Влияние вакцинопрофилактики на заболеваемость ветряной оспой в России
- Авторы: Афонина Н.М.1, Михеева И.В.1
-
Учреждения:
- Центральный научно-исследовательский институт эпидемиологии Роспотребнадзора
- Выпуск: Том 99, № 6 (2022)
- Страницы: 651-660
- Раздел: ОРИГИНАЛЬНЫЕ ИССЛЕДОВАНИЯ
- URL: https://microbiol.crie.ru/jour/article/view/1357
- DOI: https://doi.org/10.36233/0372-9311-338
- ID: 1357
Цитировать
Аннотация
Введение. Значимость проблемы ветряной оспы (ВО) для здравоохранения и экономики России обусловили внедрение вакцинации против ВО в региональные программы иммунизации некоторых субъектов Российской Федерации и календарь прививок по эпидемическим показаниям.
Цель исследования — оценка эффективности вакцинации против ВО для научного обоснования рекомендаций по расширению национального календаря профилактических прививок.
Материалы и методы. Оценка эффективности вакцинации проведена путём сопоставления объёма вакцинации с показателями заболеваемости ВО в 2006–2021 гг. по данным форм № 2 и № 5 Государственного статистического наблюдения в России в целом и на отдельных территориях.
Результаты и обсуждение. Вакцинация против ВО детей в некоторых субъектах РФ в рамках региональных программ иммунизации и групп риска в рамках календаря прививок по эпидемическим показаниям до 2019 г. практически не влияла на эпидемиологическую ситуацию. Длительное разобщение организованных коллективов в период распространения COVID-19 в 2020 г. привело к снижению заболеваемости и накоплению в популяции лиц, не иммунных к Varicella zoster, что в последующем обусловило подъём заболеваемости ВО в стране в целом. Однако в Центральном, Приволжском и Сибирском федеральных округах удалось избежать роста заболеваемости ВО благодаря значительному увеличению объёма вакцинации детей в 2020–2021 гг. При этом в большинстве субъектов РФ ежегодно вакцинировали менее 2% детей в возрасте 1–6 лет. Вследствие недостаточного охвата прививками против ВО в регионах, где имеется многолетний опыт плановой иммунизации детей, происходит сдвиг заболеваемости на старшие возрастные группы и повышение риска развития врождённых форм инфекции.
Заключение. Для повышения эффективности вакцинопрофилактики ВО рекомендуется внедрить в национальный календарь прививок двукратные прививки с охватом не менее 90% детей в возрасте 1 года, а также продолжить практику иммунизации лиц более старшего возраста из групп риска заболевания.
Ключевые слова
Полный текст
Введение
В настоящее время в России ветряная оспа (ВО) относится к числу наиболее широко распространённых инфекций [1–4].
Высокая интенсивность эпидемического процесса ВО определяет значительные экономические потери: на протяжении более 10 лет это заболевание занимает одно из ведущих мест в рейтинге экономической значимости инфекционных болезней, принося ежегодные потери в размере более 12 млрд руб. [5, 6].
В последние годы осложнённые формы ВО стали регистрироваться в 3 раза чаще, наблюдаются расширение клинического полиморфизма и нарастание доли крайне тяжёлых и летальных форм инфекции. Группами высокого риска в отношении возникновения тяжёлых клинических форм заболевания являются дети младенческого возраста [7], взрослые, беременные женщины и лица с ослабленным иммунитетом [8, 9].
Единственным эффективным средством профилактики ВО является иммунизация. Разработанная и клинически протестированная в Японии в 1970–1980-х гг. живая вакцина против ВО, а также созданные на её основе другие вакцинные препараты используются во многих странах. Прививки против ВО включены в национальные календари ряда развитых стран, на основе многолетнего опыта доказана эффективность вакцинопрофилактики этой инфекции [10, 11].
Значимость и актуальность проблемы ВО для здравоохранения и экономики России обусловили необходимость внедрения вакцинации против ВО в практику здравоохранения страны. Свердловская область и Москва стали первыми регионами, где с 2009 г., сразу после лицензирования зарубежных вакцинных препаратов для профилактики ВО в России, иммунизация против этой инфекции была включена в региональные календари профилактических прививок [12–15]. С 2014 г. вакцинация против ВО детям и взрослым из групп риска1 входит в календарь профилактических прививок по эпидемическим показаниям.
