Выявление Streptococcus agalactiae молекулярными методами у беременных женщин и частота вертикальной передачи новорождённым в провинции Вавилон
- Авторы: Marhash A.D.1, Nabat Z.N.1, Abbas N.A.1
-
Учреждения:
- Вавилонский технический институт, Технический университет Аль-Фурат Аль-Аусат
- Выпуск: Том 101, № 6 (2024)
- Страницы: 812-819
- Раздел: ОРИГИНАЛЬНЫЕ ИССЛЕДОВАНИЯ
- URL: https://microbiol.crie.ru/jour/article/view/18557
- DOI: https://doi.org/10.36233/0372-9311-515
- EDN: https://elibrary.ru/gvsihk
- ID: 18557
Цитировать
Полный текст
Аннотация
Введение. Streptococcus agalactiae — грамположительные, неподвижные и инкапсулированные кокки. На кровяном агаре они образуют узкую зону бета-гемолиза. Этот возбудитель при передаче от инфицированных матерей вызывает инвазивные бактериальные заболевания у новорождённых, в том числе сепсис, менингит, септицемию и пневмонию. Streptococcus agalactiae является патогеном, вызывающим первоочередную озабоченность общественного здравоохранения.
Цель работы — провести объективное исследование по выделению и молекулярному выявлению гена вирулентности стрептококка группы B (СГВ) и оценить частоту передачи инфекции от матери новорождённому.
Материалы и методы. В проспективное когортное исследование вошли 300 беременных женщин со сроком беременности более 35 нед. У всех участниц исследования собирали вагинальные мазки, всех женщин с диагностированным СГВ обследовали после родов, чтобы взять мазки у их новорождённых. Для оценки выделенных бактерий использовали традиционные микробиологические и молекулярные подходы.
Результаты. В исследовании приняли участие 60 (20%) из 300 беременных женщин и 16 (26,6%) их новорождённых. СГB был обнаружен с помощью культуральных методов и подтверждён с помощью ПЦР с праймерами для выявления гена atr (гена домашнего хозяйства). Положительные изоляты были на 100% чувствительны к антибиотикам, таким как цефтриаксон, пенициллин и ванкомицин, 93% были чувствительны к хлорамфениколу, 83% — к эритромицину, 13% — к тетрациклину.
Заключение. Наши данные показали высокую частоту инфицирования СГВ у беременных женщин и их новорождённых. Необходимо проводить обязательный скрининг и профилактическое лечение, чтобы свести к минимуму потенциально смертельные последствия этого заболевания.
Полный текст
Introduction
Group B Streptococcus agalactiae (GBS) are normal inhabitants of the human gastrointestinal and genitourinary systems. They are the primary cause of serious bacterial infections like neonatal meningitis, symptoms-free bacteriuria, Urinary tract infections (UTIs), bladder infection, inflammation of kidneys and pelvis, intra-amniotic infection (IAI), postpartum endometritis and pre- and postpartum bacteraemia. It is a gram-positive, opportunistic, beta-haemolytic bacteria with possible complications. It also causes infections of surgical wounds in pregnant women [1, 2]. By maternal rectovaginal colonization, GBS causes a variety of prenatal and maternal illnesses, consisting of infections in the mother, stillbirths, premature births, as well as early and late-onset sepsis in infants [3–6]. GBS-colonized mothers run the risk of vertical transmission of these bacteria to the newborn. It is one of the risk factors for early-onset sepsis in newborns [7]. This dynamic colonization constitutes the highest infectious disease risk for newborns. Notably, reports from international literature show that the rates of maternal GBS colonization were 6.5–36% in Europe [5, 6], 10–30% in North America [7, 8], 16.5–31.6% in African nations [9], and 1.4–36.7% in South America, which includes Brazil [10, 11], Chile1, Peru [12], and Argentina [13].
The first recommendations for women's intrapartum antibiotic treatment to prevent GBS infection were established in 1966 [12]. Following the introduction of these treatments, the incidence of newborn GBS disease was reduced by 80% in America [14]. According to recommendations from the CDC (Centres for Disease Control and Prevention)2, GBS can be diagnosed at 35–37 weeks of gestation using selective enrichment broth culture; however, this is not always possible in poor-income regions [5].
Maternal colonization with GBS is the greatest significant threat for newborn early-onset disease (EOD) (from 0 to 6 days) [15]. In 1973, a single infant was born to a GBS-infected woman from a group of 46 pregnant mothers, and EOD was first recorded. Among the neonates born to women with GBS infection, 2.17% were at risk for EOD at the time intrapartum antibiotic prophylaxis (IAP) has been suggested for both microbiological and risk-based screening in the United States [8]. According to the data from the World Health Organization (WHO), there were 1601 new-born fatalities in Sri Lanka in 2017, of which 0.04% were related to sepsis and other infectious diseases [11]. The convenient use of antibiotics and the detection of pathogens can further lower mortality rates in this population [15]. Consequently, monitoring maternal pathogen colonization is a crucial safeguard against infant infection. In 1980, the global prevalence of maternal GBS colonization was 18%, the Caribbean had a higher prevalence (34%), while Melanesia had the lowest (2%). Similar colonization rates (23%) were observed in North America, Europe, and Australia; Comparatively, the incidence was slightly higher in South Africa than in the Western nations, although it was lower in East Asia (9%), South and Southeast of Asia together was observed around (14%), West Africa (13%), and Central America (10%) [10]. The lack of publications in East Asia, South Asia, Southeast Asia, Western Africa and Central America may be the cause of the subpar prevalence estimates from these locations [10].
