Production of recombinant norovirus VP1 protein and its antigenic and immunogenic properties
- Authors: Lapin V.A.1, Novikov D.V.1, Mokhonova E.V.1, Melentyev D.A.1, Tsyganova M.I.1, Zaitsev D.E.1, Novikov V.V.1
-
Affiliations:
- Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology
- Issue: Vol 101, No 5 (2024)
- Pages: 661-667
- Section: ORIGINAL RESEARCHES
- URL: https://microbiol.crie.ru/jour/article/view/18687
- DOI: https://doi.org/10.36233/0372-9311-552
- EDN: https://elibrary.ru/ubmktf
- ID: 18687
Cite item
Abstract
Introduction. The importance of noroviruses in human infectious pathology and the danger of large epidemic outbreaks in organized groups determine the need to develop means of specific prevention of infection.
The aim of the study was to obtain recombinant norovirus VP1 protein and analyze its immunogenic and antigenic properties .
Materials and methods. Computer analysis of nucleotide and amino acid sequences, molecular cloning, polymerase chain reaction, electrophoresis of nucleic acids in agarose gel and proteins in polacrylamide gel, affinity chromatography, enzyme immunoassay.
Results and discussion. A genetic construct encoding recombinant VP1 of the GII genotype norovirus with codons optimized for highly effective expression in Escherichia coli has been created. The strain of E. coli Rosetta 2 (DE3) has been transformed by genetic construct. VP1 expression was carried out in E. coli cells, conditions for its production, purification and renaturation were optimized. A purified soluble recombinant VP1 protein forms virus-like particles with a diameter of 30–50 nm. Immunization of BALB/c mice by protein lead to antibodies production with a titer greater than 1 : 1000. When evaluating antigenic properties, it was shown that human IgG, IgM, and IgA antibodies interact with recombinant VP1. The total antibody detection rate was 47,4%. The results indicate the possibility of using recombinant VP1 for development of domestic vaccine for the prevention of norovirus infection.
Keywords
Full Text
Introduction
In the etiologic structure of viral acute intestinal infections, noroviruses (NV; Caliciviridae family, Norovirus genus) rank second after rotaviruses. In countries that vaccinate against rotaviruses, NVs have taken the 1st place [1, 2]. The risk groups for NV infection include children, young and elderly people. Outbreaks of NV infection are reported throughout the year with an increase in incidence in the spring and summer months.
Human NV is an non-enveloped icosahedral virus, and has a genome in the form of a single-stranded positive-sense RNA approximately 7.5-7.7 kb long, encoding 3 open reading frames. ORF1 encodes a large polyprotein, the precursor of 6 non-structural proteins (NS1/2-NS7), ORF2 encodes the major structural capsid protein, and ORF3 encodes the minor structural capsid protein VP2, which is located within the viral particle. The virus capsid is constructed of an outer (VP1) and an inner (VP2) protein. VP1 is capable of self-assembling into virus-like particles that are virtually indistinguishable from native virions and have pronounced immunogenic properties.
Ten genogroups are known for NV, and 48 genotypes have been identified based on amino acid sequence analysis of the external capsid protein VP1. The most common NV genogroup is GII, which accounts for the majority of cases of NV-gastroenteritis of children in the first years of life in Russia. Thus, in the Sverdlovsk region in 2022, the most common occurring genotypic structure of circulating NVs was a norovirus belonging to the GII genogroup (58%). Similar data were obtained during molecular epidemiologic analysis of genetic variants of NVs in a number of other European countries, Japan and China. In certain years, GII genogroup accounted for up to 80-90% of cases of pediatric NV-gastroenteritis. The dominant virus variants of this genogroup include NV GII.4 [3, 4]. The importance of NV in human infectious pathology and the danger of large epidemic outbreaks in organized groups determine the need to develop means of specific prophylaxis of infection. The example of successful introduction of rotavirus vaccine shows that vaccination programs can significantly reduce the number of cases of gastroenteritis [5]. Based on NV proteins, several candidate NV vaccines are also being developed worldwide, two of which are currently in phase II/III clinical trials for the prevention of NV infection in children and adults [6, 7].
The aim of the study was to obtain recombinant NV VP1 protein and analyze its immunogenic and antigenic properties.