С учётом современной эпидемиологической ситуации государством определены перспективы вакцинопрофилактики, документально оформленные в виде утверждённой в 2020 г. Стратегии развития иммунопрофилактики инфекционных болезней на период до 2035 г.2 В качестве одного из основных направлений оптимизации национального календаря прививок Правительством обозначено расширение перечня инфекционных болезней, против которых будет проводиться вакцинация, в частности, внедрение плановых прививок детей против ВО.
В связи с этим было проведено исследование, целью которого являлась оценка эффективности вакцинации против ВО для научного обоснования рекомендаций по расширению национального календаря профилактических прививок в Российской Федерации.
Материалы и методы
Для оценки эпидемиологической ситуации изучены данные формы № 2 Федерального государственного статистического наблюдения «Сведения об инфекционных и паразитарных заболеваниях» о заболеваемости ВО в 2006–2021 гг. в России в целом и на отдельных территориях, а также формы № 23-17 «Сведения о вспышках инфекционных заболеваний» в 2017–2021 гг. Материалами для оценки объёма вакцинации против ВО послужили данные формы № 5 Федерального государственного статистического наблюдения «Сведения о профилактических прививках» в Российской Федерации и отдельных территориях в 2013–2021 гг.
В рамках описательного эпидемиологического исследования выполнен ретроспективный анализ распределения заболеваемости во временном, возрастном и территориальном аспектах (в разрезе федеральных округов (ФО) и субъектов РФ), а также с учётом охвата вакцинацией отдельных групп населения. Из-за отсутствия статистических данных о количестве лиц, привитых против ВО, ориентировочная оценка охвата однократной вакцинацией против ВО детского населения проводилась на основании расчёта отношения (в процентах) количества проведённых прививок к численности населения в возрастной группе 1–6 лет в анализируемом году. Для статистического анализа связи между уровнем заболеваемости ВО населения и количеством привитых против этой инфекции лиц был использован метод корреляции с расчётом коэффициента линейной корреляции Пирсона (r).
Результаты
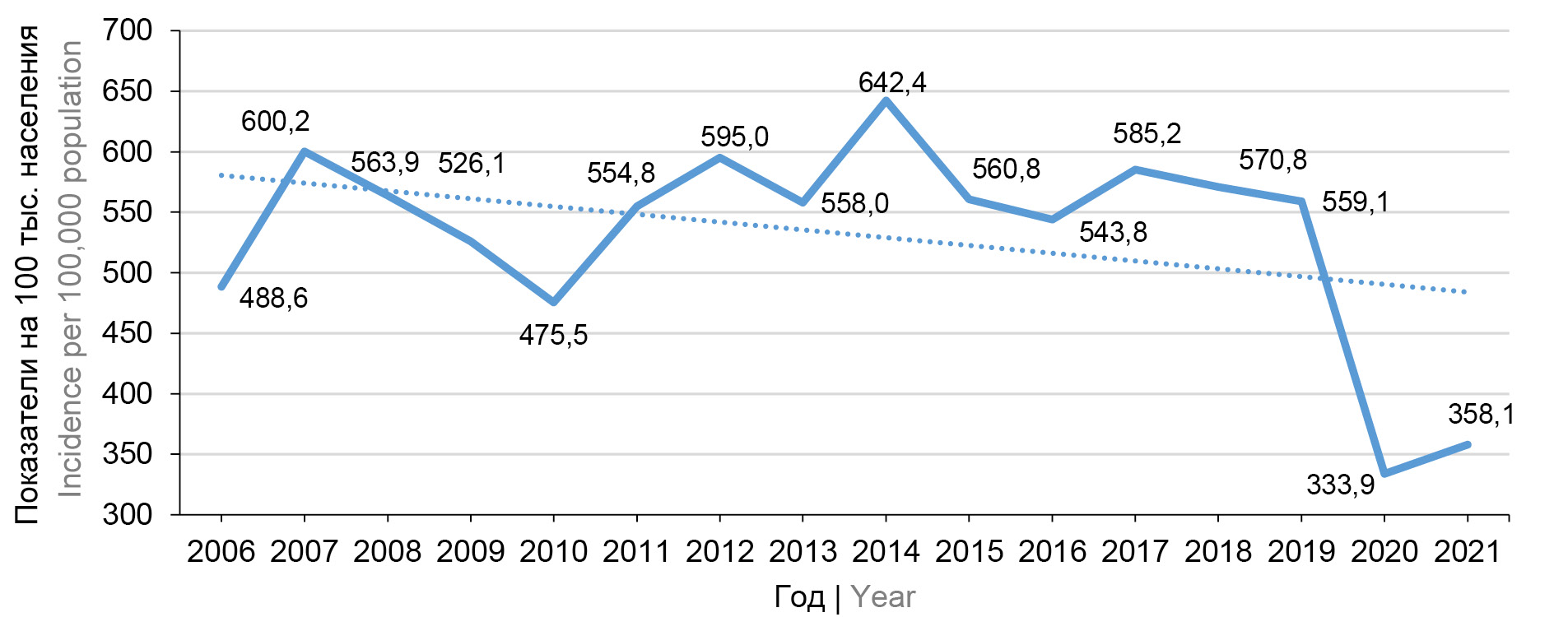
До 2019 г. в России ежегодно заболевало ВО 800–900 тыс. человек, показатели заболеваемости находились на уровне около 600 на 100 тыс. населения и не наблюдалось тенденции к снижению заболеваемости (рис. 1).