Polymerase chain reaction (PCR) assays provide an additional option for the quick identification of GBS colonization [11]. The aim of our study was the isolation of GBS strains and detection of the virulence gene by molecular method from pregnant mothers and their neonates, evaluation of the ratio of vertical transmission from infected women to their neonates, and reduction of the mortality and morbidity rates associated with GBS infection by using appropriate prophylactic antibiotics in Iraq, where neither screening for GBS nor an IAP protocol existed until the beginning of this study.
Materials and methods
Study Design
A prospective cohort study was designed that involved 300 pregnant women who were at 35 weeks or more of gestation. Three hundred vaginal swabs were taken before labor from all expectant mothers by the gynaecologist and 60 swabs from neonates born to GBS-positive women were taken shortly (to avoid contamination from other sources) after birth in the delivery room, including neonatal swabs from three sites (oral cavity, ear, and umbilicus). These neonates were born healthy.
Inclusion and Exclusion Criteria
Pregnant women who were in the final weeks of gestation (≥ 35 weeks) and were attending the Al-Zahraa Hospital of Obstetrics in Babylon (Iraq) were included in this study. This study excluded pregnant women who took antibiotics within 10 days of delivery and those who underwent caesarean surgery.
Bacteriological Identification of Isolates
All swab isolates from pregnant women and their neonates were screened for the period from November 2021 to June 2022. It was done by incubating specimens directly in the Todd-Hewitt Broth selective media overnight at 37ºC and subsequently subculturing them on blood agar to select the proper colony. The colonies were inspected and identified using the following criteria: a narrow beta haemolysis zone, gram positive cocci, bacitracin resistance, catalase negativity, sodium hippurate hydrolysis positivity, and CAMP positivity, in order to determine whether or not the plates contained GBS organisms.
Molecular identification of isolates
Samples that were positive for GBS in the culture method were sent for molecular detection after DNA extraction using the bacterial DNA extraction kit Geneaid (Taiwan). The primers and PCR steps used in the experiment to amplify atr housekeeping gene are listed in Table 1 and Table 2. 1 μl of each upstream and downstream primer, 16 μl of nuclease-free water and 2 μl of extracted DNA were added to Master Mix to total volume of 25 μl in each reaction. Electrophoresis in a 2% agarose gel was used for detection of the PCR amplification product.
Table 1. Primer sequence for atr genes [16]
Gene | Primer Sequence (5ʹ–3ʹ) | Size, bp |
Atr | F-5’CAACGATTCTCTCAGCTTTGTTAA3' R-5’TAAGAAATCTCTTGTGCGGATTTC3' | 780 |
Table 2. PCR programme
Steps | Temperature, °C | Time, min | Cycles |
Initial denaturation | 94 | 1.00 | 1 |
Denaturation | 94 | 1.00 | |
Annealing | 55 | 0.75 | 30 |
Elongation | 72 | 1.00 | |
Final extension | 72 | 10.00 | 1 |
Hold | 4 | 7.00 | 1 |
Antibiotic susceptibility test
The CLSI-recommended Kirby–Bauer disc diffusion technique with modifications on Muller Hinton agar and 5% blood was used to conduct the antibiotic sensitivity test [12]. In this method, antibiotics such as ciprofloxacin, ampicillin, penicillin, chloramphenicol, erythromycin, levofloxacin, vancomycin, tetracycline and levofloxacin were used.
Statistical Analysis
Student t-test was employed for quantitative variables and Chi-squared test was performed whenever available for binomial variables. P values less than 0.05 were considered to be statistically significant.
Results
The GBS colonization rate was 20% (60/300) among three hundred pregnant women and 26.6% (16/60) in neonates born of GBS-positive mothers (Table 3). In this study, the youngest woman was 15 years old, and the oldest woman was 44 years old, with the average age of participants being 28 years. The various sociodemographic variables are shown in Table 4 and Table 5. On comparing the variations in GBS colonization rates between rural and urban residents, a statistically significant difference was found for GBS colonization among GBS-positive women (78.3% in rural residents versus 21.7% in urban residents; p = 0.015).
