnerable groups of children.
1 WHO. Global Health Sector Strategy for Viral Hepatitis for 2016–2021: Toward Ending Viral Hepatitis; 2016. URL: https://apps.who.int/iris/handle/10665/250042
2 WHO Monitoring and evaluation for viral hepatitis B and C: recommended indicators and framework. Technical report. Geneva; 2016. URL: http://apps.who.int/iris/bitstream/10665/204790/1/9789241510288_eng.pdf
3 WHO. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. Geneva; 2018. URL: https://www.ncbi.nlm.nih.gov/books/NBK531733
About the authors
Elena R. Meskina
Moscow Regional Research and Clinical Institute
Author for correspondence.
Email: meskinaelena@rambler.ru
ORCID iD: 0000-0002-1960-6868
D. Sci. (Med.), Head, Сhildren's infectious disease department
Russian Federation, MosсowLidiya A. Galkina
Moscow Regional Research and Clinical Institute
Email: meskinaelena@rambler.ru
ORCID iD: 0000-0002-0052-2867
Cand. Sci. (Med.), senior researcher, Childrens infections disease department
Russian Federation, MosсowElena E. Tselipanova
Moscow Regional Research and Clinical Institute
Email: meskinaelena@rambler.ru
ORCID iD: 0000-0002-0586-8402
Cand. Sci. (Med.), senior researcher, Childrens infections disease department
Russian Federation, MosсowNuriniso D. Odinaeva
Moscow Regional Research and Clinical Institute; Research Clinical Institute of Childhood
Email: meskinaelena@rambler.ru
ORCID iD: 0000-0001-5214-8072
D. Sci. (Med.), Professor, Chair of рediatrics, M.F. Vladimirsky Moscow Regional Research and Clinical Institute; Director, Research Clinical Institute of Childhood
Russian Federation, Mosсow; MytishchiReferences
- WHO. Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis; 2016. Available at: https://apps.who.int/iris/handle/10665/246177
- Lazarus J.V., Wiktor S., Colombo M., Thursz M.; EASL International Liver Foundation. Micro-elimination — A path to global elimination of hepatitis C. J. Hepatol. 2017; 67(4): 665–6. https://doi.org/10.1016/j.jhep.2017.06.033.
- Indolfi G., Bailey H., Serranti D., Giaquinto C., Thorne C.; PENTAHep Study Group. Treatment and monitoring of children with chronic hepatitis C in the Pre-DAA era: A European survey of 38 paediatric specialists. J. Viral. Hepat. 2019; 26(8): 961–8. https://doi.org/10.1111/jvh.13111
- Schmelzer J., Dugan E., Blach S., Coleman S., Cai Z., DePaola M., et al. Global prevalence of hepatitis C virus in children in 2018: a modelling study. Lancet Gastroenterol. Hepatol. 2020; 5(4): 374–92. https://doi.org/10.1016/S2468-1253(19)30385-1
- Indolfi G., Easterbrook P., Dusheiko G., El-Sayed M.H., Jonas M.M., Thorne C., et al. Hepatitis C virus infection in children and adolescents. Lancet Gastroenterol. Hepatol. 2019; 4(6): 477–87. https://doi.org/10.1016/S2468-1253(19)30046-9
- Mukomolov S., Trifonova G., Levakova I., Bolsun D., Krivanogova E. Hepatitis C in the Russian Federation: challenges and future directions. Hepat. Med. 2016; 8: 51–60. https://doi.org/10.2147/HMER.S50172
- Pimenov N.N., Komarova S.V., Karandashova I.V., Tsapkova N.N., Volchkova E.V., Chulanov V.P. Hepatitis С and its outcomes in Russia: analysis of incidence, prevalence and mortality rates before the start of the programme of infection elimination. Infektsionnye bolezni. 2018; 16(3): 37–45. https://doi.org/10.20953/1729-9225-2018-3-37-45 (in Russian)
- Turkova A., Volynets G.V., Crichton S., Skvortsova T.A., Panfilova V.N., Rogozina N.V., et al. Advanced liver disease in Russian children and adolescents with chronic hepatitis C. J. Viral. Hepat. 2019; 26(7): 881–92. https://doi.org/10.1111/jvh.13093
- Mari P.C., Gulati R., Fragassi P. Adolescent hepatitis C: Prevalence, impact, and management challenges. Adolesc. Health Med. Ther. 2021; 12: 45–53. https://doi.org/10.2147/AHMT.S263864
- Epstein R.L., Wang J., Hagan L., Mayer K.H., Puro J., Linas B.P., et al. Hepatitis C virus antibody testing among 13- to 21-year-olds in a large sample of US Federally Qualified Health Centers. JAMA. 2019; 322(22): 2245–8. https://doi.org/10.1001/jama.2019.16196
- Kravchenko I.E., Ginyatullin R.R., Amon E.P., Malinniko- va E.Yu. Status of medical care for patients with chronic hepatitis C in the Russian Federation. Infektsionnye bolezni: novosti, mneniya, obuchenie. 2019; 8(4): 48–57. https://doi.org/10.24411/2305-3496-2019-14007 (in Russian)
