Вирусингибирующая активность комплекса антигенов условно-патогенных бактерий в отношении коронавируса SARS-CoV-2
- Авторы: Свитич О.А.1, Нагиева Ф.Г.1, Курбатова Е.А.1, Баркова Е.П.1, Харченко О.С.1, Строева А.Д.1, Пашков Е.А.1, Лисаков А.Н.1, Грачева А.В.1, Потапова М.Б.2, Файзулоев Е.Б.1, Зверев В.В.1
-
Учреждения:
- Научно-исследовательский институт вакцин и сывороток им. И.И. Мечникова
- Первый Московский государственный медицинский университет им. И.М. Сеченова (Сеченовский Университет)
- Выпуск: Том 100, № 2 (2023)
- Страницы: 143-152
- Раздел: ОРИГИНАЛЬНЫЕ ИССЛЕДОВАНИЯ
- URL: https://microbiol.crie.ru/jour/article/view/1251
- DOI: https://doi.org/10.36233/0372-9311-309
- EDN: https://elibrary.ru/swcktv
- ID: 1251
Цитировать
Аннотация
Введение. Комплекс антигенов условно-патогенных бактерий (КАУПБ) обладает протективным эффектом в отношении вируса гриппа птиц, вируса герпеса 2-го типа и других вирусов, вызывающих острые респираторные вирусные заболевания. В связи с пандемией COVID-19 актуально выяснить, обладает ли КАУПБ протективным эффектом в отношении коронавируса SARS-CoV-2.
Цель работы — изучить in vitro вирусингибирующую активность КАУПБ в отношении лабораторного штамма коронавируса SARS-CoV-2 Dubrovka.
Материалы и методы. В работе использовали клеточную линию Vero CCL-81, мононуклеарные клетки периферической крови человека (МКПК), мышиные моноклональные антиидиотипические антитела, структурно имитирующие биологические эффекты интерферонов (ИФН) человека, лабораторный штамм вируса SARS-CoV-2 Dubrovka. Инфекционную активность вируса определяли двумя методами: титрованием вируса методом предельных разведений на клеточных культурах с оценкой результатов по цитопатическому действию и методом бляшкообразования. Реакция ингибирования вируса поставлена in vitro на клеточной культуре, чувствительной к вирусу SARS-CoV-2, с внесением в клеточную культуру cмеси определённой дозы вируса к двукратным разведениям КАУПБ после предварительного 2-часового взаимодействия препарата с вирусом при 4ºС. Вирусингибирующую активность КАУПБ в отношении SARS-CoV-2 определяли по показателям функциональной активности α/β- и γ-рецепторов ИФН (РИФН) на МКПК человека, индуцированных in vitro КАУПБ и смесью КАУПБ с определённой дозой вируса SARS-CoV-2. Уровень экспрессии РИФН оценивали в реакции непрямой мембранной иммунофлуоресценции.
Результаты. Для выявления ингибирующего белка SARS-CoV-2 поставлена реакция гемагглютинации с эритроцитами кур, мышей, морских свинок и человека. В лизате клеток Vero CCL-81, инфицированных SARS-CoV-2 Dubrovka, обнаружены максимальная гемагглютинирующая активность с эритроцитами морской свинки и низкие титры гемагглютинации в вируссодержащей жидкости. В реакции ингибирования вируса на культуре клеток Vero CCL-81 КАУПБ ингибировал 10 доз SARS-CоV-2 Dubrovka с титром 1 : 32 cо 100% защитой клеточной культуры в течение 8 сут (период наблюдения). КАУПБ индуцировал экспрессию РИФН-α/β и -γ на мембранах МКПК человека при культивировании in vitro и снижал экспрессию РИФН-α/β и -γ при предварительном взаимодействии с SARS-CoV-2 Dubrovka.
Заключение. На основе экспериментальных исследований, включающих реакцию ингибирования вируса на культуре клеток, чувствительных к SARS-CoV-2 Dubrovka, и в реакции непрямой мембранной иммунофлуоресценции с использованием для детекции моноклональных антиидиотипических антител, имитирующих ИФН-подобные свойства, продемонстрировано, что КАУПБ обладает иммуномодулирующей и вирусингибирующей активностью.
Полный текст
Введение
В последние десятилетия достигнут значительный прорыв в исследованиях клеточных и молекулярных механизмов иммунитета. Пересмотрены ранее сложившиеся представления о роли врождённого иммунитета в реализации резистентности к широкому кругу патогенов и активации адаптивного иммунитета. Установлено, что одним из инструментов управления системой врождённого иммунитета могут быть некоторые микробные антигены (липополисахариды, пептидогликан, белковые антигены клеточной стенки и др.) [1, 2].
Активация TLR-опосредованных механизмов врождённого иммунитета через лиганды Тоll-подобных рецепторов приводит к защите организма от различного рода патогенов. Комплекс антигенов условно-патогенных бактерий (КАУПБ) используется для активации врождённого иммунитета и защиты от вирусных инфекций. Ранее была дана экспериментальная оценка протективного эффекта препарата КАУПБ в отношении вируса гриппа птиц серотипа H5N2, вируса герпеса 2-го типа [3–5]. КАУПБ применяется для профилактики и лечения острых респираторных заболеваний, хронических воспалительных заболеваний верхних и нижних дыхательных путей, бронхиальной астмы, атопического дерматита, поллиноза, латексной аллергии и др. В клинических исследованиях показано, что иммунотерапия пациентов при использовании КАУПБ на фоне базисной терапии приводила к коррекции фагоцитарной активности мононуклеарных клеток периферической крови (МКПК), повышению синтеза интерферонов (ИФН)-α и -γ и др. [6–9].
Коронавирусная инфекция, вызвавшая в 2019 г. пандемию, связана с вирусом SARS-CoV-2. Тяжёлое течение и летальные исходы при данной инфекции связаны с поражением лёгких, сердечно-сосудистой системы, почек, центральной нервной системы. В связи с этим создаются новые противовирусные как терапевтические, так и профилактические лекарственные средства [10–12].
Целью работы являлось изучение in vitro вирусингибирующего действия КАУПБ в отношении штамма коронавируса SARS-CoV-2 Dubrovka.
Материалы и методы
Работа выполнена с использованием штаммов коллекции центра коллективного пользования НИИВС им. И.И. Мечникова при финансовой поддержке Минобрнауки России (Соглашение № 075-15-2021-676 от 28.07.2021).