Table 3. GBS Isolates from Women and Their Newborns
Isolates | Frequency | Percentage | Total |
Pregnant women | 60 | 20.0 | 300 |
Newborns | 16 | 26.6 | 60 |
Table 4. Age, residence and parity of patients
Variables | Culture result | Total | p | ||
negative (n = 240) | positive (n = 60) | ||||
Age (mean ± SD), years Range | 28.44 ± 8.20 (15–44) | 29.92 ± 8.60 (15–41) | 28.60 ± 8.20 (15–44) | 0.3 | |
Residence | Urban n (%) | 92 (28.4) | 13 (21.7) | 105 (100.0) | 0.015* |
Rural n (%) | 148 (61.6) | 47 (78.3) | 195 (100.0) | ||
Parity | 1 n (%) | 26 (10.8) | 7 (11.6) | 33 (100.0) | 0.2856 |
2 n (%) | 64 (26.6) | 15 (25.0) | 79 (100.0) | ||
> 3 n (%) | 150 (61.6) | 38 (63.3) | 188 (100.0) | ||
Note. *Represent a significant difference at p ≤ 0.05.
Table 5. Age groups of female participants
Age group, years | Culture | Total | p | |
negative, n (%) | positive, n (%) | |||
15–24 | 68 (28.4) | 15 (20.0) | 83 (27.7) | 0.784 |
25–34 | 148 (61.6) | 37 (61.1) | 185 (61.6) | |
35–44 | 24 (10.0) | 8 (18.9) | 32 (10.7) | |
Total | 240 (100.0) | 60 (100.0) | 300 (100.0) | |
Molecular detection of all GBS isolates
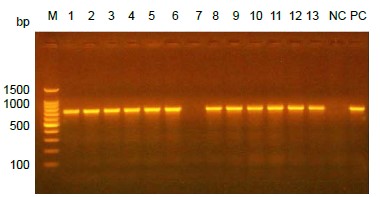
Conventional PCR was used to detect a specific gene (atr) to confirm the bacteriological identification of Streptococcus agalactiae. This gene had a molecular size of 780 bp in gel electrophoresis as shown in Figure.
Electrophoresis of PCR product for atr gene on agarose gel. Lane M represents 100 bp DNA ladder, every lane (1–13) except lane 7 represented positive results. Lanes NC and PC represented negative and positive controls respectively.
Antimicrobial sensitivity
The susceptibility of the isolates towards antibiotics is shown in Table 6. Based on the results of antimicrobial sensitivity, it was found that there is more than one antibiotic capable of inhibiting the growth of this bacteria, so they can be used as alternatives in the event of a drug allergy in the patient.
Table 6. Antimicrobial sensitivity of maternal and neonate isolates
Antimicrobial agent | Sensitivity of GBS Isolates | |
mothers (n = 60), n (%) | neonates (n = 16), n (%) | |
Penicillin | 60 (100.0) | 16 (100.0) |
Ampicillin | 60 (100.0) | 16 (100.0) |
Levofloxacin | 55 (91.6) | 15 (93.7) |
Vancomycin | 60 (100.0) | 16 (100.0) |
Tetracycline | 8 (13.3) | 4 (25.0) |
Ceftriaxone | 60 (100.0) | 16 (100.0) |
Erythromycin | 50 (83.3) | 13 (81.2) |
Chloramphenicol | 56 (93.3) | 15 (93.7) |
Ciprofloxacin | 58 (96.6) | 16 (100.0) |
Discussion
Due to the possibility of transmission from the mother to the fetus throughout the pregnancy and the postpartum period, resulting in serious illness or death, research into maternal GBS colonization is crucial. The effect of GBS infection is not restricted to childhood only but may continue to adulthood and may lead to dangerous neurological disorders. The prevalence of GBS maternal colonization worldwide is variable because this prevalence depends on many variable factors like hygienic conditions, socio demographic conditions, sample population, diagnostic techniques, and others. Our research is a conformational vertical transmission study conducted in Babylon (Iraq). This is also the first GBS cohort study that has been done in Babylon. 20% of pre-delivery mothers and 26.6% of neonates born of GBS-positive mothers were found to be colonized with GBS. The colonization rate in mothers observed in our study was comparable to the global data [8, 11].
The percentage of vertical transmission of GBS from infected mothers to neonates in the present study (26.6%) falls within the global report ranges. Compared to other global publications, however, this fraction of vertical transmission is lower than in studies conducted in Kuwait (35.5%), Bangladesh (38.0%) [17], China (7.6–16.7%) [19–21], the United States (53.8%) [22], and Eastern Ethiopia (53.8%) [23]. Other studies, such as those conducted by A. Joachim et al. in 2009 in Dar es Salaam, Tanzania (8.9%) [24] and by M. Gizachew et al. in Northwest Ethiopia was 10.4% [25], have shown results lower than that in the current study. Our explanation for this variation can be attributed to many reasons such as sample size, techniques used in diagnosis, period of stay in the delivery canal, premature rupture of membrane, and prophylaxis treatment by mothers.