Рис. 1. Динамика заболеваемости ВО в России в 2006–2021 гг. / Fig. 1. Dynamics of CP incidence in Russia in 2006–2021.
В 2020 г., с началом пандемии COVID-19, в России впервые произошло снижение заболеваемости ВО на 40%: было зарегистрировано 490 тыс. случаев против 821 тыс. в 2019 г., показатель заболеваемости составил 333,9 на 100 тыс. населения. В 2021 г. показатель заболеваемости также оставался невысоким — 358,1 на 100 тыс. населения, но вновь наметилась тенденция к росту (рис. 1).
Наиболее высокие уровни заболеваемости ВО обычно регистрировались среди детей дошкольного возраста: в возрастной группе 3–6 лет ежегодные показатели достигали 6–8 тыс. на 100 тыс. детей данной возрастной группы, среди детей 1–2 лет — 2,5–3,0 тыс. на 100 тыс. детей данного возраста. В 2020 г. снижение заболеваемости наблюдалось во всех возрастных группах населения, в 2021 г. отмечен рост заболеваемости на 15% также во всех возрастных группах (рис. 2).
Рис. 2. Динамика заболеваемости ВО в России в разрезе возрастных групп в 2006–2021 гг. / Fig. 2. Dynamics of CP incidence in Russia in 2006–2021, by age groups.
В организованных коллективах формировались крупные очаги инфекции. Ежегодно в детских дошкольных образовательных организациях регистрировалось 2,1–2,8 тыс. эпидемических вспышек ВО. На 2-м ранговом месте по числу крупных вспышек находились школы (662–826 множественных очагов инфекции ежегодно), что свидетельствовало о наличии значительной доли не иммунных к возбудителю детей в более старших возрастных группах. Так, только в 2017–2019 гг. в России было зарегистрировано 9785 эпидемических вспышек ВО с вовлечением в них 153 313 человек. При этом в 2019 г. отмечено наибольшее число крупных очагов (3663). Дать оценку вспышечной заболеваемости ВО в 2020 и 2021 гг. не представилось возможным в связи с тем, что в форме статистического наблюдения № 23-17 множественные очаги ВО и новой коронавирусной инфекции учитывались суммарно.
Согласно официальным статистическим данным, с 2013 по 2021 г. в России было проведено более 850 тыс. прививок против ВО. Ежегодно вакцинировали 32,0–199,8 тыс. человек (рис. 3).
Рис. 3. Динамика числа выполненных прививок против ВО в России в 2013–2021 гг. / Fig. 3. Dynamics of people vaccinated against CP in Russia in 2013–2021.
Темпы вакцинации против ВО не имели стабильной тенденции к росту. Напротив, в некоторые годы происходило снижение объёма иммунизации. Так, в 2014 г. было сделано вдвое меньше прививок, чем в 2013 г. (85 и 41 тыс. прививок соответственно), а в 2015 г. — на 25% меньше, чем в 2014 г. После незначительного увеличения объёма иммунизации в 2016 г. в последующие 2 года вновь наметилась тенденция его снижения. С 2019 г. отмечается ежегодное наращивание объёма иммунизации против ВО — в 2020 г. количество сделанных прививок возросло на 30% по сравнению с 2019 г., а в 2021 г. произошел рост числа выполненных прививок на 40% по сравнению с 2020 г. (рис. 3).