КАУПБ — препарат нового поколения, несущий набор антигенов — активаторов экспрессии рецепторов на клетках системы врождённого иммунитета (Тоll-подобных рецепторах 1/2, 4, 5, 2/6, 9), что обусловливает её эффективность против широкого круга патогенов и аллергии. При конструировании препарата использованы антигенные компоненты, извлечённые из Staphylococcus aureus, Klebsiella pheumoniae, Proteus vulgaris, Escherichia coli.
Клеточные культуры
В работе использовали перевиваемую клеточную линию Vero CCL-81 почек зелёных мартышек из American Type Culture Collection. Клетки культивировали в питательной среде DMEM/F12 («PanEco») с 5% эмбриональной телячьей сывороткой (ЭТС; «HyClone») и 40 мкг/мл гентамицина.
МКПК получали от доноров с 1-й группой крови и положительным резусом. Исследование проводилось при добровольном информированном согласии пациентов.
Моноклональные антиидиотипические антитела к рецепторам ИФН (РИФН)-α и -γ получали путём введения мышиных лимфоцитарных гибридом, продуцирующих антитела с «внутренним образом» человеческих ИФН-α/β и -γ, сингенным мышам линии BALB/c с последующим получением и очисткой асцитических жидкостей, содержащих указанные антитела [14, 15].
Коронавирус SARS-CoV-2
Вариант вируса SARS-CoV-2 Dubrovka получили путём изоляции вируса в культуре клеток Vero из клинического образца. С этой целью использовали мазок из ротоглотки пациентки в возрасте 61 года, в котором методом полимеразной цепной реакции с обратной транскриптазой в режиме реального времени (ОТ-ПЦР-РВ) выявлено высокое содержание РНК вируса SARS-CoV-2 (8,82 lg ТЦД50/мл). Впоследствии у пациентки развилось заболевание с клиническими признаками COVID-19: кашель, одышка, фебрильная температура, потеря вкуса и обоняния. При компьютерной томографии органов грудной полости выявлено характерное уплотнение лёгочной ткани с общей площадью поражения с обеих сторон 50% и поставлен диагноз: COVID-19, вирус идентифицирован (U07.1, МКБ-10); внебольничная двусторонняя полисегментированная вирусная пневмония. Исследование проводилось при добровольном информированном согласии пациентки.
Клиническим материалом заразили культуру клеток Vero и инкубировали в СО2-инкубаторе в течение 5 сут до появления признаков цитопатического действия (ЦПД), проявляющегося в округлении клеток, после чего проводили следующий пассаж. С целью идентификации вируса материал, полученный на разных пассажных уровнях, анализировали на наличие РНК SARS-CoV-2 методом ОТ-ПЦР-РВ с праймерами к гену N На 2, 7, 14 и 21-м пассажах в культуральной жидкости выявлена РНК коронавируса SARS-CoV-2 в высокой концентрации (9,0; 9,7; 9,2 и 9,9 lg копий РНК/мл соответственно). Таксономическая принадлежность изолята к виду SARS-CoV-2 (клада GH) была установлена путём секвенирования гена S (идентификационный номер GenBank MW514307) [13].
Инфекционную активность вируса определяли двумя методами: методом предельных разведений на клеточных культурах, выращенных на 48-луночных планшетах («Thermo Scientific/Nunc»), по ЦПД и методом бляшкообразования (БОЕ50) [18] в нашей модификации. Оба метода определения инфекционной активности вирусов, вызывающих ЦПД в культуре клеток в виде округления клеток, требуют подтверждения другим методом оценки инфекционности вируса для дифференциации ЦПД от апоптоза клеток.
Десятикратные разведения вируссодержащей жидкости (ВСЖ) готовили с разведения 10–1 до 10–8 с использованием поддерживающей питательной среды DMEM с 2% ЭТС. Из 48-луночных планшетов с монослоем клеток Vero CCL-81 удаляли ростовую среду, монослой клеток однократно промывали раствором Хэнкса. Затем каждое разведение ВСЖ вносили по 0,2 мл в 6 лунок планшета, в контрольные лунки — по 0,2 поддерживающей питательной среды. Контакт ВСЖ с клетками продолжался в инкубаторе в течение 1 ч при 36,5ºС и 5% СО2. После завершения контакта в каждую лунку планшета, включая лунки с контрольными клетками, вносили по 0,8 или 0,9 мл поддерживающей среды (среда DMEM без сыворотки).
Результаты титрования учитывали на 4–5-е сутки с начала заражения клеточной культуры. За титр вируса принимали максимальное разведение вируса, вызывающее ЦПД в 50% инфицированных клеточных культур при отсутствии деструкции клеток в неинфицированных клеточных культурах.
Для подготовки к исследованию методом бляшкообразования клеточную культуру Vero CCL-81 выращивали на 12-луночных планшетах в ростовой питательной среде. После формирования сплошного монослоя клеток через 24–48 ч питательную среду удаляли, клеточный монослой промывали раствором Хэнкса. Десятикратные разведения ВСЖ по 0,1 или 0,2 мл вносили в центр 3 лунок. Незаражённой оставляли 1 лунку для контроля клеточной культуры. Контакт вируса с клетками продолжался в инкубаторе 1,5 ч при 36,5ºС и 5% СО2. Затем из всех лунок, включая контрольную, удаляли остатки жидкости и вносили туда по 2 мл агарового покрытия, состоящего из 0,5% агара Noble и питательной среды DMEM c 4% ЭТС. После застывания агарового покрытия при комнатной температуре планшеты культивировали в инкубаторе при 36,5ºС и 5% СО2. На 5-е сутки культивирования на агаровое покрытие наносили по 1 мл 10% раствора трихлоруксусной кислоты или 3% раствора параформальдегида и культивировали в инкубаторе в течение 1 ч. После фиксации клеток агаровое покрытие удаляли встряхиванием, монослой клеток промывали проточной водой, панель высушивали и в лунки вносили по 1 мл 0,1% раствора генцианвиолета и оставляли на 2–3 мин. Затем панель промывали проточной водой и подсушивали. В каждой лунке подсчитывали количество бляшек. Устанавливали среднее число бляшек из 3 лунок для каждого разведения вируса. Титр вируса выражали в логарифмах БОЕ50/0,1мл, вычисленной по формуле:
Х = А × В/0,2,
где Х — титр в БОЕ в 1 мл; А — среднее количество бляшек; В — разведение вируса; 0,2 — объём инокулята (в мл), внесённого в 1 лунку.