Materials and methods
Nucleotide sequence analysis, oligonucleotide design, gene construction, calculation of protein molecular weight, isoelectric point and extinction coefficient were performed using the Lasergene 7.1.0 software package (Dnastar, Inc.). Codon optimization was performed using the Codonusage database1. The nucleotide sequence of VP1 of the epidemic variant of NV with genotype GII.4, prevalent in the territory of Nizhny Novgorod region, was used. Nucleotide sequences were sequenced using the ABI Prism 310 genetic analyzer (Thermo Fisher Scientific).
Escherichia coli cells, strain Rosetta 2 (DE3), transformed with the obtained genetic construct based on plasmid pET22b and encoding VP1 NV, were grown in LB-Miller medium, pH 7.0. Induction of protein synthesis was performed by adding isopropyl-β-D-1-thiogalactopyranoside to a final concentration of 0.5 mM to each culture. Cell biomasses were obtained by centrifugation, lysed in a solution containing 25 mM HEPES (pH 7.5), 1 M NaCl, 10% glycerol, 1% Triton X-100, DNase I (10 μg/mL), RNaseA (10 μg/mL), lysozyme (50 μg/mL), and 0.2 mM phenylmethylsulfonyl fluoride, disintegrated by ultrasound using a QSonica Q55 (QSonica sonicators), a centrifuged and washed buffer containing 25 mM HEPES pH 7.5, 1 M NaCl, 10% glycerol, 1 M urea was added, followed by centrifugation.
The recombinant norovirus VP1 protein was purified by metal chelate chromatography under denaturing conditions using Ni-NTA Superflow sorbent (GEHealthcare). Renaturation of VP1 was performed by dialysis. Protein electrophoresis in 12% polyacrylamide gel in the presence of sodium dodecyl sulfate was carried out by the conventional method, immunoblotting was performed using human serum antibodies against NV VP1 and horseradish peroxidase-conjugated monoclonal antibodies to human IgG Hytest. After transfer, proteins on the membrane were stained in Super Signal West Dura Extended Duration Substrate solution (Thermo Scientific) and chemical luminescence was measured using a C-DiGit Blot Scanner (Li-Cog). Microphotographs of virus-like particles formed by NV VP1 were obtained using an NT7700 electron microscope (Hitachi). Female BALB/c mice 8 weeks old and weighing 16-18 g were used for immunization. Animals were kept in vivarium conditions in accordance with interstate standards GOST 33216-2014 and GOST 33215-2014. Biomaterial for the study was taken from mice in compliance with the principles of humanity set forth in the European Community directives (86/609/EC).
The studies were conducted according to the bioethical and ethical principles established by the Declaration of Helsinki (adopted in June 1964 and revised in October 2013). To evaluate the antigenic properties of recombinant proteins, we used 637 blood plasma samples obtained from the Hemohelp diagnostic center (TIAS LOTUS LLC) from individuals aged 19–44 years who applied for diagnostic studies and gave written consent for the use of their biomaterial in the study.
Antibodies to NV VP1 was determined by solid-phase enzyme-linked immunosorbent assay. VP1 was sorbed into wells of plates at a concentration of 1 μg/mL for 18 h at 20°C. Mouse serum to be tested was diluted in increments of 2, and volunteer plasma was diluted before testing in increments of 10. Serum from unimmunized mice was used as a negative control. When antibodies were determined in the blood of laboratory animals, rabbit antibodies against mouse immunoglobulins conjugated with horseradish peroxidase were used. When determining human antibodies, horseradish peroxidase-conjugated rabbit antibodies against immunoglobulins of classes G, M and A were used. The value of optical density greater than the average value of the negative control multiplied by three was taken as a cut-off for reactivity.
The obtained data were analyzed using Microsoft Excel software (Microsoft). Statistical processing of data was performed using the Graph Pad Prism 8 program (Graph Pad Software). Differences in data were considered statistically significant at p ≤ 0.05.
Results
A site for the NdeI restriction endonuclease was added to the beginning of the nucleotide sequence encoding the VP1 protein of the epidemically significant strain of NV genotype GII.4. To the sequence encoding the C-terminal part of the protein, a nucleotide sequence encoding 6 histidines, a stop codon (TAA), and a site for the XhoI restriction endonuclease was added and used for subsequent molecular cloning. The schematic structure of the gene is shown in Figure 1. The resulting sequence was synthesized at Eurogen. The sequence encoding VP1 was transferred to the plasmid pET22b (Thermo Fisher Scientific), which allows high efficiency expression of recombinant proteins in E. coli strains containing DE3 lysogen in the genome [8].