Таким образом, на фоне пандемии COVID-19 объём вакцинопрофилактики ВО не только не снизился, но, напротив, отмечена активизация профилактической работы в отношении этой инфекции.
Результаты корреляционного анализа зависимости показателей заболеваемости ВО от числа проведённых прививок против этой инфекции в России показали обратную средней степени связь в возрастных группах детей 3–6 и 7–14 лет (коэффициенты корреляции r = –0,54 ± 0,37 и r = –0,53 ± 0,37 соответственно; p < 0,01) и слабой степени отрицательную зависимость среди взрослых (коэффициент корреляции r = –0,37 ± 0,41; p < 0,01) и детей в возрасте до 1 года (r = –0,34 ± 0,42; p < 0,01). При статистической обработке данных получены большие значения ошибок коэффициентов корреляции из-за слабой степени связи и недостаточной длительности наблюдения. Для количественной оценки силы связи необходимы дальнейший сбор данных и раздельный учёт выполненных прививок по возрастным группам.
Оценка распределения прививок по возрастным группам не продемонстрировала рост объёма вакцинации взрослых из групп риска в 2019–2021 гг.: в целом по стране ежегодно вакцинировали 45–49 тыс. взрослых. Подобная ситуация была характерна для всех ФО.
Напротив, объём вакцинации детей значительно увеличился — с 62 тыс. в 2019 г. до 154,5 тыс. в 2021 г. При этом в 2021 г. рост количества прививок отмечен практически во всех ФО, за исключением Уральского ФО (рис. 4).
Рис. 4. Число выполненных прививок против ВО детскому населению России и в ФО РФ в 2019–2021 гг. / Fig. 4. The number of children vaccinated against CP in Russia and its FDs in 2019–2021.
Учитывая, что субъекты РФ автономно внедряют региональные программы иммунизации населения, дополнительно была проведена оценка объёма вакцинопрофилактики ВО в отдельных субъектах РФ в последние годы. Установлено, что в 2020–2021 гг. возросло число регионов РФ, которые активно расширяют свои региональные календари профилактических прививок путём внедрения в них плановых прививок против ВО детскому населению. В то же время в 2020 г. в абсолютном большинстве ФО суммарно прививали менее 1% детей в возрасте 1–6 лет, за исключением Центрального и Сибирского ФО, где этот показатель составил 1,69 и 1,1% соответственно. В 2021 г. охват вакцинацией детей возрос во всех ФО, за исключением Уральского ФО (таблица).
Удельный вес детей в возрасте от 1 до 6 лет, привитых против ВО в 2020–2021 гг., в разрезе ФО РФ / Percentage of children aged 1–6 years, vaccinated against CP in 2020–2021, by FDs
ФО Federal District | 2020 | 2021 | ||
абсолютное число прививок детям number of vaccinations | охват вакцинацией детского населения от 1 до 6 лет, % vaccination coverage of children from 1 to 6 years old, % | абсолютное число прививок детям number of vaccinations | охват вакцинацией детского населения от 1 до 6 лет, % vaccination coverage of children from 1 to 6 years old, % | |
Центральный | Central | 43 902 | 1,69 | 65 602 | 2,53 |
Сибирский | Siberian | 14 687 | 1,10 | 28 476 | 2,14 |
Северо-Западный Northwestern | 8250 | 0,85 | 14 405 | 1,48 |
Дальневосточный Far Eastern | 4725 | 0,72 | 8494 | 1,30 |
Уральский | Urals | 12 187 | 1,22 | 11 122 | 1,11 |
Приволжский | Volga | 6981 | 0,33 | 20 707 | 0,97 |
Южный | Southern | 2592 | 0,22 | 4736 | 0,41 |
Северо-Кавказский North Caucasian | 581 | 0,06 | 810 | 0,09 |
Россия | Russian Federation | 93 905 | 0,87 | 154 352 | 1,44 |
В Центральном ФО в 2021 г. по сравнению с 2020 г. произошло увеличение объёма вакцинации на 50%, приблизительный уровень охвата прививками детей дошкольного возраста в 2021 г. составил 2,53%; в Сибирском ФО отмечен рост числа прививок в 2 раза, охват составил 2,1%; в Северо-Западном ФО — увеличение на 75%, охват — 1,25%; в Дальневосточном ФО — рост на 80%, охват — 1,3%; в Приволжском ФО — рост в 3 раза, охват — 0,97%; в Южном ФО — рост на 83%, охват — 0,41%, (рис. 4, таблица).