Реакция гемагглютинации
Под термином «гемагглютинация» подразумевается способность некоторых вирусов избирательно вызывать агглютинацию эритроцитов отдельных видов млекопитающих и птиц. Вирусы проявляют свои гемагглютинирующие свойства избирательно в отношении эритроцитов определённых видов животных. Для одних вирусов круг этих животных широк, для других — весьма ограничен. Имеется определённая зависимость между инфекционными и гемагглютинирующими свойствами вируса. Не безразличным для результатов реакции гемагглютинации (РГА) является источник вируса. Гемагглютинирующие свойства вируса проявляются не во всяком вируссодержащем материале. Например, вирус гриппа с помощью РГА может быть обнаружен как в смыве из носоглотки больного человека (нерегулярно), так и в эмбриональных тканевых культурах, а также во взвеси инфицированных мышиных лёгких [16].
РГА применяли для оценки наличия или отсутствия вирусного гемагглютинина в инфицированных SARS-CoV-2 клетках и в ВСЖ. Для этой цели использовали четыре вида эритроцитов: куриные, мышиные, морской свинки и человека. В основе реакции лежала агглютинация эритроцитов вирусным гемагглютинирующим антигеном. Постановка РГА проводилась в круглодонных 96-луночных планшетах («SPL Life Sciences, Ltd.») и состояла из двукратного разведения антигенов в физиологическом растворе в объёме 100 мкл и внесения в каждое разведение равного объёма 0,25% взвеси эритроцитов. Контакт образцов с эритроцитами осуществляли при 4ºС в течение 1,0–1,5 ч до оседания эритроцитов в контрольных лунках и затем учитывали РГА. За гемагглютинирующий титр вируса принимали наибольшее разведение антигена, при котором ещё наблюдается агглютинация.
Реакция ингибиции репликации вируса
Многие виды микроорганизмов продуцируют биологические соединения, активные в отношении вирусов. Так, спорообразующие бактерии Bacullus pumilus при культивировании на оптимальной питательной среде NEW продуцируют биологически активные соединения, обладающие антивирусной активностью в отношении энтеровирусов (полиовируса 1-го типа, Коксаки В (1–6), ECHO-3 и ECHO-6 [17].
Для препарата КАУПБ выбрана схема эксперимента по выявлению внеклеточного вирулицидного действия препарата in vitro в отношении SARS-CoV-2. Предварительно было установлено отсутствие вирулицидного действия КАУПБ in vitro в отношении 100 доз (2,5 lg БОЕ50/0,2 мл SARS-CoV-2 при титре вируса 4,55 lg БОЕ50/0,2 мл).
Реакция ингибиции репликации вируса (ИРВ) поставлена на двух 24-луночных планшетах с монослоем клеток Vero CCL-81. На одном планшете поставлена реакция ИРВ, на другом — протитрован SARS-СoV-2. В среде ДМЕМ без сыворотки приготовлены двукратные разведения препарата КАУПБ (разведения с 1 : 2 до 1 : 32). В каждое разведение добавлен SARS-CoV-2, равный по объёму 10 дозам (3,5 lg ТЦД50/0,2 мл или 3,55 lg БОЕ50/0,2 мл при титре вируса 4,5 lg ТЦД50/0,2 мл и 4,55 БОЕ50/0,2 мл). Контакт препарата КАУПБ с вирусом проходил при 4ºС в течение 2 ч с периодическим встряхиванием ингредиентов. По завершении контакта каждое разведение смеси препарата с вирусом вносили по 0,2 мл на 24-луночный планшет, однократно промытый раствором Хэнкса. Каждое разведение смеси вносили в 4 лунки планшета, в 2 лунках поставлен контроль клеток, и в других 2 лунках — контроль 10 доз вируса. Контакт вируса с клетками проводили в инкубаторе с 5% СО2 в течение 1,5 ч, затем во все лунки 24-луночных планшетов вносили по 0,8 мл поддерживающей среды ДМЕМ и продолжали культивирование. После установления литической деструкции клеток в лунках с контролем 10 доз вируса (как правило, на 2–3-и сутки с начала инфицирования), учитывали результаты реакции ИРВ. За титр реакции ИРВ принимали то максимальное разведение препарата, при котором наблюдается 100% защита клеток от SARS-CoV-2.
Непрямой мембранный иммунофлуоресцентный анализ
МКПК выделяли из венозной гепаринизированной (20 ЕД/мл) крови человека в градиенте плотности фиколла 1,077 г/см3 («PanEco») путём центрифугирования в течение 25 мин при 1500 об/мин. Отбирали клеточную фракцию и трижды промывали её охлаждённым фосфатно-буферным раствором, осадок клеток ресуспендировали в питательной среде DMEM/F12 с 2% ЭТС с таким расчётом, чтобы в лунке было не менее 1 млн лимфоцитов. Взвесь лимфоцитов по 1 мл распределяли в лунки 12-луночных планшетов.
За 2 ч до выделения лимфоцитов из венозной крови к 0,2 мл двукратных разведений КАУПБ вносили равный объём 10 доз SARS-CoV-2 и оставляли на контакт при 4ºС с периодическим встряхиванием смеси.
В лунки 12-луночных планшетов с лимфоцитами вносили по 0,1 мл 3 разведения (1 : 10, 1 : 20 и 1 : 40) КАУПБ и по 0,1 мл смеси КАУПБ с SARS-CoV-2. После внесения препаратов из лунок извлекали пробы образцов лимфоцитов в разные временны́е интервалы, начиная с 1 ч, в объёме 5 мкл (3 образца на каждый временной отрезок) и наносили равномерно на предметные стёкла с окнами, образцы клеток высушивали при комнатной температуре в течение ночи. Затем образцы фиксировали дважды фильтрованным 3% параформальдегидом с 0,2% бычьим сывороточным альбумином в течение 1 ч при комнатной температуре. Фиксатор дважды промывали фосфатно-буферным раствором и проводили блокировку образцов 10% нормальной козлиной сывороткой в течение 1 ч. Далее на каждое окно с образцами препаратов наносили по 20 мкл мышиных моноклональных антиидиотипических антител для ИФН-а/β и -γ и инкубировали во влажной камере в течение 1 ч при 36,5ºС в термостате. После завершения контакта с антителами и двукратной промывки и просушивания на образцы наносили антимышиный ФИТЦ-конъюгат в рабочем разведении («BioRad»). Инкубация — 1 ч во влажной камере. ФИТЦ-конъюгат перед использованием разводили в 0,1% растворе сапонина на растворе Хэнкса с 0,01 М HEPES-буфером. После завершения контакта препаратов с конъюгатом предметные стекла промывали дважды раствором Хэнкса и подсушивали.
Уровень экспрессии РИФН-а/β- и -γ на МКПК оценивали под люминесцентным микроскопом «Optica» (Италия) под масляной иммерсией с объективом 100 и окуляром 10 по процентному соотношению светящихся лимфоцитов на 200 анализируемых клеток (в повторах на каждый временной отрезок).