- Malik F., Chulanov V., Pimenov N., Fomicheva A., Lundin R., Levina N., et al. Treatment and monitoring of children and adolescents with hepatitis C in Russia: Results from a multi-centre survey on policy and practice. J. Virus. Erad. 2022; 8(1): 100063. https://doi.org/10.1016/j.jve.2022.100063
- Sanitary rules and norms SanPiN 3.3686—21. Sanitary and epidemiological requirements for the prevention of infectious diseases. Moscow; 2021. (in Russian)
- Nobili V., Monti L., Alisi A., Lo Zupone C., Pietrobattista A., Toma P. Transient elastography for assessment of fibrosis in paediatric liver disease. Pediatr. Radiol. 2011; 41(10): 1232–8. https://doi.org/10.1007/s00247-011-2143-y
- Nobili V., Vizzutti F., Arena U., Abraldes J.G., Marra F., Pietrobattista A., et al. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008; 48(2): 442–8. https://doi.org/10.1002/hep.22376
- European Association for the Study of the Liver; Clinical Practice Guidelines Panel: Chair; EASL Governing Board representative; Panel members. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020; 73(5): 1170–218. https://doi.org/10.1016/j.jhep.2020.08.018
- WHO Monitoring and evaluation for viral hepatitis B and C: recommended indicators and framework. Technical report. Geneva; 2016. Available at: http://apps.who.int/iris/bitstream/10665/204790/1/9789241510288_eng.pdf
- Baykova T.A., Lopatkina T.N. A variety of extrahepatic manifestations of chronic viral hepatitis B and C: basic treatment principles. Terapevticheskiy arkhiv. 2013; 85(4): 106–10. (in Russian)
- Rogozina N.V., Goryacheva L.G., Shilova I.V., Lutskiy A.A., Zheleznikova G.F., Lapin S.V. The course of chronic hepatitis C and the prevalence of extrahepatic manifestations in children. Epidemiologiya i infektsionnye bolezni. Aktual'nye voprosy. 2012; (5): 34–9. (in Russian)
- Soboleva N.V., Karlsen A.A., Kozhanova T.V., Kichatova V.S., Klushkina V.V., Isaeva O.V., et al. The prevalence of the hepatitis C virus among the conditionally healthy population of the Russian Federation. Zhurnal infektologii. 2017; 9(2): 56–64. https://doi.org/10.22625/2072-6732-2017-9-2-56-64 (in Russian)
- Beech B.M., Myers L., Beech D.J. Hepatitis B and C infections among homeless adolescents. Fam. Community Health. 2002; 25(2): 28–36. https://doi.org/10.1097/00003727-200207000-000057
- Benova L., Mohamoud Y.A., Calvert C., Abu-Raddad L.J. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin. Infect. Dis. 2014; 59(6): 765–73. https://doi.org/10.1093/cid/ciu447
- European Paediatric HIVHCV Co-infection Study Group in the European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC) in EuroCoord. Coinfection with HIV and hepatitis C virus in 229 children and young adults living in Europe. AIDS. 2017; 31(1): 127–35. https://doi.org/10.1097/QAD.0000000000001285
- Stinco M., Bartolini E., Veronese P., Rubino C., Moriondo M., Ricci S., et al. Epidemiology and natural history of childhood- acquired chronic hepatitis C: A single-center long-term prospective study. J. Pediatr. Gastroenterol. Nutr. 2022; 75(2): e2–7. https://doi.org/10.1097/MPG.0000000000003481
- Tovo P.A., Calitri C., Scolfaro C., Gabiano C., Garazzino S. Vertically acquired hepatitis C virus infection: Correlates of transmission and disease progression. World J. Gastroenterol. 2016; 22(4): 1382–92. https://doi.org/10.3748/wjg.v22.i4.1382
- WHO. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. Geneva; 2018. Available at: https://www.ncbi.nlm.nih.gov/books/NBK531733/
- Malik F., Bailey H., Chan P., Collins I.J., Mozalevskis A., Thorne C., et al. Where are the children in national hepatitis C policies? A global review of national strategic plans and guidelines. JHEP Rep. 2021; 3(2): 100227. https://doi.org/10.1016/j.jhepr.2021.100227
- Delgado-Borrego A., Smith L., Jonas M.M., Hall C.A., Negre B., Jordan S.H., et al. Expected and actual case ascertainment and treatment rates for children infected with hepatitis C in Florida and the United States: epidemiologic evidence from statewide and nationwide surveys. J. Pediatr. 2012; 161(5): 915–21. https://doi.org/10.1016/j.jpeds.2012.05.002
Supplementary files