Наибольшие охваты прививками детей дошкольного возраста в 2021 г. были достигнуты в следующих субъектах РФ: Сахалинская область — 11,6% (Дальневосточный ФО), Ямало-Ненецкий автономный округ — 10,1% (Уральский ФО), Новосибирская область — 9,6% (Сибирский ФО), Пензенская область — 9,3% (Приволжский ФО). В Москве показатель охвата вакцинацией детей дошкольного возраста в 2021 г. составил 5,3%, в Свердловской области — менее 1%.
Сравнительный анализ заболеваемости ВО в разрезе ФО в течение 3 последних лет показал, что бремя ВО различно в разных регионах РФ: традиционно в Северо-Западном и Уральском ФО показатели заболеваемости значительно превышают среднероссийские уровни, а в Южном и Северо-Кавказском ФО регистрируемая заболеваемость относительно невысокая (в 1,5–3,0 раза ниже среднероссийских показателей; рис. 5).
Рис. 5. Заболеваемость ВО в России и в ФО РФ в 2019–2021 гг. / Fig. 5. CP incidence in Russia and its FDs in 2019–2021.
В 2020 г. снижение заболеваемости ВО на 40% произошло на всех территориях России, независимо от реализуемых на территориях программ детской иммунизации, что позволило сделать вывод о влиянии на заболеваемость введённых ограничительных мероприятий в отношении COVID-19.
Вместе с тем проведённый анализ заболеваемости в разрезе ФО и субъектов РФ показал, что в 2021 г. после снятия ограничений в ряде ФО произошёл достоверно значимый рост уровней заболеваемости ВО (Северо-Западный, Южный, Уральский и Дальневосточный ФО), а в некоторых округах сохранилось эпидемиологическое благополучие по ВО (Центральный, Приволжский, Сибирский, Северо-Кавказский ФО; рис. 5). Причём подъём заболеваемости не наблюдался именно в тех округах, где в 2020–2021 гг. наиболее активно внедрялись в практику здравоохранения региональные программы иммунизации детского населения против ВО.
Так, в Центральном ФО нарастание объёма иммунизации произошло в подавляющем большинстве субъектов, и на верхних позициях в рейтинге субъектов по ориентировочному годовому охвату вакцинацией находились Москва, Курская, Ярославская, Брянская и Тверская области (охват более 2%). В Приволжском ФО в 2020–2021 гг. включили прививки детям в региональные календари в Пензенской, Нижегородской, Оренбургской областях, в Удмурдской Республике, Республике Татарстан и Пермском крае. Активно наращивали объём иммунизации детям дошкольного возраста в наиболее крупных субъектах Сибирского ФО — Новосибирской, Омской и Иркутской областях, где суммарно за 2 последних года было вакцинировано около 40 тыс. детей.
При этом опыт некоторых субъектов РФ свидетельствует о важности поддержания стабильно высокого уровня охвата плановой профилактической иммунизацией детей во избежание в последующие годы ухудшения эпидемиологической обстановки и сдвига заболеваемости на более старшие возрастные группы.
Так, в Свердловской области и в Москве в первые годы вакцинации детей против ВО (2009–2013 гг.) в рамках утверждённых на этих территориях региональных календарей прививок наблюдалась тенденция к снижению заболеваемости. Однако позднее в обоих регионах произошло ухудшение эпидемиологической ситуации: в Свердловской области — с 2015 г. на фоне резкого сокращения объёма вакцинации, в Москве — в 2019 г. на фоне многолетней вакцинации с низкими уровнями охвата (рис. 6).
Рис. 6. Заболеваемость ВО и объём профилактической иммунизации в Москве и в Свердловской области в 2006–2021 гг. Примечание: *столбики без окраски за 2009–2012 гг. — данные из неофициальных отчётов и экстраполированные данные, полученные на основе неполных опубликованных данных. / Fig. 6. CP incidence and preventive immunization coverage in Moscow and the Sverdlovsk Region in 2006–2021. Note. *non-highlighted columns show data for 2009–2012 from unofficial reports and extrapolated data from incomplete published information.