ВСЖ была обработана по E. Norrby [19]. К 100 мл ВСЖ добавляли 2 мл Твин-80, разведённого 1 : 10. Смесь встряхивали на льду в течение 5–10 мин. К смеси добавляли эфир в количестве, равном 1/2 объёма обрабатываемой смеси. Смесь встряхивали 15–20 мин, затем центрифугировали 20 мин при 3000 об/мин. После центрифугирования смесь расслоилась, мутноватую плёнка наверху аккуратно протыкали пипеткой, чтобы не взмутить, и отсасывали нижний слой. Раствор оставляли на 24 ч при комнатной температуре во флаконе с ватно-марлевой пробкой для испарения эфира. Затем проверяли гемагглютинирующий титр антигена, разливали по ампулам (1,0 мл) и сушили. Высушенный антиген хранится в холодильнике несколько лет без потери активности. В качестве контроля использовали культуральную жидкость из неинфицированных клеток, приготовленную по той же методике.
Статистическая обработка данных
Статистическую значимость полученных данных определяли с помощью U-критерия Манна–Уитни. Разница считалась достоверной при уровне статистической значимости р ≤ 0,05. Показатели достоверности рассчитывали с использованием программного обеспечения «GraphPad Prism 4» («Graph Red»).
Требования к безопасности работ
Все работы с коронавирусом SARS-CoV-2 проводили в условиях, отвечающих требованиям безопасности работ с патогенными биологическими объектами 11-й группы патогенности. Сотрудники, работающие с вирусом, прошли инструктаж по технике безопасности и имеют действующее удостоверение о повышении квалификации по программе «Бактериология. Вирусология. Биологическая безопасность», выданное Российским противочумным институтом «Микроб» Роспотребнадзора.
Результаты
На 1-м этапе работы исследовали наличие или отсутствие гемагглютинирующих свойств вируса SARS-CoV-2 Dubrovka. С этой целью в РГА было изучено проявление гемагглютинирующих свойств вируса в отношении эритроцитов куриных, мышиных, морской свинки и человека с использованием взвеси клеток Vero CCL-81, инфицированных SARS-CoV-2 Dubrovkа, и ВСЖ с инфицированных клеток.
Представленные в табл. 1 результаты чётко демонстрируют наличие гемагглютинирующих свойств SАRS-CoV-2 Dubrovkа в инфицированных клетках и низкие титры в ВСЖ, возможно, в связи с действительно низкой концентрацией гемагглютинина или из-за неспецифической маскировки этого феномена. Максимальная гемагглютинирующая активность проявляется в лизате инфицированных клеток с эритроцитами морских свинок.
Таблица 1. Гемагглютинирующая активность вируса SARS-CoV-2 Dubrovka
Исследуемые образцы Studied specimens | Титр вируса в РГА с 0,25% взвесью эритроцитов Virus titer in HA with 0.25% suspension of red blood cells | |||
куриных chicken | мышиных mouse | морской свинки guinea pig | человека human | |
Лизат инфицированных клеток Vero CCL-81 Lysate of infected Vero CCL-81 cells | 1 : 64 | 1 : 16 | ˃ 1 : 256 | 1 : 32 |
ВСЖ с клеток Vero CCL-81 VCF from Vero CCL-81 cells | 1 : 16 | 1 : 2 | 1 : 2 | 1 : 2 |
Неинфицированные клетки Vero CCL-81 Uninfected Vero CCL-81 cells | 0 | 0 | 0 | 0 |
Контроль эритроцитов Red blood cell control | 0 | 0 | 0 | 0 |
Результаты РГА демонстрируют, что клеточные линии Vero CCL-81, инфицированные SАRS-CoV-2 Dubrovkа, содержат вирусный гемагглютинин — один из важных вирусных белков, являющихся мишенью для антивирусных препаратов.
Для постановки реакции ИРВ важно точно установить дозу SARS-CoV-2 Dubrovka, используемую для ИРВ антивирусным препаратом КАУПБ. Инфекционную дозу вируса определяли методом предельного разведения на клеточной культуре Vero CCL-81 и методом бляшкообразования на тех же клеточных культурах.
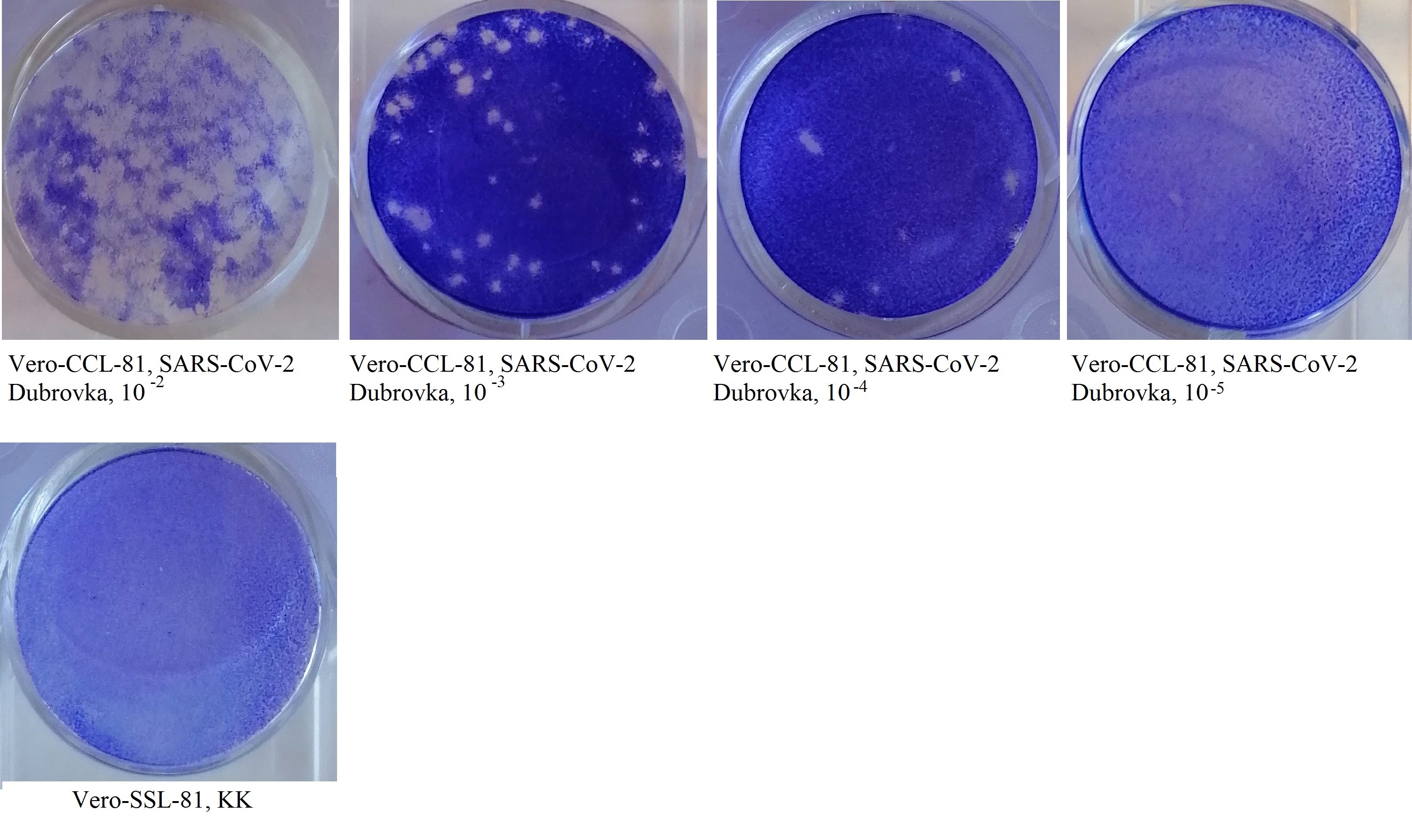
Титр вируса SARS-CoV-2 при оценке по ЦПД составил 4,50 lg ТЦД50/0,2 мл, по методу бляшкообразования — 4,55 БОЕ50/0,2 мл. Реакция ИРВ поставлена с 10 дозами вируса, титр вируса составил 3,5 lg ТЦД50/0,2 мл или 3,5 lg БОЕ50/0,2 мл (рисунок).