Fig. 1. Schematic representation of the genetic construct encoding VP1 of norovirus in pET22b.
The obtained genetic construct encoding NV VP1 was transformed into E. coli cells, Rosetta 2 strain (DE3). The efficiency of protein production was evaluated, which amounted to 20–40 mg of protein per 1 liter of cell culture. The protein formed inclusion bodies. The optimal medium composition and culture conditions for transformed E. coli cells were determined. The maximum cell culture density (OD600 = 2.8) corresponded to 5 g of biomass per 1 L of culture in LB medium containing 0.5% glycerol and 25 mM phosphate buffer pH 7.4. The optimal concentration of isopropyl-β-D-1-thiogalactopyranoside was 0.5 mM, the optimal temperature for protein expression was 30ºC, and the induction time was 4–8 h.
Subsequent purification by metal-chelate chromatography in the presence of 8 M urea and refolding by dialysis against a solution containing 25 mM HEPES pH 7.5, 150 mM NaCl, and 5% glucose resulted in a soluble protein consisting of 560 amino acids, having a calculated molecular mass of 60.6 kDa, an isoelectric point equal to 6.15 and an extinction coefficient of 1.04 (Fig. 2, a).
Fig. 2. Characteristics of the recombinant VP1 protein.
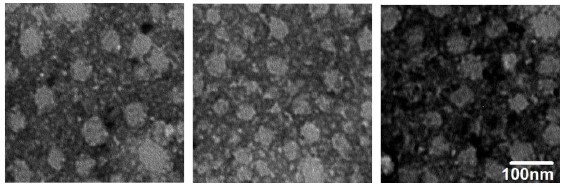
a — electrophoregram of purified norovirus VP1 protein; b — Western-blot of purified norovirus VP1 protein; 1 — VP1; 2 — molecular weight marker; с — electron microscopic photographs of virus-like particles formed by recombinant norovirus VP1. Contrast with 3% uranyl acetate, pH 4.6.
The protein was produced in preparative quantities and used to assess the ability to form virus-like particles and for immunization of laboratory mice. Fig. 2, b shows an electron microscopic photograph indicating the ability of recombinant VP1 protein to form virus-like particles with a diameter of 30-50 nm, which is consistent with the data of other authors [9]. At the same time, western blot (Fig. 2, c) showed the ability of recombinant VP1 to interact with serum antibodies of seropositive individuals.
Two intraperitoneal immunizations of 10 laboratory mice with an interval of 2 weeks and subsequent obtaining of blood serum 3 weeks after the second immunization at a dose of 10 µg (0.5 ml) resulted in the formation of antibodies against NV VP1 in the blood of animals. Antibodies in the blood of animals were detected in titers from 1 : 1024 to 1 : 4096, the average titer was 1 : 1536. Immunization of animals with the same dose of protein according to the same scheme, but mixed with 100 mg of aluminum hydroxide caused the appearance of antibodies against NV VP1 in titers up to 1 : 32,768. On average, the antibodies titer was 1 : 13,720, which was almost an order of magnitude higher than the antibodies titers in animals immunized without aluminum hydroxide (Fig. 3). Thus, it was shown that the obtained recombinant protein is able to induce a pronounced antisense response, which significantly increases in the presence of the used adjuvant.
Fig. 3. Antibody titers in mice immunized with recombinant norovirus VP1.
1 — without aluminum hydroxide; 2 — with aluminum hydroxide.
Since NV is widely circulating among the population, it is natural to expect the presence of antibodies to its proteins in human blood. We evaluated the presence of antibodies of different classes to the obtained recombinant protein in the blood of individuals living in the middle belt of Russia. The frequency of detection of antibodies to VP1 in blood plasma samples of 637 volunteers was determined. As shown in Fig. 4, IgG antibodies were detected in 14.8% of volunteers, IgM antibodies — in 7.1%, IgA antibodies — in 38.5%. The cumulative occurrence of antibodies was 47.4%.
Fig. 4. Detection rate of antibodies of different classes to recombinant norovirus VP1 in the blood of healthy volunteers.