The high vertical transmission rate of GBS contributes to substantial newborn and maternal morbidity and fatality. Vertical transmission of GBS can be prevented, hence healthcare practitioners and government officials must take this into account when developing initiatives to reduce maternal and newborn mortality [26]. Vertical transmission of GBS from a colonized woman to her neonate has not been explored properly, particularly in low income countries. A study investigating the risk variables that could be linked to vertical transmission would therefore help with the formulation of prevention measures. In the present study, we found that three maternal risk factors, mother work and antenatal care follow-up, were strongly associated with the vertical transmission of GBS from asymptomatic colonized mothers to their newborns. Women who had 4–5 ANC visits throughout their current pregnancy had a 20.9% lower risk of vertical GBS transmission to their neonates [17]. According to several studies, GBS colonization in pregnancy may be linked to a variety of factors including education, parity, mother's age, status of marriage, occupation, and an elevated body mass index [27, 28]. A substantial threat for morbidity and death rate in neonates with early-onset GBS illness has been linked to the colonization of the mother's birth canal.
This study showed statistically significant differences between GBS colonization in women in rural areas compared to those in urban areas (78.3% for rural versus 21.7% for urban; p = 0.015). This data disagrees with a study conducted in China by S. Li et al. in 2018, which showed no significant difference between women residing in rural and urban areas [29]. The plausible explanation for this difference is a lot of variables that make it easier for GBS to spread in rural regions such as low educational status, lack of health care facilities, and contamination from water and other sources. Furthermore, in this study, the neonatal samples were taken from three sites to increase the chance of GBS detection. The sample locations may influence vertical transmission rates. According to a study conducted in Pakistan [30], the risk of acquiring newborn GBS infection was much higher in sites like abdominal skin (53%) than in ear canals (18%). Furthermore, two Turkish studies published in the same year found that the rate of vertical transmission was 54.2% for three sites (throat, ear canal, and umbilicus) and 15.2% for two sites (throat and umbilicus) [31, 32]. In terms of parity, the prevalence of GBS colonization in our study was greater in more than three parities; the colonization rate of GBS in relation to parity was the highest during the reproductive years [33, 34]. The actual causes of such variable colonization are unknown and require additional research [35]. Thus, further research should be conducted to analyze the association of parity of women with GBS colonization.
The majority (61.6%) of participants in this study were between the ages of 25 and 34 years and had been pregnant at least once. This data differed from a similar study conducted by C. Turner et al. in 2012 on a population of refugees along the Thailand-Myanmar border in Southeast Asia, in which, most of the carriers were in their 20s [36]. These outcomes are similar to those of C. Adware et al. from Cameroon in 2008 [37], P. Foumane et al. in 2002 in Cameroon [38], A. Mengist et al. in 2016 in Ethiopia [39], and N.M. Nkembe et al. in 2018 [40], who reported the values to be 75%, 60%, 64%, and 65%, respectively. Our explanations for this age range consist of two reasons, firstly, some women delay pregnancy due to the presence of health issues that prevent pregnancy at the start of a marriage, and secondly, some married couples delay childbearing in the initial years of marriage. In particular, each and every GBS strain was susceptible to penicillin, ampicillin, vancomycin, and ceftriaxone indicating that these antibiotics could be used for preventative purposes. The majority of isolates obtained from mothers were sensitive to ciprofloxacin (96.6%), chloramphenicol (93.3%), levofloxacin (91.6%), and erythromycin (83.3%). In 87% (50/60) of isolates, tetracycline resistance was observed. In other nations such as Tunisia (97.3%) [41] and Iran (96%) [42], tetracycline resistance was extremely prevalent. Its use is currently restricted since the emergence of resistance appears to be linked to the extensive administration of antibiotics [43] and efficient plasmid transfer [43]. Penicillin is the drug of choice for treatment. In the case of penicillin allergy and anaphylaxis, ampicillin or vancomycin might be used as an alternative for treatment.
Nonetheless, the use of PCR to identify GBS and other pathogenic bacteria is crucial. This technique is considered a crucial method in the medical field, it is utilized in a number of medical fields to identify clinical diseases [44–48] and other dangerous genetic diseases such as cancers [49–64].
Conclusion
According to our findings which have demonstrated a high frequency of GBS infection in pregnant women and their newborns, a mandatory screening test for all pregnant women should be implemented, as well as preventive medication should be provided, to avoid the potentially fatal effects of this illness.
1 WHO. Number of infant deaths (between birth and 11 months); 2022. URL: https://who.int/data/gho/data/indicators/indicator-details/GHO/number-of-infant-deaths
2 Verani J.R., McGee L., Schrag S.J. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC; 2010. URL: https://cdc.gov/Mmwr/preview/mmwrhtml/rr5910a1.htm
Об авторах
Ali D. Marhash
Вавилонский технический институт, Технический университет Аль-Фурат Аль-Аусат
Автор, ответственный за переписку.