Бляшкообразование на монослое клеток Vero CCL-81, инфицированных коронавирусом SARS-CoV-2 Dubrovka.
КАУПБ при взаимодействии с 10 дозами вируса SARS-CoV- 2 Dubrovkа обладал вирусингибирующей активностью в разведении 1 : 32 со 100% защитой клеток на протяжении 8 сут культивирования (период наблюдения).
Для подтверждения вирусингибирующей активности КАУПБ в отношении SARS-CoV-2 Dubrovka были поставлены эксперименты по выявлению уровней экспрессии РИФН-α/β- и -γ на мембранах МКПК человека. Параллельно с помощью данной методики выясняли генез протективной активности бактериальных антигенов, входящих в состав КАУПБ. В качестве высокоспецифичного маркера в данном эксперименте выступали мышиные моноклональные антиидиотипические антитела, имитирующие биологические свойства ИФН-α/β и -γ человека, т.е. антирецепторные антитела.
Предварительно нами было установлено, что при индукции МКПК in vitro КАУПБ экспрессия РИФН-α/β и -γ более эффективно начинается с разведения препарата 1 : 10, или при концентрации 10 мкг препарата. В связи с этим было принято решение выяснить оптимальную дозу КАУПБ, индуцирующую in vitro максимальную экспрессию РИФН на мембранах МКПК.
В табл. 2 отражены данные уровня экспрессии РИФН-α/β на МКПК человека, индуцированной разными разведениями препарата, а также смесью КАУПБ с 10 дозами коронавируса SARS-CoV-2, предварительно взаимодействующих в течение 2 ч при 4°С до введения в культивируемые лимфоциты.
Таблица 2. Экспрессия РИФН-α/β (%) на МКПК человека, индуцированных in vitro КАУПБ и комплексом КАУПБ с SARS-CoV-2 штамм Dubrovka, ч
Время индукции, ч Induction time, hours | Разведение КАУПБ (доза) | ACOPB dilution (dose) | Контроль (средние значения) РИФН-α Control (mean values) RINF-α | |||||||||
1 : 10 (10 мкг | µg) | снижение, % decrease, % | 1 : 20 (5 мкг | µg) | снижение, % decrease, % | 1 : 40 (2,5 мкг | µg) | снижение, % decrease, % | ||||||
КАУПБ ACOPB | КАУПБ + SARS-CoV-2 ACOPB + SARS-CoV-2 | КАУПБ ACOPB | КАУПБ + SARS-CoV-2 ACOPB + SARS-CoV-2 | КАУПБ ACOPB | КАУПБ + SARS-CoV-2 ACOPB + SARS-CoV-2 | МКПК human peripheral blood mononuclear cells | SARS-CoV-2 | ||||
1 | 0,7 ± 0,001 | 0,4 ± 0,02 | 0,3* | 0,5 ± 0,04 | 0,3 ± 0,01 | 0,2* | 0,5 ± 0,03 | 0,2 ± 0,07 | 0,3* | 0,60 ± 0,03 | 0,60 ± 0,05 |
6 | 3,1 ± 0,05 | 2,6 ± 0,01 | 0,5* | 4,1 ± 0,09 | 2,4 ± 0,04 | 1,7* | 2,8 ± 0,1 | 2,0 ± 0,04 | 0,8* | ||
24 | 5,3 ± 0,07 | 3,8 ± 0,04 | 1,5* | 7,8 ± 0,08 | 6,0 ± 0,07 | 1,8* | 7,3 ± 0,01 | 5,0 ± 0,09 | 2,3* | ||
30 | 6,0 ± 0,09 | 5,0 ± 0,07 | 1,0* | 10,0 ± 0,05 | 6,0 ± 0,05 | 4,0* | 6,5 ± 0,04 | 5,0 ± 0,03 | 1,5* | ||
44 | 3,5 ± 0.01 | 2,5 ± 0,09 | 1,0* | 8,0 ± 0,04 | 5,5 ± 0,07 | 2,5* | 3,0 ± 0,03 | 2,5 ± 0,05 | 0,5* | ||
48 | 3,0 ± 0,08 | 2,0 ± 0,04 | 1,0* | 5,0 ± 0,02 | 3,0 ± 0,04 | 2,0* | 3,0 ± 0,05 | 2,0 ± 0,02 | 1,0* | ||
50 | 2,5 ± 0,01 | 2,0 ± 0,06 | 0,5* | 4,5 ± 0,02 | 3,0 ± 0,03 | 1,5* | 3,0 ± 0,05 | 1,5 ± 0,03 | 1,5* | ||
Примечание | Note. *р ≤ 0,05.
Анализ уровня экспрессии РИФН-α/β, индуцированных на мембранах лимфоцитов КАУПБ, показывает (табл. 2), что более эффективная индукция всеми разведениями препарата начинается с 6 ч с момента индукции, достигает максимума к 24–30 ч и затем постепенно снижается. При этом все дозы препарата вызывают на мембранах лимфоцитов in vitro индукцию РИФН-α/β, т.е. синтез ИФН-α/β человека. Необходимо отметить, что максимальный синтез ИФН-α/β при in vitro исследованиях достигается при разведении препарата 1 : 20, т.е. при концентрации препарата в 5 мкг.