Проведённый авторами анализ заболеваемости ВО в разрезе возрастных групп в Москве [14] в многолетней динамике показал рост показателя заболеваемости взрослых с 2016 г., повышение заболеваемости детей младенческого возраста и рост доли заболевших ВО детей до 1 мес в 2020–2021 гг.
Обсуждение
Несмотря на проводимую с 2009 г. в отдельных субъектах РФ (Москва, Свердловская область) вакцинацию против ВО детей дошкольного возраста [15, 16], а также осуществляемую с 2014 г. вакцинацию групп риска в рамках календаря прививок по эпидемическим показаниям, заболеваемость этой инфекцией в России до 2019 г. не имела тенденции к снижению, а в организованных коллективах регистрировались эпидемические вспышки инфекции (в 2017–2019 гг. — более 3 тыс. крупных очагов ежегодно с отсутствием тенденции к уменьшению их числа).
Формирование множественных очагов ВО в детских организованных коллективах, медицинских организациях, учреждениях социального типа и других организациях обусловлено чрезвычайно высокой контагиозностью возбудителя. Отечественными авторами ранее отмечалось, что вспышки ВО зачастую не удаётся купировать с помощью противоэпидемических мероприятий без использования такого инструмента, как постконтактная иммунизация в очагах инфекции [2].
Экстренная вакцинопрофилактика в очаге ВО с 2014 г. регламентирована календарем прививок по эпидемическим показаниям, поэтому высокая вспышечная заболеваемость в 2017–2019 гг. может быть обусловлена недостаточным её проведением.
В связи с тем, что в России отсутствует отечественный вакцинный препарат для профилактики ВО, а закупки иностранных вакцин для регионов сопровождаются трудностями, темпы вакцинации против ВО в стране до 2019 г. не имели стабильной тенденции к росту.
При этом оценить уровни охвата прививками против ВО в субъектах РФ невозможно из-за отсутствия этих данных в статистической форме учета № 6 «Сведения о контингентах детей и взрослых, привитых против инфекционных заболеваний».
Учитывая высокое бремя ВО, в настоящее время субъекты РФ включают прививки против ВО детям в свои региональные календари профилактических прививок. Так, с 2019 г., несмотря на высокую нагрузку на учреждения здравоохранения в связи с распространением COVID-19, на значительном числе территорий РФ вырос объём вакцинопрофилактики ВО. При этом объём вакцинации увеличился не за счёт вакцинации взрослых лиц из групп повышенного риска в рамках календаря прививок по эпидемическим показания, а за счёт плановой вакцинации детского населения в соответствии с региональными программами иммунизации регионов.
На основании результатов сравнительного анализа заболеваемости ВО и темпов иммунопрофилактики в разрезе ФО было показано, что в 2020 г. улучшение эпидемиологической ситуации по ВО в большей степени произошло в результате ограничительных мероприятий в связи с распространением COVID-19, чем из-за увеличения объёма вакцинации.
С учётом того, что в связи с массовыми ограничительными мероприятиями в детских коллективах в 2020 г. в популяции произошло накопление не переболевших ВО детей, на 2021 г. прогнозировали очередной циклический эпидемический подъём заболеваемости ВО, особенно среди детей, перенесших заболевание COVID-19. Вместе с тем ожидаемый рост заболеваемости не наблюдался в тех ФО, где наибольшее число субъектов включили прививки против ВО детям в свои региональные программы иммунизации и где охват иммунизацией детей дошкольного возраста был выше среднего по стране.
Однако тот факт, что к 2021 г. в большинстве субъектов прививки против ВО ежегодно получали менее 2% детей в возрасте 1–6 лет, демонстрирует низкие темпы вакцинации, которые не позволят ещё долгое время достичь рекомендуемого ВОЗ уровня охвата прививками 85–90% [10]. В документах ВОЗ подчёркивается, что плановая иммунизация детей при уровне охвата ниже рекомендуемого может изменить эпидемиологические характеристики инфекции и привести к увеличению числа случаев заболевания среди детей более старшего возраста и взрослых [10]. Подобное наблюдается в Москве с 2016 г. [14]. А в условиях «повзросления» ВО увеличивается вероятность заболевания беременных и, следовательно, возрастает и риск внутриутробного заражения новорождённых с развитием врождённой или неонатальной ВО [16].
С подобными неблагоприятными тенденциями и аналогичными проблемами сталкивались во многих странах на начальных этапах внедрения вакцинопрофилактики ВО более 15 лет назад [17, 18].