The results indicate that the epitopes of the recombinant VP1 protein of NV GII.4 obtained by us are recognized by antibodies present in human blood. However, IgG antibodiy titers were mostly low and exceeded a value of 1 : 1000 or more in seropositive volunteers only in 4.3% of cases (4 of 94), indicating that these individuals had a long history of NV exposure. In volunteers who had IgA antibodies to NV VP1, high titers (equal to 1 : 1000 or more) were detected with similar frequency (4.9% of cases). In contrast, of the 25 volunteers who had IgM antibodies to NV VP1, titers equal to 1 : 1000 or more were detected in 16% of cases. It is likely that these individuals had recent exposure to the virus.
Discussion
The data obtained on the frequency of antibodies against the recombinant VP1 protein of NV GII.4 and their titers are consistent with the results found by other authors. A wide variation in the frequency of antibodies detection and their titers in individuals of different ages living in different countries has been reported. The cumulative detection rate ranges from 25 to 95%. Cross-reactivity with NV of other genogroups is observed [10, 11].
The NV VP1 protein consists of 2 domains involved in the self-assembly of virus-like particles [12]. The ability of NV VP1 to self-assemble can be used to obtain chimeric proteins forming virus-like particles, consisting of the S-domain of NV VP1 and fragments of other proteins acting as antigen. That is, there is a possibility of decorating virus-like particles of NV with different antigens, which has been demonstrated in the works of a number of authors [13–15]. The genetic construct encoding the recombinant protein we obtained can be used as a molecular platform for the creation of chimeric virus-like particles based on NV VP1.
Conclusion
The recombinant NV VP1 protein expressed in E. coli obtained by us is capable of forming virus-like particles, shows immunogenicity in mice in the absence and presence of adjuvant, and is recognized by human antibodies of IgG, IgM and IgA classes. The results of this work indicate the possibility of using recombinant VP1 as an antigen in the design of a vaccine for the prevention of NV infection based on virus-like particles.
1 URL: http://www.kazusa.or.jp/codon/
About the authors
Vladislav A. Lapin
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology
Email: mbre@mail.ru
ORCID iD: 0000-0002-5905-5722
junior researcher, Laboratory of immunochemistry
Russian Federation, Nizhny NovgorodDmitry V. Novikov
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology
Email: mbre@mail.ru
ORCID iD: 0000-0001-7049-6935
Cand. Sci. (Biol.), leading researcher, Laboratory of immunochemistry
Russian Federation, Nizhny NovgorodEkaterina V. Mokhonova
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology
Email: mbre@mail.ru
ORCID iD: 0000-0002-9742-7646
researcher, Laboratory of immunochemistry
Russian Federation, Nizhny NovgorodDmitry A. Melentyev
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology
Email: mbre@mail.ru
ORCID iD: 0000-0002-2441-6874
junior researcher, Laboratory of immunochemistry
Russian Federation, Nizhny NovgorodMaria I. Tsyganova
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology
Email: mbre@mail.ru
ORCID iD: 0000-0002-2811-6844
Cand. Sci. (Biol.), leading researcher, Laboratory of immunochemistry
Russian Federation, Nizhny NovgorodDmitry E. Zaitsev
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology
Email: mbre@mail.ru
ORCID iD: 0000-0002-7663-6924
senior laboratory assistant, Laboratory of immunochemistry
Russian Federation, Nizhny NovgorodViktor V. Novikov
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology
Author for correspondence.