Уровень экспрессии РИФН-α/β, индуцируемых на мембранах лимфоцитов in vitro смесью КАУПБ c SARS-CoV-2 Dubrovka, снижается начиная с 6 ч с момента индукции и продолжается на протяжении всего периода исследования (табл. 2). Максимальное снижение уровня экспрессии РИФН-α/β достигается к 30 ч с момента индукции, наиболее эффективное снижение уровня экспрессии отмечается при индукции лимфоцитов смесью КАУПБ с коронавирусом в разведении 1 : 20, или 5 мкг (р ≤ 0,05). Полученные результаты означают, что КАУПБ ингибирует репликацию SARS-CoV-2 Dubrovka, нейтрализуя его инфекционную активность. Этот вывод был подтверждён результатами реакции ИРВ, поставленной в культуре клеток Vero CCL-81, чувствительных к SARS-CoV-2 Dubrovka.
Данные результаты чётко демонстрируют, что КАУПБ является эффективным иммуномодулирующим препаратом и одновременно обладает вирусингибирующим эффектом. Этот вывод подтверждается и данными, относящимися к синтезу уровня РИФН-γ (табл. 3). Уровень экспрессии РИФН-γ, т.е. синтез иммунного ИФН лимфоцитами, индуцированными in vitro КАУПБ, а также смесью препарата с SARS-CoV-2 Dubrovka происходила по тем же принципам, что и индукция РИФН-α/β. Анализ данных, представленных в табл. 3, показывает, что КАУПБ в изучаемых концентрациях вызывает in vitro на мембранах лимфоцитов, культивируемых in vitro, экспрессию РИФН-γ начиная с 6 ч с момента индукции и до 50 ч (период наблюдения). Активно экспрессируются РИФН-γ с 24 до 44 ч с момента индукции КАУПБ с максимальной индукцией при концентрациях 10 и 5 мкг. Экспрессия РИФН-γ снижается при индукции лимфоцитов препаратом КАУПБ после предварительного 2-часового взаимодействия с SAPS-CoV-2 Dubrovka, что свидетельствует о вирусингибирующей активности КАУПБ.
Таблица 3. Экспрессия РИФН-γ (%) на МКПК человека, индуцированных in vitro КАУПБ и комплексом КАУПБ с SARS-CoV-2 штамм Dubrovka, ч
Время индукции, ч Induction time, hours | Разведение КАУПБ (доза) | ACOPB dilution (dose) | Контроль (средние значения) РИФН-α Control (mean values) RINF-α | |||||||||
1 : 10 (10 мкг | µg) | снижение, % decrease, % | 1 : 20 (5 мкг | µg) | снижение, % decrease, % | 1 : 40 (2,5 мкг | µg) | снижение, % decrease, % | ||||||
КАУПБ ACOPB | КАУПБ + SARS-CoV-2 ACOPB + SARS-CoV-2 | КАУПБ ACOPB | КАУПБ + SARS-CoV-2 ACOPB + SARS-CoV-2 | КАУПБ ACOPB | КАУПБ + SARS-CoV-2 ACOPB + SARS-CoV-2 | МКПК human peripheral blood mononuclear cells | SARS-CoV-2 | ||||
1 | 0,6 ± 0,07 | 0,6 ± 0,05 | 0 | 0,6 ± 0,04 | 0,8 ± 0,03 | 0 | 0,7 ± 0,02 | 0,6 ± 0,05 | 0,1* | 0,57 ± 0,03 | 0, 55 ± 0,03 |
6 | 2,4 ± 0,01 | 2,4 ± 0,09 | 0 | 3,8 ± 0,05 | 3,2 ± 0,05 | 0,6* | 3,4 ± 0,01 | 3,0 ± 0,07 | 0,4* | ||
24 | 4,5 ± 0,03 | 3,5 ± 0,01 | 1,0* | 5,5 ± 0,02 | 4,5 ± 0,06 | 1,0* | 4,8 ± 0,04 | 3,8 ± 0,03 | 1,0* | ||
30 | 6,5 ± 0,01 | 3,5 ± 0,09 | 3,0* | 7,5 ± 0,05 | 3,5 ± 0,05 | 4,0* | 4,0 ± 0,07 | 3,0 ± 0,04 | 1,0* | ||
44 | 4,5 ± 0,02 | 3,0 ± 0,03 | 1,0* | 5,0 ± 0,01 | 3,5 ± 0,05 | 1,5* | 4,0 ± 0,06 | 3,0 ± 0,01 | 1,0* | ||
48 | 3,5 ± 0,03 | 2,5 ± 0,02 | 1,0* | 4,0 ± 0,07 | 2,5 ± 0,07 | 1,5* | 3,5 ± 0,01 | 2,5 ± 0,05 | 1,0* | ||
50 | 3,0 ± 0,05 | 2,5 ± 0,09 | 0,5* | 3,5 ± 0,04 | 2,5 ± 0,01 | 1,0* | 3,0 ± 0,07 | 2,0 ± 0,06 | 1,0* | ||
Примечание | Note. *р ≤ 0,05.
Обсуждение
КАУПБ используется для эффективной профилактики и лечения большинства хронических заболеваний верхних и нижних дыхательных путей. Установлен протективный эффект применения КАУПБ при вирусных инфекциях — гриппозной и герпетической. В связи с пандемией COVID-19 появилась необходимость выяснить, обладает ли данный препарат протективным эффектом при профилактике и лечении коронавирусной инфекции человека. Для решения данной проблемы использовали отечественный штамм коронавируса SARS-CoV-2 Dubrovka, изолированный от больного COVID-19 и адаптированный к культуре клеток Vero CCL-81.
В РГА в клеточной культуре Vero CCL-81, инфицированной SARS-CoV-2 штамм Dubrovka, были установлены высокий титр гемагглютинина, являющегося мишенью для антивирусных препаратов, — более 1 : 256 и низкое его содержание в ВСЖ.
Для постановки реакции ИРВ КАУПБ необходимо установить точный титр вируса. SARS-CoV-2 оттитрован двумя методами: методом предельных разведений в культуре клеток Vero CCL-81 и методом бляшкообразования на этой же клеточной культуре. Вирусингибирующий титр КАУПБ с 10 дозами SARS-CoV-2 составил 1 : 32 со 100% защитой клеток в течение 8 сут (период наблюдения).