Email: alidmarhash@gmail.com
ORCID iD: 0000-0003-1078-0541
отдел медицинских лабораторий
Ирак, ВавилонZainab N. Nabat
Вавилонский технический институт, Технический университет Аль-Фурат Аль-Аусат
Email: alidmarhash@gmail.com
ORCID iD: 0000-0001-5517-8146
отдел медицинских лабораторий
Ирак, ВавилонNawras A. Abbas
Вавилонский технический институт, Технический университет Аль-Фурат Аль-Аусат
Email: alidmarhash@gmail.com
ORCID iD: 0000-0002-5818-5525
отдел общественного здравоохранения
Ирак, ВавилонСписок литературы
- Edwards M.S., Baker C.J. Streptococcus agalactiae (Group B Streptococcus). In: Mandell G.L., Bennett J.E., Dolin R., eds. Principles and Practices of Infectious Diseases. Elsevier;2010.
- Seale A.C., Bianchi-Jassir F., Russell N.J., et al. Estimates of the burden of group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin. Infect. Dis. 2017;65(Suppl. 2):S200–19. DOI: https://doi.org/10.1093/cid/cix664
- Parks T., Barrett L., Jones N. Invasive streptococcal disease: a review for clinicians. Br. Med. Bull. 2015;115(1):77–89. DOI: https://doi.org/10.1093/bmb/ldv027
- Lawn J.E., Cousens S., Zupan J.; Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? where? why? Lancet. 2005;365(9462):891–900. DOI: https://doi.org/10.1016/s0140-6736(05)71048-5
- Centers for Disease Control and Prevention (CDC). Early-onset and late-onset neonatal group B streptococcal disease – United States, 1996–2004. MMWR Morb. Mortal. Wkly. Rep. 2005;54(47):1205–8.
- Russell N.J., Seale A.C., O’Sullivan C., et al. Risk of early-onset neonatal group B streptococcal disease with maternal colonization worldwide: systematic review and meta-analyses. Clin. Infect. Dis. 2017;65(Suppl. 2):S152–9. DOI: https://doi.org/10.1093/cid/cix655
- Bianchi-Jassir F., Seale A.C., Kohli-Lynch M., et al. Preterm birth associated with group B Streptococcus maternal colonization worldwide: systematic review and meta-analyses. Clin. Infect. Dis. 2017;65(Suppl. 2):S133–42. DOI: https://doi.org/10.1093/cid/cix661
- Le Doare K., O’Driscoll M., Turner K., et al. Intrapartum antibiotic chemoprophylaxis policies for the prevention of group B streptococcal disease worldwide: systematic review. Clin. Infect. Dis. 2017;65(Suppl. 2):S143-51. DOI: https://doi.org/10.1093/cid/cix654
- Baker C.J., Barrett F.F. Transmission of group B streptococci among parturient women and their neonates. J. Pediatr. 1973;83(6):919–25. DOI: https://doi.org/10.1016/S0022-3476(73)80524-4
- Verani J.R., McGee L., Schrag S.J. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease – revised guidelines from CDC, 2010. MMWR Recomm. Rep. 2010; 59(RR-10):1–36.
- Russell N.J., Seale A.C., O’Driscoll M., et al. GBS Maternal Colonization Investigator Group. Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin. Infect. Dis. 2017;65(Suppl. 2):S100–11. DOI: https://doi.org/10.1093/cid/cix658
- CLSI. Performance standards for antimicrobial susceptibility testing. 27th ed. CLSI supplement M100. Wayne; 2017.
- Dilrukshi G.N., Kottahachchi J., Dissanayake D.M., et al. Group B Streptococcus colonisation and their antimicrobial susceptibility among pregnant women attending antenatal clinics in tertiary care hospitals in the Western Province of Sri Lanka. J. Obstet. Gynaecol. 2021;41(1):1–6. DOI: https://doi.org/10.1080/01443615.2020.1716313
- Kudagammana H.D., Rathnayaka R.M., Weerasooriya B.W., et al. Group B streptococcal colonisation among Sri Lankan mothers. SCIREA J. Clin. Med. 2019;4(6):209–15.