В связи с этим в 2006 г. Консультативный комитет по практике иммунизации Центра по контролю заболеваний США принял рекомендации по проведению двукратной вакцинации детей, «подчищающей» вакцинации лиц, ранее получивших одну дозу вакцины, однократной вакцинации всех здоровых людей в возрасте старше 13 лет, ранее не болевших и не привитых, обязательной вакцинации при поступлении в школу, в колледж [19].
С учётом опыта других стран и рекомендаций ВОЗ [10] важно минимизировать риск негативных последствий, возможных на начальном этапе внедрения вакцинопрофилактики ВО, с помощью планирования региональных программ иммунизации на основе результатов эпидемиологического анализа, выполненного на достаточном методическом уровне. Необходимо обеспечить охват вакцинацией на уровне не менее 90%, решить вопрос о внедрении двукратной прививки против ВО, а также создать адекватный эпидемиологический надзор как за всеми формами инфекции Varicella zoster (ВО, врождённой формой инфекции, опоясывающим лишаем), так и за вакцинопрофилактикой этой инфекции3.
Выводы
Вакцинация против ВО в рамках календаря прививок по эпидемическим показаниям в России практически не влияла на заболеваемость этой инфекцией, в то время как длительное разобщение организованных коллективов в период распространения COVID-19 в 2020 г. привело к существенному улучшению эпидемиологической ситуации по ВО. При этом объём иммунизации детей против ВО остаётся крайне низким, охват прививками детей от 1 до 6 лет составляет менее 2%.
В 2021 г. не произошёл прогнозируемый рост заболеваемости ВО в Центральном, Приволжском и Сибирском ФО благодаря активному включению в региональные программы иммунизации субъектов этих округов прививок против этой инфекции детскому населению.
На основании регионального опыта рекомендуется внедрить в национальный календарь профилактических прививок двукратные прививки против ВО с охватом не менее 90% детей в возрасте 1 года.
При внедрении вакцинопрофилактики необходимо усиление эпидемиологического надзора за инфекцией, вызванной вирусом Varicella zoster: совершенствование статистического учёта вспышечной заболеваемости, а именно внедрение отдельного учёта вспышек ВО в форме № 23-17, внедрение учёта врождённых форм инфекции и дополнение формы № 6 сведениями о привитости детей и взрослых против ВО.
1 Приказ Министерства здравоохранения РФ № 1122н от 06.12.2021 «Об утверждении национального календаря профилактических прививок, календаря профилактических прививок по эпидемическим показаниям и порядке проведения профилактических прививок».
2 Распоряжение Правительства РФ от 18.09.2020 № 2390-р «Об утверждении Стратегии развития иммунопрофилактики инфекционных болезней на период до 2035 года».
3 МР 3.1.0224-20. 3.1. Профилактика инфекционных болезней. Эпидемиологический надзор за инфекцией, вызываемой вирусом Varicella Zoster. Методические рекомендации (утв. Главным государственным санитарным врачом РФ 14.12.2020)
Об авторах
Наталия Михайловна Афонина
Центральный научно-исследовательский институт эпидемиологии Роспотребнадзора
Email: irina_mikheeva@mail.ru
ORCID iD: 0000-0002-3205-4025
к.м.н., н.с. лаб. иммунопрофилактики
Россия, МоскваИрина Викторовна Михеева
Центральный научно-исследовательский институт эпидемиологии Роспотребнадзора
Автор, ответственный за переписку.
Email: irina_mikheeva@mail.ru
ORCID iD: 0000-0001-8736-4007
д.м.н., профессор, зав. лаб. иммунопрофилактики
Россия, МоскваСписок литературы
- Каира А.Н., Лавров В.Ф., Свитич О.А., Соломай Т.В., Волосникова А.В. Особенности эпидемиологии ветряной оспы на отдельно взятой территории. Эпидемиология и вакцинопрофилактика. 2020; 19(2): 63–9. https://doi.org/10.31631/2073-3046-2020-19-2-63-69
- Ситник Т.Н., Штейнке Л.В., Габбасова Н.В. Ветряная оспа: «повзрослевшая» инфекция. Эпидемиология и вакцинопрофилактика. 2018; 17(5): 54–9. https://doi.org/10.31631/2073-3046-2018-17-5-54-59
- Харченко Г.А., Кимирилова О.Г. Ветряная оспа: клиника, лечение, профилактика. Эпидемиология и инфекционные болезни. 2017; (2): 72–5.