Далее необходимо было выяснить, чем обусловлен протективный эффект КАУПБ. Известно, что ИФН в организме необходимы для полного выражения иммунного ответа на антигенный стимул и что любой антиген является интерфероногеном [10]. Ранее нами с помощью мышиных моноклональных антиидиотипических антител, структурно имитирующих ИФН-α/β и -γ человека, показано, что активация иммунной системы, связанная с инфекцией, отражается на уровне экспрессии РИФН-α/β и -γ на иммунокомпетентных клетках человека [12, 13]. В реакции непрямой мембранной иммунофлуоресценции показано, что КАУПБ при введении в культивируемые лимфоциты человека in vitro индуцирует РИФН-α/β и -γ, что свидетельствует об эффективном иммуномодулирующем эффекте препарата.
В случае предварительного взаимодействия КАУПБ с 10 дозами коронавируса SARS-CoV-2 штамм Dubrovka в течение не менее 2 ч и с последующим внесением этого комплекса в культивируемые in vitro лимфоциты происходит снижение уровня экспрессии РИФН-α/β и -γ. Данный результат свидетельствует о том, что КАУПБ обладает вирусингибирующим эффектом и протективный эффект препарата обеспечивается синтезированными в организме эндогенными ИФН-α/β и -γ.
Об авторах
Оксана Анатольевна Свитич
Научно-исследовательский институт вакцин и сывороток им. И.И. Мечникова
Email: svitich_o_a@staff.sechenov.ru
ORCID iD: 0000-0003-1757-8389
д.м.н., член-корр. РАН, зав. отделом иммунологии и аллергологии, директор
Россия, МоскваФирая Галиевна Нагиева
Научно-исследовательский институт вакцин и сывороток им. И.И. Мечникова
Автор, ответственный за переписку.
Email: fgn42@yandex.ru
ORCID iD: 0000-0001-8204-4899
д.м.н., доцент, зав. лаб. гибридных клеточных культур отдела вирусологии
Россия, МоскваЕкатерина Алексеевна Курбатова
Научно-исследовательский институт вакцин и сывороток им. И.И. Мечникова
Email: kurbatova6162@yandex.ru
ORCID iD: 0000-0002-4474-7531
д.м.н., проф., зав. лаб. терапевтических вакцин
Россия, МоскваЕлена Петровна Баркова
Научно-исследовательский институт вакцин и сывороток им. И.И. Мечникова
Email: e.barkova2012@yandex.ru
ORCID iD: 0000-0002-3369-8869
к.б.н., в.н.с. лаб. гибридных клеточных культур отдела вирусологии
Россия, МоскваОльгга Сергеевна Харченко
Научно-исследовательский институт вакцин и сывороток им. И.И. Мечникова
Email: bio139@yandex.ru
ORCID iD: 0000-0002-2169-9610
н.с. лаб. ДНК-содержащих вирусов отдела вирусологии
Россия, МоскваАлексадра Дмитриевна Строева
Научно-исследовательский институт вакцин и сывороток им. И.И. Мечникова
Email: aleksandra.26@mail.ru
ORCID iD: 0000-0002-4179-931X
м.н.с. лаб. гибридных клеточных культур отдела вирусологии
Россия, МоскваЕвгений Алексеевич Пашков
Научно-исследовательский институт вакцин и сывороток им. И.И. Мечникова
Email: svitich_o_a@staff.sechenov.ru
ORCID iD: 0000-0002-5682-4581
м.н.с. лаб. молекулярной иммунологии отдела иммунологии и аллергологии
Россия, МоскваАлексей Николаевич Лисаков
Научно-исследовательский институт вакцин и сывороток им. И.И. Мечникова
Email: lisakov@mail.ru
ORCID iD: 0000-0001-9374-5842
н.с. лаб. гибридных клеточных культур отдела вирусологии
Россия, МоскваАнастасия Вячеславовна Грачева
Научно-исследовательский институт вакцин и сывороток им. И.И. Мечникова
Email: gr54@yandex.ru
ORCID iD: 0000-0001-8428-4482
м.н.с. лаб. молекулярной вирусологии
Россия, МоскваМария Борисовна Потапова
Первый Московский государственный медицинский университет им. И.М. Сеченова (Сеченовский Университет)
Email: svitich_o_a@staff.sechenov.ru
ORCID iD: 0000-0001-9647-1322
аспирант кафедры кожных венерических болезней им. В.А. Рахманова
Россия, МоскваЕвгений Бихтиерович Файзулоев
Научно-исследовательский институт вакцин и сывороток им. И.И. Мечникова
Email: feb@mail.ru
ORCID iD: 0000-0001-7385-5083
к.б.н., зав. лаб. молекулярной вирусологии
Россия, МоскваВиталий Васильевич Зверев
Научно-исследовательский институт вакцин и сывороток им. И.И. Мечникова
Email: vitalyzverev@outlook.com
ORCID iD: 0000-0001-5808-2246
д.б.н., проф., академик РАН, зав. лаб. молекулярной биотехнологии
Россия, МоскваСписок литературы
- Ахматова Н.К. Молекулярные и клеточные механизмы действия иммуномодуляторов микробного происхождения на функциональную активность эффекторов врожденного иммунитета: Автореф. дисс. … д-ра мед. наук. М.; 2006. Akhmatova N.K. Molecular and cellular mechanisms of action of immunomodulators of microbial origin on the functional activity of the effectors of innate immunity: Diss. Moscow; 2006.
- Пащенко М.В., Пинегин Б.В. Физиология клеток врожденной иммунной системы: дендритные клетки. Иммунология. 2006;27(6):368–78. Pashenkov M.V., Pinegln B.V. Cell physiology of innate immune system: dendritic cells. Immunology. 2006;27(6):368–78. EDN: https://www.elibrary.ru/hykkzd
- Егорова Н.Б., Курбатова Е.А., Ахматова Н.К., Семенова И.Б. Протективная активность Иммуновак — ВП-4 в отношении вируса гриппа птиц H5N2 при разных методах введения. Журнал микробиологии, эпидемиологии и иммунологии. 2011;88(1): 49–53. Egorova N.B., Kurbatova E.A., Akhmatova N.K., Semenova I.B. Protective activity of immunovacvp-4 vaccine against avian influenza virus H5N2 administered by different methods. Journal of Microbiology, Epidemiology and Immunobiology. 2011;88(1):49–53. EDN: https://www.elibrary.ru/qbbzsx
- Егорова Н.Б., Курбатова Е.А., Семенова И.Б. Вакцины и вакцинация: национальное руководство. М.; 2011:693–714. Egorova N.B., Kurbatova E.A., Semenova I.B. Vaccines and Vaccination: National Guidelines. Moscow; 2011:693–714.