- Sapugahawatte D.N., Li.C., Liyanapathirana V., et al. Colonization of group B Streptococcus in pregnant women and their neonates from a Sri Lankan hospital. Pathogens. 2022;11(4):386. DOI: https://doi.org/10.3390/pathogens11040386
- Schörner M.A., Feuershuette O.H., Scheffer M.C., et al. Detection of group B Streptococcus agalactiae from anorectal and vaginal screening tests. Clin. Microbiol. 2014;3(5). DOI: https://doi.org/10.4172/2327-5073.1000169
- Al-Sweih N., Hammoud M., Al-Shimmiri M., et al. Serotype distribution and mother-to-baby transmission rate of Streptococcus agalactiae among expectant mothers in Kuwait. Arch. Gynecol. Obstet. 2005;272(2):131–5. DOI: https://doi.org/10.1007/s00404-004-0705-z
- Saha S.K., Ahmed Z.B., Modak J.K., et al. Group B Streptococcus among pregnant women and newborns in Mirzapur, Bangladesh: colonization, vertical transmission, and serotype distribution. J. Clin. Microbiol. 2017;55(8):2406–12. DOI: https://doi.org/10.1128/jcm.00380-17
- Chen J., Fu J., Du W., et al. Group B streptococcal colonization in mothers and infants in western China: prevalences and risk factors. BMC Infect. Dis. 2018;18(1):291. DOI: https://doi.org/10.1186/s12879-018-3216-4
- Chen Z., Wu C., Cao X., et al. Risk factors for neonatal group B streptococcus vertical transmission: a prospective cohort study of 1815 mother–baby pairs. J. Perinatol. 2018;38(10):1309–17. DOI: https://doi.org/10.1038/s41372-018-0182-z
- Yang M.J., Sun P.L., Wen K.C., et al. Prevalence of maternal group B streptococcus colonization and vertical transmission in low-risk women in a single institute. J. Chin. Med. Assoc. 2012;75(1):25–8. DOI: https://doi.org/10.1016/j.jcma.2011.10.011
- Hickman M.E., Rench M.A., Ferrieri P., et al. Changing epidemiology of group B streptococcal colonization. Pediatrics. 1999;104(2 Pt. 1):203–9. DOI: https://doi.org/10.1542/peds.104.2.203
- Yadeta T.A., Worku A., Egata G., et al. Vertical transmission of group B Streptococcus and associated factors among pregnant women: a cross-sectional study, Eastern Ethiopia. Infect. Drug Resist. 2018;11:397–404. DOI: https://doi.org/10.2147/idr.s150029
- Joachim A., Matee M.I., Massawe F.A., et al. Maternal and neonatal colonisation of group B streptococcus at Muhimbili National Hospital in Dar es Salaam, Tanzania: prevalence, risk factors and antimicrobial resistance. BMC Public Health. 2009;9:437. DOI: https://doi.org/10.1186/1471-2458-9-437
- Gizachew M., Tiruneh M., Moges F., et al. Proportion of Streptococcus agalactiae vertical transmission and associated risk factors among Ethiopian mother-newborn dyads, Northwest Ethiopia. Sci. Rep. 2020;10(1):3477. DOI: https://doi.org/10.1038/s41598-020-60447-y
- Islam M.S., Saha S.K., Islam M., et al. Prevalence, serotype distribution and mortality risk associated with group B Streptococcus colonization of newborns in rural Bangladesh. Pediatr. Infect. Dis. J. 2016;35(12):1309–12. DOI: https://doi.org/10.1097/INF.0000000000001306
- Schuchat A., Oxtoby M., Cochi S., et al. Population-based risk factors for neonatal group B streptococcal disease: results of a cohort study in metropolitan Atlanta. J. Infect. Dis. 1990;162(3):672–7. DOI: https://doi.org/10.1093/infdis/162.3.672
- Stapleton R.D., Kahn J.M., Evans L.E., et al. Risk factors for group B streptococcal genitourinary tract colonization in pregnant women. Obstet. Gynecol. 2005;106(6):1246–52. DOI: https://doi.org/10.1097/01.AOG.0000187893.52488.4b
- Li S., Wen G., Cao X., et al. Molecular characteristics of Streptococcus agalactiae in a mother-baby prospective cohort study: implication for vaccine development and insights into vertical transmission. Vaccine. 2018;36(15):1941–8. DOI: https://doi.org/10.1016/j.vaccine.2018.02.109
- Chaudhry B.Y., Akhtar N., Balouch A.H. Vaginal carriage rate of group B Streptococcus in pregnant women and its transmission to neonates. J. Ayub Med. Coll. Abbottabad. 2010;22(4):167–70.
- Kadanali A., Altoparlak U., Kadanali S. Maternal carriage and neonatal colonisation of group B Streptococcus in eastern Turkey: prevalence, risk factors and antimicrobial resistance. Int. J. Cin. Pract. 2005;59(4):437–40. DOI: https://doi.org/10.1111/j.1368-5031.2005.00395
- Eren A., Kucukercan M., Oguzoglu N., et al. The carriage of group B streptococci in Turkish pregnant women and its transmission rate in newborns and serotype distribution. Turk. J. Pediatr. 2005;47(1):28–33.
- Berg A.W., Sprij A.J., Oostvogel P.M., et al. Prevalence of colonisation with group B streptococci in pregnant women of a multi-ethnic population in the Netherlands. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006;124(2):178–83. DOI: https://doi.org/10.1016/j.ejogrb.2005.06.007
- Munir S.K., Waheed K., Khanum A., et al. Frequency of group B streptococci in pregnant women in a tertiary care hospital. J. Coll. Physicians Surg. Pak. 2016;26(1):27–30.