- Афонина Н.М., Михеева И.В. Современная эпидемиологическая характеристика ветряной оспы в России. One Health & Risk Management. 2020; (1): 12–21. https://doi.org/10.38045/ohrm.2020.1.03
- Государственный доклад «О состоянии санитарно-эпидемиологического благополучия населения в Российской Федерации в 2019 году». М.; 2020.
- Михеева М.А., Михеева И.В. Динамика рейтинга экономического ущерба от инфекционных болезней как критерий эффективности эпидемиологического контроля. Журнал микробиологии, эпидемиологии и иммунобиологии. 2020; 97(2): 174–81. https://doi.org/10.36233/0372-9311-2020-97-2-174-181
- Михайлова Е.В. Ветряная оспа у новорожденных: симптомы, осложнения, современные методы терапии и профилактики. В кн.: Материалы XIII Конгресса детских инфекционистов России «Актуальные вопросы инфекционной патологии и вакцинопрофилактики». М.; 2014: 48–9.
- Баликин В.Ф., Философова М.С. Расширение клинического полиморфизма и нарастание тяжести инфекции Varicella zoster у детей. В кн.: Материалы XIII Конгресса детских инфекционистов России «Актуальные вопросы инфекционной патологии и вакцинопрофилактики». М.; 2014: 8.
- Зрячкин Н.И., Бучкова Т.Н., Чеботарева Г.И. Осложнения ветряной оспы (обзор литературы). Журнал инфектологии. 2017; 9(3): 117–28. https://doi.org/10.22625/2072-6732-2017-9-3-117-128
- WHO. Varicella and herpes zoster vaccine: WHO position paper. Weekly Epidemiological Record. 2014; 89(25). Available at:
- https://www.who.int/publications/i/item/who-wer-8925-265-288
- Epidemiology and Prevention of Vaccine-Preventable Diseases. In: The Pink Book: Course Textbook. 2021. Available at:
- https://www.cdc.gov/vaccines/pubs/pinkbook/index.html
- Ковтун О., Романенко В., Казакевич Н., Саввина Н. Региональная программа вакцинопрофилактики: пути создания, достижения и перспективы. Педиатрическая фармакология. 2010; 7(4): 19–23.
- Яковлева Т.В., Акимкин В.Г., Лыткина И.Н. Перспектива развития Национального календаря профилактических прививок. Эпидемиология и вакцинопрофилактика. 2011; (1): 44–50.
- Афонина Н.М., Михеева И.В. Эффективность региональных программ вакцинопрофилактики ветряной оспы. Инфекционные болезни: новости, мнения, обучение. 2022; 11(3): 95–103. https://doi.org/10.33029/2305-3496-2022-11-3-95-103
- Филиппов О.В., Большакова Л.Н., Елагина Т.Н. Региональный календарь профилактических прививок в Москве: история, развитие, перспективы. Эпидемиология и вакцинопрофилактика. 2020; 19(4): 63–75. https://doi.org/10.31631/2073-3046-2020-19-4-63-75
- Ксенофонтова О., Рожкова Л., Саввинова Т., Харитонов А. Опыт вакцинопрофилактики ветряной оспы в г. Екатеринбурге. Педиатрическая фармакология. 2010; 7(4): 34–7.
- Lamont R.F., Sobel J.D., Carrington D., Mazaki-Tovi S., Kusanovic J.P., Vaisbuch E., et al. Varicella-zoster virus (chickenpox) infection in pregnancy. BJOG. 2011; 118(10): 1155–62. https://doi.org/10.1111/j.1471-0528.2011.02983.x
- Asano Y. Varicella vaccine: the Japanese experience. J. Infect. Dis. 1996; 174(Suppl. 3): S310–3. https://doi.org/10.1093/infdis/174.supplement_3.s310
- Chaves S.S., Gargiullo P., Zhang J.X., Civen R., Guris D., Mascola L., et al. Loss of vaccine-induced immunity to varicella over time. N. Engl. J. Med. 2007; 356(11): 1121–9. https://doi.org/10.1056/nejmoa064040
- Marin M., Güris D., Chaves S.S., Schmid S., Seward J.F. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2007; 56(RR-4): 1–40.
Дополнительные файлы