- Гладько О.В., Егорова Н.Б., Масютова С.А. и др. Иммунотерапия генитального герпеса поликомпонентной вакциной ВП-4. Военно-медицинский журнал. 2002;323(5):73–6. Glad'ko O.V., Egorova N.B., Masyutova S.A., et al. Immunotherapy of genital herpes with a multicomponent VP-4 vaccine. Military Medical Journal. 2002;323(5):73–6.
- Чучалин А.Г., Осипова Г.Л., Егорова Н.Б. и др. Контролируемые исследования по эффективности поликомпонентной вакцины при иммунотерапии у больных хроническими обструктивными заболеваниями органов дыхания. Пульмонология. 1995;(2):55–61. Chuchalin A.G., Osipova G.L., Egorova N.B., et al. Controlled studies on the effectiveness of a multicomponent vaccine in immunotherapy in patients with chronic obstructive respiratory diseases. Russian Pulmonology. 1995;(2):55–61. EDN: https://www.elibrary.ru/xctiqb
- Немыкина О.Е., Егорова Н.Б., Щербакова Б.В. и др. Оптимизация лечения атопического дерматита с помощью иммунотерапии. Журнал микробиологии, эпидемиологии и иммунобиологии. 2005;85(4):53–7. Nemykina O.E., Egorova N.B., Shcherbakova B.V., et al. Optimization of treatment of atopic dermatitis with the help of immunotherapy. Journal of Microbiology, Epidemiology and Immunobiology. 2005;85(4):53–7. EDN: https://www.elibrary.ru/ymzeiw
- Антонова Л.П., Маркова Т.П., Курбатова Е.А. Применение поликомпонентной вакцины ВП-4 комбинированным назально-подкожным методом в лечении больных бронхиальной астмой и хроническим обструктивным бронхитом. Журнал микробиологии, эпидемиологии и иммунобиологии. 2004;84(6):36–40. Antonova L.P., Markova T.P., Kurbatova E.A. Application of polycomponent vaccine VP-4 by combined nasal-subcutaneous method in the treatment of patients with bronchial asthma and chronic obstructive bronchitis. Journal of Microbiology, Epidemiology and Immunobiology. 2004;84(6):36–40.
- Немыкина О.Е., Егорова Н.Б., Курбатова Е.А. и др. Иммунологические показатели при терапии атопического дерматита у детей поликомпонентной вакциной Иммуновак ВП-4. Журнал микробиологии, эпидемиологии и иммунобиологии. 2005; 85(5):45–9. Nemykina O.E., Egorova N.B., Shcherbakova B.V., et al. Immunological characteristics in the therapy of atopic dermatitis in children with polycomponent vaccine Immunovac ВП-4. Journal of Microbiology, Epidemiology and Immunobiology. 2005;85(5):45–9. EDN: https://www.elibrary.ru/hsuwwp
- Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19(3):141–54. DOI: https://doi.org/10.1038/s41579-020-00459-7
- Harrison A.G., Lin T., Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020;41(12): 1100–5. DOI: https://doi.org/10.1016/j.it.2020.10.004
- Tu Y.F., Chien C.S., Yarmishyn A.A., et al. A review of SARS — CoV-2 and the ongoing clinical trials. Int. J. Mol. Sci. 2020;21(7):2657. DOI: https://doi.org/10.3390/ijms21072657
- Грачева А.В., Корчевая Е.Р., Кудряшова А.М. и др. Адаптация МТТ – теста для определения нейтрализующих антител к вирусу SARS-CoV-2. Журнал микробиологии, эпидемиологии и иммунобиологии. 2021;98(3):253–65. Gracheva A.V., Korchevaya E.R., Kudryashova A.M., et al. Adaptation of the MTT assay for detection of neutralizing antibodies against the SARS-CoV-2 virus. Journal of Microbiology, Epidemiology and Immunobiology. 2021;98(3):253–65. DOI: https://doi.org/10.36233/0372-9311-136 EDN: https://www.elibrary.ru/jglovv
- Баркова Е.П., Нагиева Ф.Г., Кузнецов В.П. и др. Экспрессия рецепторов для человеческих интерферонов альфа и гамма на поверхности мононуклеарных клеток периферической крови при некоторых вирусных инфекциях. Вопросы вирусологии. 1998;43(6):16–8. Barkova E.P., Nagieva F.G., Kuznetsov V.P., et al. Expression of receptors for human interferons alpha and gamma on the surface of peripheral blood mononuclear cells in some viral infections. Problems of Virology. 1998;43(6):16–8. EDN: https://www.elibrary.ru/wiapnb
- Лисаков А.Н., Нагиева Ф.Г., Баркова Е.П. и др. Исследование in vitro интерфероновых рецепторов иммунокомпетентных клеток при экспериментальных гриппозных инфекциях. Инфекция и иммунитет. 2015;5(3):273–8. Lisakov A.N., Nagieva F.G., Barkova E.P., et al. The immunocompetent cells receptors research under experimental influenza infection in vitro. Russian Journal of Infection and Immunity. 2015;5(3):273–8. DOI: https://doi.org/10.15789/2220-7619-2015-3-273-278 EDN: https://www.elibrary.ru/ukjpgj
- Шубладзе А.К., Гайдамович С.Я. Краткий курс практической вирусологии. М.;1954:92–3. Shubladze A.K., Gaidamovich S.Ya. A Short Course of Practical Virology. Moscow;1954:92–3.
- Михайлова Н.А., Нагиева Ф.Г., Гринько О.Н., Зверев В.В. Изучение противовирусной активности спорообразующих бактерий Bacillus pumilus (штамм Пашков) при экспериментальной энтеровирусной инфекции in vitro. Журнал микробиологии, эпидемиологии и иммунобиологии. 2010;87(2): 69–74. Mikhaylova N.A., Nagieva F.G., Grinko O.M., Zverev V.V. Experimental study of antiviral activity of spore-forming bacterium Bacillus pumilus "Pashkov". Journal of Microbiology, Epidemiology and Immunobiology. 2010; 87(2):69–74. EDN: https://www.elibrary.ru/rurigl
- Bracci N., Pan H.C., Lehman C., et al. Improved plaque assay for human coronaviruses 229 E and OC43. PeerJ. 2020;8:e10639. DOI: https://doi.org/10.7717/peerj.10639
- Norrby E. Hemagglutination by measles virus. 4. A simple procedure for production of high potency antigen for hemagglutination-inhibition (HI) tests. Proc. Soc. Exp. Biol. Med. 1962; 111:814–8. DOI: https://doi.org/10.3181/00379727-111-27930
Дополнительные файлы