- Khan M.A., Faiz A., Ashshi A.M. Maternal colonization of group B Streptococcus prevalence, associated factors and antimicrobial resistance. Ann. Saudi Med. 2015;35(6):423–7. DOI: https://doi.org/10.5144/0256-4947.2015.423
- Turner C., Turner P., Po L., et al. Group B streptococcal carriage, serotype distribution and antibiotic susceptibilities in pregnant women at the time of delivery in a refugee population on the Thai-Myanmar border. BMC Infect. Dis. 2012;12:34. DOI: https://doi.org/10.1186/1471-2334-12-34
- Adware C., Michel T., Paul A.J., et al. Vaginal colonization and resistance profile of group B Streptococcus among pregnant women in Yaoundé Gynaecology, Obstetric and Paediatric Hospital in Cameroon. J. Clin. Med. Res. 2014;6(3):16–21. DOI: https://doi.org/10.5897/JCMR2014.0249
- Foumane P., Mboudou E., Dohbit J.S, et al. Group B beta hemolytic Streptococcus in pregnancy and its effect on maternal and foetal outcome in the Yaounde General Hospital: a descriptive study. Clin. Mother Child Health. 2009;6(1):995–1002.
- Mengist A., Kannan H., Abdissa A. Prevalence and antimicrobial susceptibility pattern of anorectal and vaginal group B Streptococci isolates among pregnant women in Jimma, Ethiopia. BMC Res. Notes. 2016;9:351. DOI: https://doi.org/10.1186/s13104-016-2158-4
- Nkembe N.M., Kamga H.G., Baiye W.A., et al. Streptococcus agalactiae prevalence and antimicrobial susceptibility pattern in vaginal and anorectal swabs of pregnant women at a tertiary hospital in Cameroon. BMC Res. Notes. 2018;11(1):480. DOI: https://doi.org/10.1186/s13104-018-3589-x
- Santhanam S., Jose R., Sahni R.D., et al. Prevalence of group B streptococcal colonization among pregnant women and neonates in a tertiary hospital in India. J. Turk. Ger. Gynecol. Assoc. 2017;18(4):181–4. DOI: https://doi.org/10.4274/jtgga.2017.0032
- Campbell J.R., Hillier S.L., Krohn M.A., et al. Group B streptococcal colonization and serotype-specific immunity in pregnant women at delivery. Obstet. Gynecol. 2000;96(4):498–503. DOI: https://doi.org/10.1016/s0029-7844(00)00977-7
- Burdett V. Identification of tetracycline-resistant R-plasmids in Streptococcus agalactiae (group B). Antimicrob. Agents Chemother. 1980;18(5):753–60. DOI: https://doi.org/10.1128/aac.18.5.753
- Husain A.G., Alrubaii B.A. Molecular detection and expression of virulence factor encoding genes of Pseudomonas aeruginosa isolated from clinical samples. Biomedicine. 2023;43(5):1514–9. DOI: https://doi.org/10.51248/.v43i5.3263
- Saleh T.H., Hashim S.T., Malik S.N., et al. The impact some of nutrients on swarming phenomenon and detection the responsible gene RsbA in clinical isolates of Proteus mirabilis. Int. J. Res. Pharm. Sci. 2020;11(1):437–44. DOI: https://doi.org/10.26452/ijrps.v11i1.1839
- Sabah Fakhry S., Noori Hammed Z., Abdul-elah Bakir W., Abdullah Laftaah ALRubaii B. Identification of methicillin-resistant strains of Staphylococcus aureus isolated from humans and food sources by Use of mecA 1 and mecA 2 genes in Pulsedfield gel electrophoresis (PFGE) technique. Revis Bionatura. 2022;7(2):44. DOI: https://doi.org/10.21931/RB/2022.07.02.44
- Ali M.A., Al-Rubaii B.A. Study of the effects of audible sounds and magnetic fields on Staphylococcus aureus methicillin resistance and mecA gene expression. Trop. J. Nat. Prod. Res. 2023;5(5):825–830. DOI: org/10.26538/tjnpr/v5i5.6
- Mohsin M.R., AL-Rubaii B.A. Bacterial growth and antibiotic sensitivity of Proteus mirabilis treated with anti-inflammatory and painkiller drugs. Biomedicine. 2023;43(2):728–34. DOI: https://doi.org/10.51248/.v43i02.2693
- Bassi A.G., Al-Rubaii B.A. Detection of pyocin S and the effects of lactobacillus acidophilus cell-free supernatants on multi-drug resistant Pseudomonas aeruginosa isolated from patients of Baghdad Hospitals. J. Commun. Dis. 2024;56(1):135–44. DOI: https://doi.org/10.24321/0019.5138.202418