Email: mbre@mail.ru
ORCID iD: 0000-0002-2449-7213
D. Sci. (Biol.), Professor, Head, Laboratory of immunochemistry
Russian Federation, Nizhny NovgorodReferences
- Black R.E., Perin J., Yeung D., et al. Estimated global and regional causes of deaths from diarrhoea in children younger than 5 years during 2000-21: a systematic review and Bayesian multinomial analysis. Lancet Glob. Health. 2024;12(6):e919–28. DOI: https://doi.org/10.1016/S2214-109X(24)00078-0
- Jeon K., Lee S.K., Jeong S., et al. Trends in the detection of viruses causing gastroenteritis over a 10-year period and impact of nonpharmaceutical interventions. J. Clin. Virol. 2024;172:105676. DOI: https://doi.org/10.1016/j.jcv.2024.105676
- van Beek J., de Graaf M., Al-Hello H., et al. Molecular surveillance of norovirus, 2005–16: an epidemiological analysis of data collected from the NoroNet network. Lancet Infect. Dis. 2018;18(5):545–53. DOI: https://doi.org/10.1016/s1473-3099(18)30059-8
- Быков Р.О., Скрябина С.В., Килячина А.С. и др. Молекулярно-генетическая характеристика и филогенетический анализ возбудителей норовирусной инфекции человека отдельных муниципалитетов в Свердловской области за 2022 год. Журнал микробиологии, эпидемиологии и иммунобиологии. 2023;100(4):306–313. Bykov R.O., Scriabina S.V., Kilyachina A.S., et al. Genetic characterization and phylogenetic analysis of human norovirus infection in individual municipalities of the Sverdlovsk region in 2022. Journal of Microbiology, Epidemiology and Immunobiology. 2023; 100(4):306–13. DOI: https://doi.org/10.36233/0372-9311-402 EDN: https://elibrary.ru/qiehre
- Burnett E., Parashar U., Tate J. Rotavirus vaccines: effectiveness, safety, and future directions. Paediatric Drugs. 2018;20:223–33. DOI: https://doi.org/10.1007/s40272-018-0283-3
- Treanor J., Sherwood J., Cramer J.P., et al. A phase 2 study of the bivalent VLP norovirus vaccine candidate in older adults; impact of MPL adjuvant or a second dose. Vaccine. 2020;38(36):5842–50. DOI: https://doi.org/10.1016/j.vaccine.2020.06.011
- López P., López-Medina E., Sáez-Llorens X., et al. Immunogenicity and tolerability of a bivalent virus-like particle norovirus vaccine candidate in children from 6 months up to 4 years of age: a phase 2 randomized, double-blind trial. Hum. Vaccin. Immunother. 2023;19(1):2204787. DOI: https://doi.org/10.1080/21645515.2023.2204787
- Mokhonov V.V., Vasilenko E.A., Gorshkova E.N., et al. SlyD-deficient Escherichia coli strains: a highway to contaminant-free protein extraction. Biochem. Biophys. Res. Commun. 2018;499(4):967–72. DOI: https://doi.org/10.1016/j.bbrc.2018.04.029
- Lampinen V., Gröhn S., Soppela S., et al. SpyTag/SpyCatcher display of influenza M2e peptide on norovirus-like particle provides stronger immunization than direct genetic fusion. Front. Cell Infect. Microbiol. 2023;13:1216364. DOI: https://doi.org/10.3389/fcimb.2023.1216364
- Kobayashi S., Fujiwara N., Rockx B., et al. Characterization of the homo- and heterotypic immune responses after natural norovirus infection. J. Med. Virol. 2005;77:439–46. DOI: https://doi.org/10.1002/jmv.20473
- Takeda N., Minagawa H. Seroepidemiological study of norovirus infection in Aichi Prefecture, Japan. Microbiol. Immunol. 2009;53:356–9. DOI: https://doi.org/10.1111/j.1348-0421.2009.00132.x
- Tan M., Fang P., Chachiyo T., et al. Noroviral particle: structure, function and applications in virus-host interaction. Virology. 2008;382:115–23. DOI: https://doi.org/10.1016/j.virol.2008.08.047
- Новиков Д.В., Мелентьев Д.А., Мохонов В.В., и др. Получение вирусоподобных частиц норовируса, содержащих VP1 эховируса 30. Вопросы вирусологии. 2021;66(5):383–9. Novikov D.V., Melentev D.A., Mokhonov V.V., et al. Construction of norovirus (Caliciviridae: Norovirus) virus-like particles containing VP1 of the Echovirus 30 (Piconaviridae: Enterovirus: Enterovirus B). Problems of Virology. 2021;66(5):383–9. DOI: https://doi.org/10.36233/0507-4088-79 EDN: https://elibrary.ru/mkbqet
- Tamminen K., Heinimäki S., Vesikari T., Blazevic V. Rotavirus VP6 adjuvant effect on norovirus GII.4 virus-like particle uptake and presentation by bone marrow-derived dendritic cells in vitro and in vivo. J. Immunol. Res. 2020;2020:3194704. DOI: https://doi.org/10.1155/2020/3194704
- Boonyakida J., Khoris I.M., Nasrin F., Park E.Y. Improvement of modular protein display efficiency in SpyTag-implemented norovirus-like particles. Biomacromolecules. 2023;24(1): 308–18. DOI: https://doi.org/10.1021/acs.biomac.2c01150
Supplementary files