- Abbas M.S., Ahmed A.G., Ali S.Q., et al. Immunological inflammatory factors in patients diagnosed with COVID-19. Biomedicine. 2023;43(1):230–5. DOI: https://doi.org/10.51248/.v43i1.2413
- Al-Saadi H.K., Awad H.A., Saltan Z.S., et al. Antioxidant and antibacterial activities of Allium sativum ethanol extract and silver nanoparticles. Trop. J. Nat. Prod. Res. 2023;7(6):3105–10. DOI: https://doi.org/10.26538/tjnpr/v7i6.5
- Hassoon A.H. Evaluating the role of mitochondrial DNA quantification in blastocyst transfers potential. AIP Conf. Proc. 2022;2386(1):020046. DOI: https://doi.org/10.1063/5.0067093
- Buniya H.K., Hassoon A.H., Hameed A.K. Molecular genetic variability in the d-loop region for females with breast cancer and the effect of the chemotherapy. Res. J. Pharm. Technol. 2018;11(9):3787–92. DOI: https://doi.org/10.5958/0974-360X.2018.00694.7
- Rasoul L.M., Marhoon A.A., Albaayit S.F., et al. Cytotoxic effect of cloned EGFP gene on NCI-H727 cell line via genetically engineered gene transfer system. Biomedicine. 2022;42(5): 938–42. DOI: https://doi.org/10.51248/.v42i5.1934
- Bresam S., Al-Jumaily R.M., Karim G.F., et al. Polymorphism in SNP rs972283 of the KLF14 gene and genetic disposition to peptic ulcer. Biomedicine. 2023;43(1):216–20. DOI: https://doi.org/10.51248/.v43i1.2411
- Al-Jumaily R.M., AL-Sheakli I.I., Muhammed H.J., et al. Gene expression of Interleukin-10 and Foxp3 as critical biomarkers in rheumatoid arthritis patients. Biomedicine. 2023;43(4):1183–7. DOI: https://doi.org/10.51248/.v43i4.3107
- Sultan R.S., Al Khayali B.D., Abdulmajeed G.M., et al. Exploring small nucleolar RNA host gene 3 as a therapeutic target in breast cancer through metabolic reprogramming. Opera Med. Physiol. 2023;10(4):36-47. DOI: https://doi.org/10.24412/2500-2295-2023-4-36-47
- Ismael M.K., Qaddoori Y.B., Shaban M.N., et al. The immunohistochemical staining of Vimentin and E-Cadherin in bladder cancer patients infected with hepatitis C virus. J. Pure Appl. Microbiol. 2023;17(2):1009–16. DOI: https://doi.org/10.22207/JPAM.17.2.30
- Bresam S., Alhumairi R.M.A.U., Hade I.M., et al. Genetic mutation rs972283 of the KLF14 gene and the incidence of gastric cancer. Biomedicine. 2023;43(4):1256–60. DOI: https://doi.org/10.51248/.v43i1.2411
- Hamoode R.H., Alkubaisy S.A., Sattar D.A., et al. Detection of anti-testicular antibodies among infertile males using indirect immunofluorescent technique. Biomedicine. 2022;42(5):978–82. DOI: https://doi.org/10.51248/.v42i5.1963
- Mohammed R.A., Al-Asady Z.T.S., Frayyeh M.J. et al .The influence of radiotherapy exposure on anti-TPO Ab, anti-Tg Ab, anti-nuclear Ab, anti-DSA Ab and complete blood markers in hospital physician workers in Nuclear Baghdad Hospital. Opera Med. Physiol. 2024;11(2):5–15. doi: 10.24412/2500-2295-2024-2-5-15
- Hadi S.T., Hashim S., Abdulrazaq Al-Obaidi R.A., et al. A biological study of chitinase produced by clinical isolates of Pseudomonas aeruginosa and detection of chia responsible gene. Int. J. R. Pharm. Sci. 2020;11(2):1318–30. DOI: https://doi.org/10.26452/ijrps.v11i2.1989
- Jawad N.K., Numan A.T., Ahmed A.G., et al. IL-38 gene expression: a new player in Graves’ ophthalmopathy patients in Iraq. Biomedicine. 2023;43(1):210–5. DOI: https://doi.org/10.51248/.v43i1.2027
- Шалепо К.В., Хуснутдинова Т.А., Будиловская О.В. и др. Молекулярно-генетические детерминанты вирулентности Streptococcus agalactiae, выделенных у беременных и новорождённых Санкт-Петербурга и Ленинградской области в 2010–2023 годах. Журнал микробиологии, эпидемиологии и иммунобиологии. 2024;101(2):217–26. Shalepo K.S., Khusnutdinova T.A., Budilovskaya O.V., et al. Molecular genetic determinants of virulence of Streptococcus agalactiae isolated from pregnant women and newborns in St. Petersburg and the Leningrad region in 2010–2023. Journal of Microbiology, Epidemiology and Immunobiology. 2024; 101(2):217–26. DOI: https://doi.org/10.36233/0372-9311-501 EDN: https://elibrary.ru/qnunfe
Дополнительные файлы